
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ekateina Kozuharova | -- | 6254 | 2023-07-26 00:14:30 | | | |
| 2 | Sirius Huang | -1 word(s) | 6253 | 2023-07-26 03:52:16 | | |
Video Upload Options
Systemic lupus erythematosus (SLE) is an autoimmune disease involving multiple organs and clinical manifestations. The Latin word lupus, meaning wolf, was in the medical literature prior to the 1200s to describe skin lesions that devour flesh, and the resources available to physicians to help people were limited. The present text reviews the ethnobotanical and pharmacological aspects of medicinal plants and purified molecules from natural sources with efficacy against lupus conditions. Among these molecules are artemisinin and its derivatives, antroquinonol, baicalin, curcumin, emodin, mangiferin, salvianolic acid A, triptolide, the total glycosides of paeony (TGP), and other supplements such as fatty acids and vitamins. In addition, medicinal plants, herbal remedies, mushrooms, and fungi that have been investigated for their effects on different lupus conditions through clinical trials, in vivo, in vitro, or in silico studies are reviewed. A special emphasis was placed on clinical trials, active phytochemicals, and their mechanisms of action. This discussion can be helpful for researchers in designing new goal-oriented studies. It can also help practitioners gain insight into recent updates on supplements that might help patients suffering from lupus conditions.
1. Introduction
2. Ethnobotany
3. Purified Molecules from Natural Sources
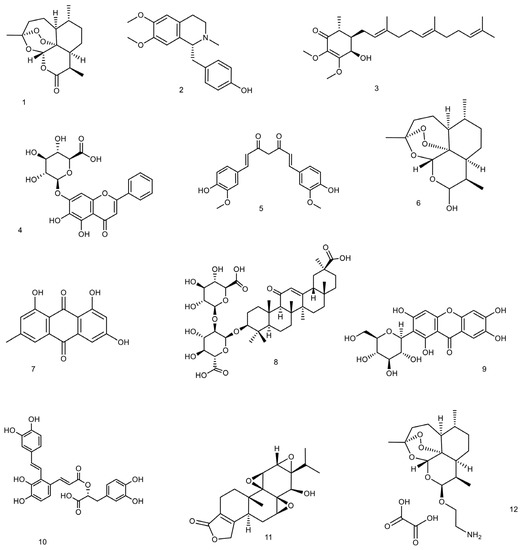
3.1. Artemisinin and Its Derivatives
Toxicity and Side Effects
3.2. Antroquinonol
Toxicity and Side Effects
3.3. Baicalin
Toxicity and Side Effects
3.4. Curcumin
3.5. Emodin
Toxicity and Side Effects
3.6. Esculetin
Toxicity and Side Effects of Esculetin
3.7. Mangiferin
Toxicity and Side Effects
3.8. Salvianolic Acid A
Toxicity and Side Effects
3.9. Triptolide
Toxicity and Side Effects
3.10. Total Glycosides of Paeony (TGP)
Toxicity and Side Effects
4. Fatty Acids, Vitamins, and Minerals
4.1. Fatty Acids
4.2. Vitamin A
4.3. Vitamin B
4.4. Vitamin C
4.5. Vitamin D
4.6. Vitamin E
4.7. Calcium
4.8. Iron
4.9. Selenium
4.10. Zinc
5. Herbal Medicines, Medicinal Plants, Mushrooms, and Fungi and Their Crude Extracts
References
- Dörner, T.; Furie, R. Novel paradigms in systemic lupus erythematosus. Lancet 2019, 393, 2344–2358.
- Veeranki, S.; Choubey, D. Systemic lupus erythematosus and increased risk to develop B cell malignancies: Role of the p200-family proteins. Immunol. Lett. 2010, 133, 1–5.
- Lightfoot, Y.L.; Blanco, L.P.; Kaplan, M.J. Metabolic abnormalities and oxidative stress in lupus. Curr. Opin. Rheumatol. 2017, 29, 442.
- Bertsias, G.K.; Pamfil, C.; Fanouriakis, A.; Boumpas, D.T. Diagnostic criteria for systemic lupus erythematosus: Has the time come? Nat. Rev. Rheumatol. 2013, 9, 687–694.
