Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Bogaczyk | -- | 2698 | 2023-07-26 21:28:24 | | | |
| 2 | Conner Chen | Meta information modification | 2698 | 2023-07-28 02:41:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bogaczyk, A.; Zawlik, I.; Zuzak, T.; Kluz, M.; Potocka, N.; Kluz, T. MicroRNAs in Endometrial Cancer Patients. Encyclopedia. Available online: https://encyclopedia.pub/entry/47328 (accessed on 08 February 2026).
Bogaczyk A, Zawlik I, Zuzak T, Kluz M, Potocka N, Kluz T. MicroRNAs in Endometrial Cancer Patients. Encyclopedia. Available at: https://encyclopedia.pub/entry/47328. Accessed February 08, 2026.
Bogaczyk, Anna, Izabela Zawlik, Tomasz Zuzak, Marta Kluz, Natalia Potocka, Tomasz Kluz. "MicroRNAs in Endometrial Cancer Patients" Encyclopedia, https://encyclopedia.pub/entry/47328 (accessed February 08, 2026).
Bogaczyk, A., Zawlik, I., Zuzak, T., Kluz, M., Potocka, N., & Kluz, T. (2023, July 26). MicroRNAs in Endometrial Cancer Patients. In Encyclopedia. https://encyclopedia.pub/entry/47328
Bogaczyk, Anna, et al. "MicroRNAs in Endometrial Cancer Patients." Encyclopedia. Web. 26 July, 2023.
Copy Citation
Endometrial cancer is one of the most common cancers in developing and developed countries. Although the detection of this cancer is high at the early stages, there is still a lack of markers to monitor the disease, its recurrence, and metastasis. MiRNAs are in charge of the post-transcriptional regulation of genes responsible for the most important biological processes, which is why they are increasingly used as biomarkers in many types of cancer.
miRNA
endometrial cancer
epigenetics
1. Introduction
1.1. Endometrial Cancer
Endometrial cancer (EC) was diagnosed in 417,367 women worldwide in 2020, with the highest burden of the disease recorded in North America and Western Europe. The incidence of EC is rapidly increasing. As of 2020, uterine cancer is the fourth most common female cancer in Europe, with an incidence of 12.9–20.2 per 100,000 women and a mortality rate of 2.0–3.7 per 100,000 women [1]. The high incidence rate in North America and Western Europe can be attributed to the high prevalence of lifestyle risk factors for EC, such as high standard of living, aging population, and obesity, which are associated with approximately 50% of EC cases [2].
In the historical morphological division (according to Bokhman’s dualistic theory), EC was classified as type I, the so-called endometroid, which is associated with excessive estrogen stimulation, develops on the basis of endometrial hyperplasia, is more common, and has a favorable prognosis. Type II (non-endometrioid) unrelated to estrogen stimulation has a poor prognosis. Type I includes stage I or II endometrioid adenocarcinoma, while type II EC includes stage III endometrioid adenocarcinoma, serous, clear cell, undifferentiated, and carcinoma [3]. The Cancer Genome Atlas (TCGA), introduced molecular profiling in 2013, which indicates a paradigm shift from morphological to molecular classification [4]. The TCGA studies identified four molecular subgroups characterized by the POLE mutation (POLEmut group), microsatellite instability (MSI group), which arises from MMRD, high somatic copy number changes (driven by the TP53 mutation, also called p53abn group), and a low number of copies without a specific molecular profile (NSMP group), each with a separate prognosis [4]. POLEmut tumors, despite their aggressive appearance, have an extremely favorable prognosis, while the group with a high copy number driven by the TP53 mutation has an unfavorable prognosis. The prognosis of tumors with a mismatch repair deficiency (MMRd) and those without a specific molecular profile (NSMP) is relatively favorable [5][6].
The basic treatment of endometrial cancer is surgery and, possibly, subsequent chemotherapy, radiotherapy, and chemoradiotherapy [5][7][8]. The risk of endometrial cancer recurrence is also present in very low-risk cases and is 2.9% within the first 3 years after the end of treatment [9]. Therefore, it is necessary to study the molecular mechanisms in the pathogenesis of endometrial cancer in order to discover new therapeutic methods.
1.2. MicroRNAs
The non-coding molecules play a particular role in the regulation of gene expression. The group of regulatory non-coding RNAs includes transport RNA (tRNA), ribosomal RNA (rRNA), antisense RNA (asRNA), microRNA (miRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), competing endogenous RNA (ceRNA), and piwi-interactive RNA (piRNA) [10].
MiRNAs are a particularly interesting group in terms of regulation of gene expression. They were discovered in 1993 and are non-coding, single-stranded, small RNA molecules about 19–25 nucleotides long. The first ones to be described were small RNA molecules encoded by the lin-4 gene, which regulates the expression of the lin-14 protein in Caenorhabditis elegans by Lee et al. [11].
MiRNA formation begins in the cell nucleus where polymerase II (Pol II) transcribes the pri-miRNA. Pri-miRNA is trimmed by the DROSHA complex and DGCR8 proteins to pre-miRNA. Then, the pre-miRNA is exported by Exportin 5 to the cytoplasm [12]. In this transport, Exportin 5 interacts with the Ran protein. In the cytoplasm, a miRNA duplex is formed from the pre-miRNA, which then separates into two mature single-stranded miRNAs. This process takes place with the participation of DICER and Argonaute 2 (AGO2) [13][14].
MiRNAs function as components of a ribonucleoprotein complex called miRISC (microRNA-induced silencing complex) [15]. Mature miRNA molecules, embedded in miRISC complexes, have the ability to bind to the 3′ untranslated regions (3′UTR) of the mRNA of the target gene. As a result of full nucleotide complementarity, they can lead to transcript degradation. In most cases, miRNAs are usually imperfectly complementary to their target gene and modulate the effect on gene expression via translational repression [16]. The mechanism of action of miRNAs involves binding to a sequence within the RNA-induced silencing complex (RISC), and then gene regulation through translational repression, mRNA degradation, poly(A) tail shortening, and removal of the 5′7-methylguanyl cap [17].
MiRNAs are involved in various cellular functions including proliferation, migration, invasion, and the epithelial–mesenchymal transition (EMT) process. EMT is an important process where epithelial cells lose cell–cell contact and undergo a gradual transformation from an epithelial to a mesenchymal phenotype, which includes, i.e., cytoskeletal remodeling and migratory activity [18]. MiRNAs affect genome instability, regulate metabolism, and influence the apoptosis process of tumor cells; in addition, they also play a role in angiogenesis and immune escape of cancer [19][20][21][22][23]. They may also regulate gene expression within the cell or may be released outside the cell. This leads to the regulation of gene expression in neighboring cells. Therefore, they are regulators of a complex network of processes occurring in the tumor microenvironment [24]. For instance, the let-7 family acts as a regulator of normal cell differentiation and proliferation and inhibits the growth of cancer cells. Let-7 levels are crucial for development cells and act directly on RAS genes via LIN28 [25]. Masood N. et al. have reported mutual inhibition of let-7 and LIN28, but let-7 also inhibits IL-6 in embryonic cells, resulting in high levels of NFKB. NFKB together with c-Myc has a stimulating effect on the formation of higher levels of LIN28 in cells. This increase in LIN28 then leads to a marked decrease in let-7 [26].
During carcinogenesis, the miRNA expression profile is significantly dysregulated. This is the result of many changes, including amplification and deletion of genes or epigenetic abnormalities. Moreover, miRNA expression is deregulated in cancer as a result of defects in their biogenesis machinery, including DICER and DROSHA [17][27]. Overexpressed miRNAs in cancers can function as oncogenes and promote cancer development through downregulated tumor suppressor genes or genes that control cell differentiation or apoptosis. Underexpressed miRNAs can function as cancer suppressor genes and can inhibit cancers by regulating oncogenes or genes that control cell differentiation or apoptosis [28]. Such examples are miR-181a, miR-181b, and miR-181c, which are downregulated in glioma [29], while miR-181a and miR-181b are overexpressed in patients with acute lymphoblastic leukemia (ALL) [30].
In addition, they are involved in the regulation of cancer-related signaling pathways, including the JAK/STAT3 transcription pathway [31], the NF-KB pathway [32], and the MAPK/ERK pathway [33]. They may also affect other miRNAs and may be subject to mutual regulation of miRNAs: miRNAs [34].
MiRNAs can be regulators of the above processes, but they can also be regulated by such molecules as circular RNAs, long ncRNAs, or pseudogenes. CircRNA molecules act as ”sponges” for miRNA and thus regulate the amount of free miRNA. They are post-transcriptional regulators of gene expression regulation. A single circRNA molecule can bind to several miRNAs [35][36].
Currently, miRNAs are attractive candidates for therapeutic targets in the treatment of malignancies. Therefore, identifying their targets is essential for cancer research. They are used to assess response to treatment. MiRNAs have also been found to induce chemoresistance in various cancers [37]. A relationship has also been found between miRNA expression and response to treatment, for example, in breast cancer, miRNA-205 was upregulated in tamoxifen resistance cells MCF-7/TAMR-1 (M/T) and M/T cell-derived exosomes (M/ T-Exo) [38]. In lung cancer, the relationship between miRNA levels and cisplatin resistance has also been demonstrated, and miR33b-3p, miR-425-3p, miR-124, miR-295-5p are overexpressed, while miR-98, miR-26a, miR-107 or miR-17 [39]. In advanced colorectal cancer, resistance to FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) has been shown to correlate with miR-19a overexpression [40]. A similar situation occurs when treating patients with advanced CRC with anti-VEGF or anti-EGFR inhibitors, e.g., overexpression of miR-126 has been correlated with resistance to bevacizumab [41].
The phenomenon of chemoresistance also occurs in endometrial cancer. MiR-222-3p has been shown to increase raloxifene resistance by suppressing Erα expression in cancer cells. MiR-222-3p may be a potential target for restoring ERα expression and response to antiestrogen therapy in the EC. With the upregulation of miR-222-3p, RL95-2 cells were less sensitive to raloxifene. In contrast, AN3CA cells were more sensitive after miR-222-3p inhibition [42].
An interesting direction of research is resistance to cisplatin. Cisplatin has been used in the treatment of various cancers as an effective chemotherapeutic agent for several decades. Wang et al. showed that overexpression of miR-135a increased the survival of endometrial cancer cells after cisplatin treatment. And the decrease in miR-135a expression reduced the survival of endometrial cancer cells after cisplatin treatment. Researchers indicated that miR-135a regulated cisplatin resistance in EC cells. The expression level of miR-135a was associated with cisplatin-induced apoptosis in EC cells. These findings suggest that miR-135a may affect the chemosensitivity of endometrial cancer cells to cisplatin treatment [43].
The most commonly used material for miRNA detection is tissue obtained during surgery. They can also be detected in blood, serum, urine, and other body fluids [44][45]. The method of using blood collection instead of abrasion of the uterus is much easier and carries a lower risk of complications, such as uterine infection. In the future, miRNA profile analysis may be included in routine blood tests for endometrial cancer screening in the general population. This is of great importance, especially for patients living in places with difficult access to health care.