- Bootsma, H.; Spronk, P.; de Boer, G.; Limburg, P.; Kallenberg, C.; Derksen, R.; Wolters-Dicke, J.; Gmelig-Meyling, F.; Kater, L.; Hermans, J. Prevention of relapses in systemic lupus erythematosus. Lancet 1995, 345, 1595–1599.
- Loram, L.C.; Culp, M.E.; Connolly-Strong, E.C.; Sturgill-Koszycki, S. Melanocortin peptides: Potential targets in systemic lupus erythematosus. Inflammation 2015, 38, 260–271.
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25.
- Mikdashi, J.; Nived, O. Measuring disease activity in adults with systemic lupus erythematosus: The challenges of administrative burden and responsiveness to patient concerns in clinical research. Arthritis Res. Ther. 2015, 17, 1–10.
- Almaani, S.; Meara, A.; Rovin, B.H. Update on lupus nephritis. Clin. J. Am. Soc. Nephrol. 2017, 12, 825–835.
- Hoover, P.J.; Costenbader, K.H. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist’s perspective. Kidney Int. 2016, 90, 487–492.
- Wallace, D.J. The evolution of drug discovery in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2015, 11, 616–620.
- Basta, F.; Fasola, F.; Triantafyllias, K.; Schwarting, A. Systemic lupus erythematosus (SLE) therapy: The old and the new. Rheumatol. Ther. 2020, 7, 433–446.
- Thomas, D.E. The Lupus Encyclopedia: A Comprehensive Guide for Patients and Families; JHU Press: Baltimore, MD, USA, 2014.
- Fu, S.M.; Gaskin, F. History of systemic lupus erythematosus with an emphasis on certain recent major issues. In Systemic Lupus Erythematosus; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–8.
- Tene, V.; Malagon, O.; Finzi, P.V.; Vidari, G.; Armijos, C.; Zaragoza, T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007, 111, 63–81.
- Kyaw, M.S.; Aye, M.M.; Grinnell, M.; Rabach, M. Traditional and ethnobotanical dermatology practices in Myanmar. Clin. Dermatol. 2018, 36, 320–324.
- Magee, A.; Van Wyk, B.-E.; Van Vuuren, S. Ethnobotany and antimicrobial activity of sieketroos (Arctopus species). S. Afr. J. Bot. 2007, 73, 159–162.
- Orhan, N.; Akkol, E.; Ergun, F. Evaluation of antiinflammatory and antinociceptive effects of some Juniperus species growing in Turkey. Turk. J. Biol. 2012, 36, 719–726.
- Gilca, M.; Tiplica, G.S.; Salavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other Eastern European countries. Clin. Dermatol. 2018, 36, 338–352.
- Verma, R.; Singh, H.; Thakur, A.; Kohli, S. Ethnobotanical survey of medicinal and aromatic plants of Bhagalpur Region. Int. J. Appl. Sci. Biotechnol. 2020, 8, 216–222.
- Vahedi-Mazdabadi, Y.; Saeedi, M. Treatment of Lupus Nephritis from Iranian Traditional Medicine and Modern Medicine Points of View: A Comparative Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 6645319.
- Sharififar, F.; Yassa, N.; Mozaffarian, V. Bioactivity of major components from the seeds of Bunium persicum (Boiss.) Fedtch. Pak. J. Pharm. Sci. 2010, 23, 300–304.
- Abou El-Soud, N.H.; El-Lithy, N.A.; El-Saeed, G.; Wahby, M.S.; Khalil, M.Y.; Morsy, F.; Shaffie, N. Renoprotective effects of caraway (Carum carvi L.) essential oil in streptozotocin induced diabetic rats. J. Appl. Pharm. Sci. 2014, 4, 027–033.
- Aghili, M.H. Makhzan-al-Advia; Tehran University of Medical Sciences: Tehran, Iran, 2009; pp. 227–228. (In Persian)
- Ardakani, M.R.S.; Farjadmand, F.; Rahimi, R. Makhzan al adviyeh and pointing to the scientific names of medicinal plants for the first time in a persian book. Tradit. Integr. Med. 2018, 3, 186–195.