2. MicroRNAs in Endometrial Cancer Patients
2.1. The Process of Carcinogenesis
The development of endometrial cancer is a complex process involving multiple oncogenes and tumor suppressor genes, although the molecular mechanisms are not clear. In recent years, many studies have been conducted on the expression and function of miRNAs in endometrial cancer [46][47][48][49]. Cancer progression involves several key steps (Figure 1), including primary tumor growth, migration, and local invasion, transendothelial migration of cancer cells into vessels known as intravasation, survival in the circulatory system, extravasation, and niche formation (pre-metastatic niche). This is followed by the recruitment of tumor-promoting immune cells and metastasis. Each stage of carcinogenesis is regulated by many miRs.
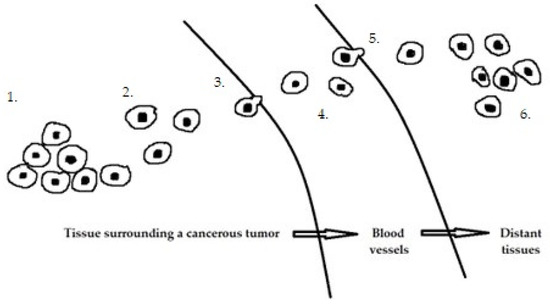
Figure 1. Stages of cancer progression: 1. Primary tumor growth. 2. Migration and local invasion. 3. Transendothelial migration of cancer cells into vessels 4. Survival in the circulatory system. 5. Extravasation. 6. Pre-metastatic niche formation.
2.2. Risk Factors and Prognostic Factors
Risk factors for EC include genetic predisposition to Lynch syndrome and Cowden syndrome, polycystic ovary syndrome (PCOS), use of tamoxifen, infertility, diabetes, and obesity [50][51][52]. On the other hand, prognostic factors for EC include the patient’s age, stage of endometrial cancer, involvement of lymph nodes, and lymphatic space (LVSI) [53].
2.2.1. Polycystic Ovary Syndrome (PCOS)
PCOS is the most common endocrine disorder among young women of reproductive age. It is characterized by rare or absent ovulation and hyperandrogenism. In patients with PCOS, other factors of endometrial cancer are often diagnosed, such as diabetes, obesity, and nulliparous status [54]. MiRNAs have been studied in patients with polycystic ovary syndrome (PCOS) [55]. This is a large group of mostly young women. The worldwide prevalence of PCOS ranges from 4–21% [56]. Obesity with or without concomitant diabetes often coexists in these women [57]. Many abnormalities and overexpression of many miRNAs have been found in them. It has been discovered that changes in their expression occurring in PCOS are also often associated with metabolic syndrome, which includes hypertension, dyslipidemia, central obesity, and impaired glucose tolerance [58].
PCOS increases the risk of developing endometrial cancer 2.7 times. It has been shown that women with PCOS also change the level of miRNAs, e.g., miR-27a-5p, the level of which is increased in serum-derived exosomes. MiR-27a-5p plays a role in EC cell migration and invasion by regulating SMAD4 [59].
2.2.2. Obesity and Diabetes
Altered expression patterns of miRNAs are not only associated with cancer development but also with comorbidities that are common in patients with EC. These diseases include obesity, type 2 diabetes, and cardiovascular diseases [60]. They are risk factors for many cancers, including endometrial cancer. Another important risk factor in EC carcinogenesis is excessive estrogenic stimulation of the endometrium with a simultaneous lack of progesterone effect. This is the case with polycyclic ovary syndrome (PCOS), obesity, functional tumors, and iatrogenic use of estrogens [54][61][62]. Serum miRNA levels were abnormal in obese women or women with type 2 diabetes, data are summarized in Table 1.
Table 1. MiRNA changes in obese women.
2.2.3. Aging of the Body
Aging is a natural and multifactorial phenomenon characterized by the accumulation of degenerative processes, which in turn are underpinned by multiple changes and damages in molecular pathways [70][71]. Despite many theories that have been proposed to explain the phenomenon of aging, none has been able to fully explain the mechanisms that drive the underlying process so far [71]. MiRNA expression also changes with the age of patients [72]. Some of these were downregulated in long-lived individuals, such as let-7, miR-17, and miR-34 (known as longevity miRNAs). They are conserved in humans and probably promote life extension. Conversely, they are upregulated in age-related diseases such as cancer [73]. MiR-151a-3p, miR-181a-5p, and miR-1248 are downregulated with age [71][74]. In contrast, miR-21 and miR-23a expression increases in middle-aged humans and decreases in advanced age [75].
2.2.4. Involvement of the Lymph Nodes Metastasis
One of the most important prognostic factors used to determine the stage of EC and possible adjuvant treatment is the presence of neoplastic cells in the lymph nodes. Lymphadenectomy is associated with significant surgical and postoperative risks. The use of sentinel lymph node mapping (SLNM) has emerged as an alternative method for total lymphadenectomy in the EC [76]. However, controversy remains over the use of SLNM in high-risk diseases and its false-negative rate (3%) [77]. Reliable SLNM mapping requires surgeons and institutions to be equipped with appropriate knowledge and skills. In addition, SLNM mapping is performed during the operation. It is also worth remembering that the involvement of lymph nodes may also engage paraaortic nodes. Isolated involvement of the paraaortic nodes in patients without pelvic nodal metastases was only 1% [78]. Therefore, finding pre-operative methods that can accurately identify LNM (lymph node metastasis) would be of great clinical value. MiRNA mapping may prove to be such a way. A correlation of miRNAs depending on the presence of relapses in lymph nodes has been demonstrated. Table 2 summarizes the above data.
Table 2. MicroRNAs changes in metastatic lymph nodes.
2.2.5. The Impact of miRNA Changes on Survival and Recurrence in Patients with Endometrial Cancer
Mortality related to endometrial cancer continues to increase [92]. Although most patients with endometrial cancer have a tumor confined to the uterus that is treated by hysterectomy with or without adjuvant therapy, the advanced disease has a poor prognosis [93]. Although early-stage endometrial cancer is associated with a favorable 5-year relative survival rate (96%), the rate is only 18% in patients with distant metastases [94]. Patients with an increase in the recurrence-free period were examined and changes in microRNA levels were also found here (Table 3).
Table 3. MicroRNA changes occurring in patients with good prognosis, with long PFS (progression-free survival).
On the other hand, the factors associated with shortening the recurrence-free period are a high expression of miR-21 [79] or miR-205 [102].
Changes in miRNA expression levels are clearly visible in tumor tissue but can also be seen in plasma/serum. In patients with endometrial cancer, two groups—increased and decreased expression—were distinguished. Disorders of miRNA expression in plasma/serum are summarized in Table 4. Such studies are particularly important due to the ease of obtaining material for research.
Table 4. Serum miRNA changes in patients with endometrial cancer.
| Upregulation | miR-15a-5p [103] miR-20b-5p [104] miR-27a [105] miR-106b-5p [103] miR-107 [103] miR-143 [44][104] miR-143-3p [104] miR-150-5p [105] miR-186 [106][107] miR-195-5p [104] miR-200a [106] miR-203 [106] miR-204 [106] miR-204-5p [104] miR-222 [106][107] miR-223 [106][107] miR-423-3p [104] miR-449 [106] miR-484 [104] miR-887-5p [108] |
| Downregulation | miR-16 [44] miR-99b [44] miR-125 [44] miR-145 [44] miR-204 [107] |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428.
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Primers 2021, 7, 88.
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73.
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397.
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224.
- Dowdy, S.C.; Glaser, G.E. Adjuvant therapy for women with high-risk endometrial carcinoma. Lancet Oncol. 2018, 19, 268–269.
- Matei, D.; Filiaci, V.; Randall, M.E.; Mutch, D.; Steinhoff, M.M.; DiSilvestro, P.A.; Moxley, K.M.; Kim, Y.M.; Powell, M.A.; O’Malley, D.M.; et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N. Engl. J. Med. 2019, 380, 2317–2326.
- Stasenko, M.; Feit, N.; Lee, S.S.K.; Shepherd, C.; Soslow, R.A.; Cadoo, K.A.; Alektiar, K.; Da Silva, E.M.; Martins Sebastião, A.P.; Leitao, M.M.; et al. Clinical patterns and genomic profiling of recurrent ‘ultra-low risk’ endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 717–723.
- Vallone, C.; Rigon, G.; Gulia, C.; Baffa, A.; Votino, R.; Morosetti, G.; Zaami, S.; Briganti, V.; Catania, F.; Gaffi, M.; et al. Non-Coding RNAs and Endometrial Cancer. Genes 2018, 9, 187.
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854.
- Kim, V.N. MicroRNA precursors in motion: Exportin-5 mediates their nuclear export. Trends Cell Biol. 2004, 14, 156–159.
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333.
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target Ther. 2016, 1, 15004.
- Fabian, M.R.; Sonenberg, N. The mechanics of miRNA-mediated gene silencing: A look under the hood of miRISC. Nat. Struct. Mol. Biol. 2012, 19, 586–593.
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297.
- Grzywa, T.M.; Klicka, K.; Włodarski, P.K. Regulators at Every Step-How microRNAs Drive Tumor Cell Invasiveness and Metastasis. Cancers 2020, 12, 3709.
- Chiu, H.C.; Li, C.J.; Yiang, G.T.; Tsai, A.P.; Wu, M.Y. Epithelial to Mesenchymal Transition and Cell Biology of Molecular Regulation in Endometrial Carcinogenesis. J. Clin. Med. 2019, 8, 439.
- Vincent, K.; Pichler, M.; Lee, G.W.; Ling, H. MicroRNAs, genomic instability and cancer. Int. J. Mol. Sci. 2014, 15, 14475–14491.
- Shirjang, S.; Mansoori, B.; Asghari, S.; Duijf, P.H.G.; Mohammadi, A.; Gjerstorff, M.; Baradaran, B. MicroRNAs in cancer cell death pathways: Apoptosis and necroptosis. Free Radic. Biol. Med. 2019, 139, 1–15.
- Pedroza-Torres, A.; Romero-Córdoba, S.L.; Justo-Garrido, M.; Salido-Guadarrama, I.; Rodríguez-Bautista, R.; Montaño, S.; Muñiz-Mendoza, R.; Arriaga-Canon, C.; Fragoso-Ontiveros, V.; Álvarez-Gómez, R.M.; et al. MicroRNAs in Tumor Cell Metabolism: Roles and Therapeutic Opportunities. Front. Oncol. 2019, 9, 1404.
- Wang, Y.; Wang, L.; Chen, C.; Chu, X. New insights into the regulatory role of microRNA in tumor angiogenesis and clinical implications. Mol. Cancer 2018, 17, 22.
- Yi, M.; Xu, L.; Jiao, Y.; Luo, S.; Li, A.; Wu, K. The role of cancer-derived microRNAs in cancer immune escape. J. Hematol. Oncol. 2020, 13, 25.