- Seri, A.; Khorsand, M.; Rezaei, Z.; Hamedi, A.; Takhshid, M.A. Inhibitory effect of bunium persicum hydroalcoholic extract on glucose-induced albumin glycation, oxidation, and aggregation in vitro. Iran. J. Med. Sci. 2017, 42, 369.
- Mehrabadi, M.M.; Zarshenas, M.M. A Concise Overview of Phytochemistry, Pharmacology and Clinical Aspects of Persian Cumin; Bunium persicum (Boiss.) B. Fedtsch. Curr. Drug Discov. Technol. 2021, 18, 485–491.
- Bansal, S.; Sharma, K.; Gautam, V.; Lone, A.A.; Malhotra, E.V.; Kumar, S.; Singh, R. A comprehensive review of Bunium persicum: A valuable medicinal spice. Food Rev. Int. 2021, 39, 1184–1202.
- Hamedi, A.; Sakhteman, A.; Moheimani, S.M. An in silico approach towards investigation of possible effects of essential oils constituents on receptors involved in cardiovascular diseases (CVD) and associated risk factors (Diabetes Mellitus and Hyperlipidemia). Cardiovasc. Hematol. Agents Med. Chem. (Former. Curr. Med. Chem.-Cardiovasc. Hematol. Agents) 2021, 19, 32–42.
- Ebrahimi-Najafabadi, H.; Kazemeini, S.S.; Pasdaran, A.; Hamedi, A. A novel similarity search approach for high-performance thin-layer chromatography (HPTLC) fingerprinting of medicinal plants. Phytochem. Anal. 2019, 30, 405–414.
- Mojab, F.; Hamedi, A.; Nickavar, B.; Javidnia, K. Hydrodistilled volatile constituents of the leaves of Daucus carota L. subsp. sativus (Hoffman.) Arcang. (Apiaceae) from Iran. J. Essent. Oil Bear. Plants 2008, 11, 271–277.
- D’Cunha, N.M.; Peterson, G.; Baby, K.; Thomas, J. Impetigo: A need for new therapies in a world of increasing antimicrobial resistance. J. Clin. Pharm. Ther. 2017, 43, 150–153.
- Corson, T.W.; Crews, C.M. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell 2007, 130, 769–774.
- Garcia, L.C. A Review of Artemisia annua L.: Its genetics, biochemical characteristics, and anti-malarial efficacy. Int. J. Sci. Technol. 2015, 5, 38–46.
- Krishna, S.; Bustamante, L.; Haynes, R.K.; Staines, H.M. Artemisinins: Their growing importance in medicine. Trends Pharmacol. Sci. 2008, 29, 520–527.
- Li, Y.; Xu, T.; Qiu, X.; Tian, B.; Bi, C.; Yao, L. Effectiveness of Bailing capsules in the treatment of lupus nephritis: A meta-analysis. Mol. Med. Rep. 2020, 22, 2132–2140.
- Wang, C.; Fortin, P.; Li, Y.; Panaritis, T.; Gans, M.; Esdaile, J. Discontinuation of antimalarial drugs in systemic lupus erythematosus. J. Rheumatol. 1999, 26, 808–815.
- Mu, X.; Wang, C. Artemisinins—A promising new treatment for systemic lupus erythematosus: A descriptive review. Curr. Rheumatol. Rep. 2018, 20, 1–10.
- Golenser, J.; Waknine, J.H.; Krugliak, M.; Hunt, N.H.; Grau, G.E. Current perspectives on the mechanism of action of artemisinins. Int. J. Parasitol. 2006, 36, 1427–1441.
- Efferth, T.; Oesch, F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med. Res. Rev. 2021, 41, 3023–3061.
- Dang, W.-Z.; Li, H.; Jiang, B.; Nandakumar, K.S.; Liu, K.-F.; Liu, L.-X.; Yu, X.-C.; Tan, H.-J.; Zhou, C. Therapeutic effects of artesunate on lupus-prone MRL/lpr mice are dependent on T follicular helper cell differentiation and activation of JAK2–STAT3 signaling pathway. Phytomedicine 2019, 62, 152965.
- Li, D.; Qi, J.; Wang, J.; Pan, Y.; Li, J.; Xia, X.; Dou, H.; Hou, Y. Protective effect of dihydroartemisinin in inhibiting senescence of myeloid-derived suppressor cells from lupus mice via Nrf2/HO-1 pathway. Free Radic. Biol. Med. 2019, 143, 260–274.
- Chen, Y.; Tao, T.; Wang, W.; Yang, B.; Cha, X. Dihydroartemisinin attenuated the symptoms of mice model of systemic lupus erythematosus by restoring the Treg/Th17 balance. Clin. Exp. Pharmacol. Physiol. 2021, 48, 626–633.
- Efferth, T.; Kaina, B. Toxicity of the antimalarial artemisinin and its dervatives. Crit. Rev. Toxicol. 2010, 40, 405–421.
- Yin, J.; Wang, H.; Ding, R. Artemisinin and its derivatives: Progress in toxicology. Chin. J. Pharmacol. Toxicol. 2014, 6, 309–314.
- Lee, W.-T.; Lee, T.-H.; Cheng, C.-H.; Chen, K.-C.; Chen, Y.-C.; Lin, C.-W. Antroquinonol from Antrodia Camphorata suppresses breast tumor migration/invasion through inhibiting ERK-AP-1-and AKT-NF-κB-dependent MMP-9 and epithelial-mesenchymal transition expressions. Food Chem. Toxicol. 2015, 78, 33–41.
- Tsai, P.-Y.; Ka, S.-M.; Chao, T.-K.; Chang, J.-M.; Lin, S.-H.; Li, C.-Y.; Kuo, M.-T.; Chen, P.; Chen, A. Antroquinonol reduces oxidative stress by enhancing the Nrf2 signaling pathway and inhibits inflammation and sclerosis in focal segmental glomerulosclerosis mice. Free Radic. Biol. Med. 2011, 50, 1503–1516.
- Geethangili, M.; Tzeng, Y.-M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid.-Based Complement. Altern. Med. 2011, 2011, 212641.
- Zhang, B.-B.; Hu, P.-F.; Huang, J.; Hu, Y.-D.; Chen, L.; Xu, G.-R. Current advances on the structure, bioactivity, synthesis, and metabolic regulation of novel ubiquinone derivatives in the edible and medicinal mushroom Antrodia cinnamomea. J. Agric. Food Chem. 2017, 65, 10395–10405.
- Villaume, M.T.; Sella, E.; Saul, G.; Borzilleri, R.M.; Fargnoli, J.; Johnston, K.A.; Zhang, H.; Fereshteh, M.P.; Dhar, T.M.; Baran, P.S. Antroquinonol a: Scalable synthesis and preclinical biology of a phase 2 drug candidate. ACS Cent. Sci. 2016, 2, 27–31.
- Angamuthu, V.; Shanmugavadivu, M.; Nagarajan, G.; Velmurugan, B.K. Pharmacological activities of antroquinonol-Mini review. Chem.-Biol. Interact. 2019, 297, 8–15.
- Tsai, P.Y.; Ka, S.M.; Chang, J.M.; Lai, J.H.; Dai, M.S.; Jheng, H.L.; Kuo, M.T.; Chen, P.; Chen, A. Antroquinonol differentially modulates T cell activity and reduces interleukin-18 production, but enhances Nrf2 activation, in murine accelerated severe lupus nephritis. Arthritis Rheum. 2012, 64, 232–242.
- Chang, J.-M.; Lee, Y.-R.; Hung, L.-M.; Liu, S.-Y.; Kuo, M.-T.; Wen, W.-C.; Chen, P. An extract of Antrodia camphorata mycelia attenuates the progression of nephritis in systemic lupus erythematosus-prone NZB/W F1 mice. Evid.-Based Complement. Altern. Med. 2011, 2011, 465894.
- Kuang, Y.; Li, B.; Wang, Z.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102.
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine 2017, 7, 23.
- Chang, W.-H.; Chen, M.C.; Cheng, I.H. Antroquinonol lowers brain amyloid-β levels and improves spatial learning and memory in a transgenic mouse model of Alzheimer’s disease. Sci. Rep. 2015, 5, 15067.