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673.
- Iliopoulos, D.; Hirsch, H.A.; Struhl, K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009, 139, 693–706.
- Masood, N.; Basharat, Z.; Khan, T.; Yasmin, A. Entangling Relation of Micro RNA-let7, miRNA-200 and miRNA-125 with Various Cancers. Pathol. Oncol. Res. 2017, 23, 707–715.
- Klicka, K.; Grzywa, T.M.; Klinke, A.; Mielniczuk, A.; Włodarski, P.K. The Role of miRNAs in the Regulation of Endometrial Cancer Invasiveness and Metastasis—A Systematic Review. Cancers 2021, 13, 3393.
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007, 302, 1–12.
- Ciafrè, S.A.; Galardi, S.; Mangiola, A.; Ferracin, M.; Liu, C.G.; Sabatino, G.; Negrini, M.; Maira, G.; Croce, C.M.; Farace, M.G. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem. Biophys. Res. Commun. 2005, 334, 1351–1358.
- Mendiola-Soto, D.K.; Bárcenas-López, D.A.; Pérez-Amado, C.J.; Cruz-Miranda, G.M.; Mejía-Aranguré, J.M.; Ramírez-Bello, J.; Hidalgo-Miranda, A.; Jiménez-Morales, S. MiRNAs in Hematopoiesis and Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2023, 24, 5436.
- Sajjadi-Dokht, M.; Merza Mohamad, T.A.; Sulaiman Rahman, H.; Suliman Maashi, M.; Danshina, S.; Shomali, N.; Solali, S.; Marofi, F.; Zeinalzadeh, E.; Akbari, M.; et al. MicroRNAs and JAK/STAT3 signaling: A new promising therapeutic axis in blood cancers. Genes Dis. 2022, 9, 849–867.
- Wang, Y.; Ma, H.; Li, Y.; Su, R. MiR-192-5p-Modified Tumor-Associated Macrophages-Derived Exosome Suppressed Endometrial Cancer Progression Through Targeting IRAK1/NF-κB Signaling. Reprod Sci. 2022, 29, 436–447.
- Chen, H.X.; Xu, X.X.; Tan, B.Z.; Zhang, Z.; Zhou, X.D. MicroRNA-29b Inhibits Angiogenesis by Targeting VEGFA through the MAPK/ERK and PI3K/Akt Signaling Pathways in Endometrial Carcinoma. Cell Physiol. Biochem. 2017, 41, 933–946.
- Guo, L.; Sun, B.; Wu, Q.; Yang, S.; Chen, F. miRNA-miRNA interaction implicates for potential mutual regulatory pattern. Gene 2012, 511, 187–194.
- Hsiao, K.Y.; Sun, H.S.; Tsai, S.J. Circular RNA—New member of noncoding RNA with novel functions. Exp. Biol. Med. 2017, 242, 1136–1141.
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G.; et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016, 7, 11215.
- Boussios, S.; Ozturk, M.A.; Moschetta, M.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Katsanos, K.H.; Christodoulou, D.K.; Pavlidis, N. The Developing Story of Predictive Biomarkers in Colorectal Cancer. J. Pers. Med. 2019, 9, 12.
- Zhao, Y.; Jin, L.J.; Zhang, X.Y. Exosomal miRNA-205 promotes breast cancer chemoresistance and tumorigenesis through E2F1. Aging 2021, 13, 18498–18514.
- Konoshenko, M.; Lansukhay, Y.; Krasilnikov, S.; Laktionov, P. MicroRNAs as Predictors of Lung-Cancer Resistance and Sensitivity to Cisplatin. Int. J. Mol. Sci. 2022, 23, 7594.
- Chen, Q.; Xia, H.W.; Ge, X.J.; Zhang, Y.C.; Tang, Q.L.; Bi, F. Serum miR-19a predicts resistance to FOLFOX chemotherapy in advanced colorectal cancer cases. Asian Pac. J. Cancer Prev. 2013, 14, 7421–7426.
- Hansen, T.F.; Carlsen, A.L.; Heegaard, N.H.; Sørensen, F.B.; Jakobsen, A. Changes in circulating microRNA-126 during treatment with chemotherapy and bevacizumab predicts treatment response in patients with metastatic colorectal cancer. Br. J. Cancer 2015, 112, 624–629.
- Liu, B.; Che, Q.; Qiu, H.; Bao, W.; Chen, X.; Lu, W.; Li, B.; Wan, X. Elevated MiR-222-3p promotes proliferation and invasion of endometrial carcinoma via targeting ERα. PLoS ONE 2014, 9, e87563.
- Wang, J.; Zhang, L.; Jiang, W.; Zhang, R.; Zhang, B.; Silayiding, A.; Duan, X. MicroRNA-135a promotes proliferation, migration, invasion and induces chemoresistance of endometrial cancer cells. Eur. J. Obs. Gynecol. Reprod Biol. X 2020, 5, 100103.
- Kumari, P.; Sharma, I.; Saha, S.C.; Srinivasan, R.; Bhardwaj, P. Role of serum microRNAs as biomarkers for endometriosis, endometrioid carcinoma of ovary & endometrioid endometrial cancer. Indian J. Med. Res. 2022, 156, 516–523.
- Donkers, H.; Hirschfeld, M.; Weiß, D.; Erbes, T.; Jäger, M.; Pijnenborg, J.; Bekkers, R.; Galaal, K. Detection of microRNA in urine to identify patients with endometrial cancer: A feasibility study. Int. J. Gynecol. Cancer 2021, 31, 868–874.