- Chae, B.-S. Baicalin Ameliorates Dysimmunoregulation in Pristane-Induced Lupus Mice: Production of IL-6 and PGE2 and Activation of T cells. Nat. Prod. Sci. 2011, 17, 354–362.
- Yang, J.; Yang, X.; Yang, J.; Li, M. Baicalin ameliorates lupus autoimmunity by inhibiting differentiation of Tfh cells and inducing expansion of Tfr cells. Cell Death Dis. 2019, 10, 140.
- Chae, B.S. Effect of Baicalin on the Ex vivo Production of Cytokines in Pristane-Induced Lupus Mice. YAKHAK HOEJI 2016, 60, 21–28.
- Delerue, T.; Barroso, M.F.; Dias-Teixeira, M.; Figueiredo-González, M.; Delerue-Matos, C.; Grosso, C. Interactions between Ginkgo biloba L. and Scutellaria baicalensis Georgi in multicomponent mixtures towards cholinesterase inhibition and ROS scavenging. Food Res. Int. 2021, 140, 109857.
- Cai, Y.; Ma, W.; Xiao, Y.; Wu, B.; Li, X.; Liu, F.; Qiu, J.; Zhang, G. High doses of baicalin induces kidney injury and fibrosis through regulating TGF-β/Smad signaling pathway. Toxicol. Appl. Pharmacol. 2017, 333, 1–9.
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and bioavailability enhancement of baicalin: A review. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 159–168.
- Momtazi-Borojeni, A.A.; Haftcheshmeh, S.M.; Esmaeili, S.-A.; Johnston, T.P.; Abdollahi, E.; Sahebkar, A. Curcumin: A natural modulator of immune cells in systemic lupus erythematosus. Autoimmun. Rev. 2018, 17, 125–135.
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545.
- Constantin, M.M.; Nita, I.E.; Olteanu, R.; Constantin, T.; Bucur, S.; Matei, C.; Raducan, A. Significance and impact of dietary factors on systemic lupus erythematosus pathogenesis. Exp. Ther. Med. 2019, 17, 1085–1090.
- Kumar, A.; Dogra, S.; Prakash, A. Protective effect of curcumin (Curcuma longa), against aluminium toxicity: Possible behavioral and biochemical alterations in rats. Behav. Brain Res. 2009, 205, 384–390.
- Rezaee, R.; Momtazi, A.A.; Monemi, A.; Sahebkar, A. Curcumin: A potentially powerful tool to reverse cisplatin-induced toxicity. Pharmacol. Res. 2017, 117, 218–227.
- Soetikno, V.; Sari, F.R.; Veeraveedu, P.T.; Thandavarayan, R.A.; Harima, M.; Sukumaran, V.; Lakshmanan, A.P.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr. Metab. 2011, 8, 1–11.
- Shishodia, S.; Sethi, G.; Aggarwal, B.B. Curcumin: Getting back to the roots. Ann. N. Y. Acad. Sci. 2005, 1056, 206–217.
- Shishodia, S.; Singh, T.; Chaturvedi, M.M. Modulation of transcription factors by curcumin. Mol. Targets Ther. Uses Curcumin Health Dis. 2007, 595, 127–148.
- Gonzales, A.M.; Orlando, R.A. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. 2008, 5, 17.
- JIANG, C.-X. Curcumin analog exhibited anti-inflammatory activity through inhibiting ERK/JNK and NF-κB signaling pathway. Chin. Tradit. Herb. Drugs 2016, 24, 2871–2876.
- Han, S.-S.; Keum, Y.-S.; Seo, H.-J.; Surh, Y.-J. Curcumin suppresses activation of NF-κB and AP-1 induced by phorbol ester in cultured human promyelocytic leukemia cells. BMB Rep. 2002, 35, 337–342.
- Srinivas, G.; Babykutty, S.; Sathiadevan, P.P.; Srinivas, P. Molecular mechanism of emodin action: Transition from laxative ingredient to an antitumor agent. Med. Res. Rev. 2007, 27, 591–608.
- Zhang, B.; Shi, Y.; Lei, T.C. Detection of active P-glycoprotein in systemic lupus erythematosus patients with poor disease control. Exp. Ther. Med. 2012, 4, 705–710.
- Xia, Y.; Xu, S. Effect of emodin on nephritis of BXSB lupus mice and its pharmacological mechanism. Cent. China Med. J. 2003, 27, 63–64.
- Yuan, X.; Dai, B.; Yang, L.; Lin, B.; Lin, E.; Pan, Y. Emodin ameliorates renal injury in BXSB mice by modulating TNF-α/ICAM-1. Biosci. Rep. 2020, 40, BSR20202551.
- Sharifi-Rad, J.; Herrera-Bravo, J.; Kamiloglu, S.; Petroni, K.; Mishra, A.P.; Monserrat-Mesquida, M.; Sureda, A.; Martorell, M.; Aidarbekovna, D.S.; Yessimsiitova, Z. Recent advances in the therapeutic potential of emodin for human health. Biomed. Pharmacother. 2022, 154, 113555.
- Akkol, E.K.; Tatlı, I.I.; Karatoprak, G.Ş.; Ağar, O.T.; Yücel, Ç.; Sobarzo-Sánchez, E.; Capasso, R. Is emodin with anticancer effects completely innocent? Two sides of the coin. Cancers 2021, 13, 2733.
- Zhang, Y.; Li, Z.; Wu, H.; Wang, J.; Zhang, S. Esculetin alleviates murine lupus nephritis by inhibiting complement activation and enhancing Nrf2 signaling pathway. J. Ethnopharmacol. 2022, 288, 115004.
- Wang, W.; Sheng, L.; Chen, Y.; Li, Z.; Wu, H.; Ma, J.; Zhang, D.; Chen, X.; Zhang, S. Total coumarin derivates from Hydrangea paniculata attenuate renal injuries in cationized-BSA induced membranous nephropathy by inhibiting complement activation and interleukin 10-mediated interstitial fibrosis. Phytomedicine 2022, 96, 153886.
- Tubaro, A.; Del Negro, P.; Ragazzi, E.; Zampiron, S.; Della Loggia, R. Anti-inflammatory and peripheral analgesic activity of esculetin in vivo. Pharmacol. Res. Commun. 1988, 20, 83–85.
- Lum, P.T.; Sekar, M.; Gan, S.H.; Jeyabalan, S.; Bonam, S.R.; Rani, N.N.I.M.; Ku-Mahdzir, K.-M.; Seow, L.J.; Wu, Y.S.; Subramaniyan, V. Therapeutic potential of mangiferin against kidney disorders and its mechanism of action: A review. Saudi J. Biol. Sci. 2022, 29, 1530–1542.
- Jangra, A.; Arora, M.K.; Kisku, A.; Sharma, S. The multifaceted role of mangiferin in health and diseases: A review. Adv. Tradit. Med. 2021, 21, 619–643.
- Liang, C.-L.; Lu, W.; Zhou, J.-Y.; Chen, Y.; Zhang, Q.; Liu, H.; Qiu, F.; Dai, Z. Mangiferin attenuates murine lupus nephritis by inducing CD4+ Foxp3+ regulatory T cells via suppression of mTOR signaling. Cell. Physiol. Biochem. 2018, 50, 1560–1573.
- Morozkina, S.N.; Nhung Vu, T.H.; Generalova, Y.E.; Snetkov, P.P.; Uspenskaya, M.V. Mangiferin as new potential anti-cancer agent and mangiferin-integrated polymer systems—A novel research direction. Biomolecules 2021, 11, 79.
- Mei, S.; Ma, H.; Chen, X. Anticancer and anti-inflammatory properties of mangiferin: A review of its molecular mechanisms. Food Chem. Toxicol. 2021, 149, 111997.
- Reddeman, R.A.; Glávits, R.; Endres, J.R.; Clewell, A.E.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A toxicological evaluation of mango leaf extract (Mangifera indica) containing 60% mangiferin. J. Toxicol. 2019, 2019, 4763015.
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. J. Tradit. Complement. Med. 2014, 4, 82–88.
- Lin, Y.; Yan, Y.; Zhang, H.; Chen, Y.; He, Y.; Wang, S.; Fang, L.; Lv, Y.; Du, G. Salvianolic acid A alleviates renal injury in systemic lupus erythematosus induced by pristane in BALB/c mice. Acta Pharm. Sin. B 2017, 7, 159–166.