- Donkers, H.; Hirschfeld, M.; Weiß, D.; Erbes, T.; Jaeger, M.; Pijnenborg, J.M.A.; Bekkers, R.; Galaal, K.; ENITEC-consortium. Usefulness of microRNA detection in the diagnostics of endometrial cancer. Acta Obs. Gynecol. Scand 2021, 100, 1148–1154.
- Liu, J.; Li, C.; Jiang, Y.; Wan, Y.; Zhou, S.; Cheng, W. Tumor-suppressor role of miR-139-5p in endometrial cancer. Cancer Cell Int. 2018, 18, 51.
- Wang, Q.; Xu, K.; Tong, Y.; Dai, X.; Xu, T.; He, D.; Ying, J. Novel miRNA markers for the diagnosis and prognosis of endometrial cancer. J. Cell Mol. Med. 2020, 24, 4533–4546.
- Witek, Ł.; Janikowski, T.; Gabriel, I.; Bodzek, P.; Olejek, A. Analysis of microRNA regulating cell cycle-related tumor suppressor genes in endometrial cancer patients. Hum. Cell 2021, 34, 564–569.
- Braun, M.M.; Overbeek-Wager, E.A.; Grumbo, R.J. Diagnosis and Management of Endometrial Cancer. Am. Fam. Physician 2016, 93, 468–474.
- Lu, K.H.; Schorge, J.O.; Rodabaugh, K.J.; Daniels, M.S.; Sun, C.C.; Soliman, P.T.; White, K.G.; Luthra, R.; Gershenson, D.M.; Broaddus, R.R. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J. Clin. Oncol. 2007, 25, 5158–5164.
- Lee, M.; Piao, J.; Jeon, M.J. Risk Factors Associated with Endometrial Pathology in Premenopausal Breast Cancer Patients Treated with Tamoxifen. Yonsei Med. J. 2020, 61, 317–322.
- Piergentili, R.; Zaami, S.; Cavaliere, A.F.; Signore, F.; Scambia, G.; Mattei, A.; Marinelli, E.; Gulia, C.; Perelli, F. Non-Coding RNAs as Prognostic Markers for Endometrial Cancer. Int. J. Mol. Sci. 2021, 22, 3151.
- Papaioannou, S.; Tzafettas, J. Anovulation with or without PCO, hyperandrogenaemia and hyperinsulinaemia as promoters of endometrial and breast cancer. Best Prac. Res. Clin. Obs. Gynaecol. 2010, 24, 19–27.
- Chen, B.; Xu, P.; Wang, J.; Zhang, C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene 2019, 706, 91–96.
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15.
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584.
- Udesen, P.B.; Sørensen, A.E.; Svendsen, R.; Frisk, N.L.S.; Hess, A.L.; Aziz, M.; Wissing, M.L.M.; Englund, A.L.M.; Dalgaard, L.T. Circulating miRNAs in Women with Polycystic Ovary Syndrome: A Longitudinal Cohort Study. Cells 2023, 12, 983.
- Che, X.; Jian, F.; Chen, C.; Liu, C.; Liu, G.; Feng, W. PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J. Mol. Endocrinol. 2020, 64, 1–12.
- Cirillo, F.; Catellani, C.; Sartori, C.; Lazzeroni, P.; Amarri, S.; Street, M.E. Obesity, Insulin Resistance, and Colorectal Cancer: Could miRNA Dysregulation Play A Role? Int. J. Mol. Sci. 2019, 20, 2922.
- Sanderson, P.A.; Critchley, H.O.; Williams, A.R.; Arends, M.J.; Saunders, P.T. New concepts for an old problem: The diagnosis of endometrial hyperplasia. Hum. Reprod Update 2017, 23, 232–254.
- Armstrong, A.J.; Hurd, W.W.; Elguero, S.; Barker, N.M.; Zanotti, K.M. Diagnosis and management of endometrial hyperplasia. J. Minim. Invasive Gynecol. 2012, 19, 562–571.
- Wu, L.; Dai, X.; Zhan, J.; Zhang, Y.; Zhang, H.; Zeng, S.; Xi, W. Profiling peripheral microRNAs in obesity and type 2 diabetes mellitus. APMIS 2015, 123, 580–585.
- Ouni, M.; Gottmann, P.; Westholm, E.; Schwerbel, K.; Jähnert, M.; Stadion, M.; Rittig, K.; Vogel, H.; Schürmann, A. MiR-205 is up-regulated in islets of diabetes-susceptible mice and targets the diabetes gene Tcf7l2. Acta Physiol. 2021, 232, e13693.
- Pescador, N.; Pérez-Barba, M.; Ibarra, J.M.; Corbatón, A.; Martínez-Larrad, M.T.; Serrano-Ríos, M. Serum circulating microRNA profiling for identification of potential type 2 diabetes and obesity biomarkers. PLoS ONE 2013, 8, e77251.
- Pang, H.; Wang, J.; Wei, Q.; Liu, J.; Chu, X.; Yuan, C.; Yang, B.; Li, M.; Ma, D.; Tang, Y.; et al. miR-548ag functions as an oncogene by suppressing MOB1B in the development of obesity-related endometrial cancer. Cancer Sci. 2023, 114, 1507–1518.
- Kim, N.H.; Ahn, J.; Choi, Y.M.; Son, H.J.; Choi, W.H.; Cho, H.J.; Yu, J.H.; Seo, J.A.; Jang, Y.J.; Jung, C.H.; et al. Differential circulating and visceral fat microRNA expression of non-obese and obese subjects. Clin. Nutr. 2020, 39, 910–916.
- Williams, A.; Dougal, D.M.; Jenkins, W.; Greene, N.; Williams-DeVane, C.; Kimbro, K.S. Serum miR-17 levels are downregulated in obese, African American women with elevated HbA1c. J. Diabetes Metab. Disord. 2019, 18, 173–179.
- Yu, F.; Chapman, S.; Pham, D.L.; Ko, M.L.; Zhou, B.; Ko, G.Y. Decreased miR-150 in obesity-associated type 2 diabetic mice increases intraocular inflammation and exacerbates retinal dysfunction. BMJ Open Diabetes Res. Care 2020, 8, e001446.
- Kirkwood, T.B. Understanding the odd science of aging. Cell 2005, 120, 437–447.
- Wagner, K.H.; Cameron-Smith, D.; Wessner, B.; Franzke, B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients 2016, 8, 338.
- Kumar, S.; Vijayan, M.; Bhatti, J.S.; Reddy, P.H. MicroRNAs as Peripheral Biomarkers in Aging and Age-Related Diseases. Prog. Mol. Biol. Transl. Sci. 2017, 146, 47–94.
- Gerasymchuk, M.; Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. The Role of microRNAs in Organismal and Skin Aging. Int. J. Mol. Sci. 2020, 21, 5281.
- Noren Hooten, N.; Fitzpatrick, M.; Wood, W.H.; De, S.; Ejiogu, N.; Zhang, Y.; Mattison, J.A.; Becker, K.G.; Zonderman, A.B.; Evans, M.K. Age-related changes in microRNA levels in serum. Aging 2013, 5, 725–740.
- Wang, S.; Sun, Y.; Yao, L.; Xing, Y.; Yang, H.; Ma, Q. The Role of microRNA-23a-3p in the Progression of Human Aging Process by Targeting FOXO3a. Mol. Biotechnol. 2023.
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392.
- Wu, C.; Zhou, X.; Li, J.; Xiao, R.; Xin, H.; Dai, L.; Zhu, Y.; Bao, W. Serum miRNA-204-5p as a potential non-invasive biomarker for the diagnosis of endometrial cancer with sentinel lymph node mapping. Oncol. Lett. 2022, 24, 248.
- Abu-Rustum, N.R.; Gomez, J.D.; Alektiar, K.M.; Soslow, R.A.; Hensley, M.L.; Leitao, M.M.; Gardner, G.J.; Sonoda, Y.; Chi, D.S.; Barakat, R.R. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol. Oncol. 2009, 115, 236–238.
- Sato, K.; Miyamoto, M.; Takano, M.; Tsuda, H. MicroRNA-21 expression in cancer cells is an independent biomarker of progression-free survival of endometrioid endometrial carcinoma. Virchows Arch. 2021, 479, 883–891.
- Bao, W.; Zhang, Y.; Li, S.; Fan, Q.; Qiu, M.; Wang, Y.; Li, Y.; Ji, X.; Yang, Y.; Sang, Z.; et al. miR-107-5p promotes tumor proliferation and invasion by targeting estrogen receptor-α in endometrial carcinoma. Oncol. Rep. 2019, 41, 1575–1585.
- Yoneyama, K.; Ishibashi, O.; Kawase, R.; Kurose, K.; Takeshita, T. miR-200a, miR-200b and miR-429 are onco-miRs that target the PTEN gene in endometrioid endometrial carcinoma. Anticancer Res. 2015, 35, 1401–1410.
- Guo, C.M.; Liu, S.Q.; Sun, M.Z. miR-429 as biomarker for diagnosis, treatment and prognosis of cancers and its potential action mechanisms: A systematic literature review. Neoplasma 2020, 67, 215–228.
- Sun, X.; Hou, L.; Qiu, C.; Kong, B. MiR-501 promotes tumor proliferation and metastasis by targeting HOXD10 in endometrial cancer. Cell Mol. Biol. Lett. 2021, 26, 20.
- Chen, C.; Zhang, Q.; Kong, B. miRNA-576-5p promotes endometrial cancer cell growth and metastasis by targeting ZBTB4. Clin. Transl. Oncol. 2023, 25, 706–720.
- Delangle, R.; De Foucher, T.; Larsen, A.K.; Sabbah, M.; Azaïs, H.; Bendifallah, S.; Daraï, E.; Ballester, M.; Mehats, C.; Uzan, C.; et al. The Use of microRNAs in the Management of Endometrial Cancer: A Meta-Analysis. Cancers 2019, 11, 832.
- Wang, J.; Gong, X.; Yang, L.; Li, L.; Gao, X.; Ni, T.; Yang, X.; Fan, Q.; Sun, X.; Wang, Y. Loss of exosomal miR-26a-5p contributes to endometrial cancer lymphangiogenesis and lymphatic metastasis. Clin. Transl. Med. 2022, 12, e846.
- Corrado, G.; Laquintana, V.; Loria, R.; Carosi, M.; de Salvo, L.; Sperduti, I.; Zampa, A.; Cicchillitti, L.; Piaggio, G.; Cutillo, G.; et al. Endometrial cancer prognosis correlates with the expression of L1CAM and miR34a biomarkers. J. Exp. Clin. Cancer Res. 2018, 37, 139.
- Wang, Z.; Wang, W.; Huang, K.; Wang, Y.; Li, J.; Yang, X. MicroRNA-34a inhibits cells proliferation and invasion by downregulating Notch1 in endometrial cancer. Oncotarget 2017, 8, 111258–111270.
- Fu, K.; Li, Y.; Song, J.; Cai, W.; Wu, W.; Ye, X.; Xu, J. Identification of a MicroRNA Signature Associated With Lymph Node Metastasis in Endometrial Endometrioid Cancer. Front. Genet. 2021, 12, 650102.
- Li, B.L.; Lu, W.; Qu, J.J.; Ye, L.; Du, G.Q.; Wan, X.P. Loss of exosomal miR-148b from cancer-associated fibroblasts promotes endometrial cancer cell invasion and cancer metastasis. J. Cell Physiol. 2019, 234, 2943–2953.
- Chen, S.; Sun, K.X.; Liu, B.L.; Zong, Z.H.; Zhao, Y. MicroRNA-505 functions as a tumor suppressor in endometrial cancer by targeting TGF-α. Mol. Cancer 2016, 15, 11.
- Constantine, G.D.; Kessler, G.; Graham, S.; Goldstein, S.R. Increased Incidence of Endometrial Cancer Following the Women’s Health Initiative: An Assessment of Risk Factors. J. Women’s Health 2019, 28, 237–243.
- Miller, D.S.; Filiaci, V.L.; Mannel, R.S.; Cohn, D.E.; Matsumoto, T.; Tewari, K.S.; DiSilvestro, P.; Pearl, M.L.; Argenta, P.A.; Powell, M.A.; et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J. Clin. Oncol. 2020, 38, 3841–3850.
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; DiSimone, C.; et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J. Clin. Oncol. 2020, 38, 2981–2992.
- Kong, J.; He, X.; Wang, Y.; Li, J. Effect of microRNA-29b on proliferation, migration, and invasion of endometrial cancer cells. J. Int. Med. Res. 2019, 47, 3803–3817.
- Zheng, X.; Liu, M.; Song, Y.; Feng, C. Long Noncoding RNA-ATB Impairs the Function of Tumor Suppressor miR-126-Mediated Signals in Endometrial Cancer for Tumor Growth and Metastasis. Cancer Biother. Radiopharm. 2019, 34, 47–55.
- Qu, J.; Zhang, L.; Li, L.; Su, Y. miR-148b Functions as a Tumor Suppressor by Targeting Endoplasmic Reticulum Metallo Protease 1 in Human Endometrial Cancer Cells. Oncol. Res. 2018, 27, 81–88.
- Tsuruta, T.; Kozaki, K.; Uesugi, A.; Furuta, M.; Hirasawa, A.; Imoto, I.; Susumu, N.; Aoki, D.; Inazawa, J. miR-152 is a tumor suppressor microRNA that is silenced by DNA hypermethylation in endometrial cancer. Cancer Res. 2011, 71, 6450–6462.
- Xie, D.; Liang, Y.; Su, Y.; An, Y.; Qu, P. miR-152 inhibits proliferation of human endometrial cancer cells via inducing G2/M phase arrest by suppressing CDC25B expression. Biomed. Pharmacother. 2018, 99, 299–305.
- Fang, Y.Y.; Tan, M.R.; Zhou, J.; Liang, L.; Liu, X.Y.; Zhao, K.; Bao, E.C. miR-214-3p inhibits epithelial-to-mesenchymal transition and metastasis of endometrial cancer cells by targeting TWIST1. Onco. Targets Ther. 2019, 12, 9449–9458.
- Zhang, H.H.; Li, R.; Li, Y.J.; Yu, X.X.; Sun, Q.N.; Li, A.Y.; Kong, Y. eIF4E-related miR-320a and miR-340-5p inhibit endometrial carcinoma cell metastatic capability by preventing TGF-β1-induced epithelial-mesenchymal transition. Oncol. Rep. 2020, 43, 447–460.
- Karaayvaz, M.; Zhang, C.; Liang, S.; Shroyer, K.R.; Ju, J. Prognostic significance of miR-205 in endometrial cancer. PLoS ONE 2012, 7, e35158.
- Zhou, L.; Wang, W.; Wang, F.; Yang, S.; Hu, J.; Lu, B.; Pan, Z.; Ma, Y.; Zheng, M.; Lei, S.; et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer 2021, 20, 57.
- Fan, X.; Zou, X.; Liu, C.; Cheng, W.; Zhang, S.; Geng, X.; Zhu, W. MicroRNA expression profile in serum reveals novel diagnostic biomarkers for endometrial cancer. Biosci. Rep. 2021, 41, BSR20210111.
- Ghazala, R.A.; El-Attar, E.A.; Abouzeid, Z.S. Circulating miRNA 27a and miRNA150-5p; a noninvasive approach to endometrial carcinoma. Mol. Biol. Rep. 2021, 48, 4351–4360.
- Rižner, T.L. Discovery of biomarkers for endometrial cancer: Current status and prospects. Expert Rev. Mol. Diagn. 2016, 16, 1315–1336.
- Montagnana, M.; Benati, M.; Danese, E.; Giudici, S.; Perfranceschi, M.; Ruzzenenete, O.; Salvagno, G.L.; Bassi, A.; Gelati, M.; Paviati, E.; et al. Aberrant MicroRNA Expression in Patients With Endometrial Cancer. Int. J. Gynecol. Cancer 2017, 27, 459–466.
- Jiang, Y.; Wang, N.; Yin, D.; Li, Y.K.; Guo, L.; Shi, L.P.; Huang, X. Changes in the Expression of Serum MiR-887-5p in Patients With Endometrial Cancer. Int. J. Gynecol. Cancer 2016, 26, 1143–1147.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
677
Revisions:
2 times
(View History)
Update Date:
28 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