- Yang, M.-Y.; Song, Z.-Y.; Gan, H.-L.; Zheng, M.-H.; Liu, Q.; Meng, X.-T.; Pan, T.; Li, Z.-Y.; Peng, R.-X.; Liu, K. Non-clinical safety evaluation of salvianolic acid A: Acute, 4-week intravenous toxicities and genotoxicity evaluations. BMC Pharmacol. Toxicol. 2022, 23, 83.
- Du, G.; Song, J.; Du, L.; Zhang, L.; Qiang, G.; Wang, S.; Yang, X.; Fang, L. Chemical and pharmacological research on the polyphenol acids isolated from Danshen: A review of salvianolic acids. Adv. Pharmacol. 2020, 87, 1–41.
- Yuan, K.; Li, X.; Lu, Q.; Zhu, Q.; Jiang, H.; Wang, T.; Huang, G.; Xu, A. Application and mechanisms of triptolide in the treatment of inflammatory diseases—A review. Front. Pharmacol. 2019, 10, 1469.
- Wang, Q.; Meng, J.; Dong, A.; Yu, J.-z.; Zhang, G.-X.; Ma, C.-G. The pharmacological effects and mechanism of Tripterygium wilfordii Hook F in central nervous system autoimmunity. J. Altern. Complement. Med. 2016, 22, 496–502.
- Zhou, Z.-L.; Yang, Y.-X.; Ding, J.; Li, Y.-C.; Miao, Z.-H. Triptolide: Structural modifications, structure–activity relationships, bioactivities, clinical development and mechanisms. Nat. Prod. Rep. 2012, 29, 457–475.
- Zhao, X.; Tang, X.; Yan, Q.; Song, H.; Li, Z.; Wang, D.; Chen, H.; Sun, L. Triptolide ameliorates lupus via the induction of miR-125a-5p mediating Treg upregulation. Int. Immunopharmacol. 2019, 71, 14–21.
- Crews, G.; Erickson, L.; Pan, F.; Fisniku, O.; Jang, M.-S.; Wynn, C.; Benediktsson, H.; Kobayashi, M.; Jiang, H. Down-regulation of TGF-β and VCAM-1 is associated with successful treatment of chronic rejection in rats. Transplant. Proc. 2005, 1926–1928.
- Hong, Y.; Zhou, W.; Li, K.; Sacks, S.H. Triptolide is a potent suppressant of C3, CD40 and B7h expression in activated human proximal tubular epithelial cells. Kidney Int. 2002, 62, 1291–1300.
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide attenuates inflammatory response in membranous glomerulo-nephritis rat via downregulation of NF-κB signaling pathway. Kidney Blood Press. Res. 2016, 41, 901–910.
- Li, Y.; Yu, C.; Zhu, W.-M.; Xie, Y.; Qi, X.; Li, N.; Li, J.-S. Triptolide ameliorates IL-10-deficient mice colitis by mechanisms involving suppression of IL-6/STAT3 signaling pathway and down-regulation of IL-17. Mol. Immunol. 2010, 47, 2467–2474.
- Qi, Q.; Li, H.; Lin, Z.-M.; Yang, X.-Q.; Zhu, F.-H.; Liu, Y.-T.; Shao, M.-J.; Zhang, L.-Y.; Xu, Y.-S.; Yan, Y.-X. (5 R)-5-hydroxytriptolide ameliorates anti-glomerular basement membrane glomerulonephritis in NZW mice by regulating Fcγ receptor signaling. Acta Pharmacol. Sin. 2018, 39, 107–116.
- Zhang, L.-Y.; Li, H.; Wu, Y.-W.; Cheng, L.; Yan, Y.-X.; Yang, X.-Q.; Zhu, F.-H.; He, S.-J.; Tang, W.; Zuo, J.-P. (5R)-5-hydroxytriptolide ameliorates lupus nephritis in MRL/lpr mice by preventing infiltration of immune cells. Am. J. Physiol.-Ren. Physiol. 2017, 312, F769–F777.
- Fan, D.; Guo, Q.; Shen, J.; Zheng, K.; Lu, C.; Zhang, G.; Lu, A.; He, X. The effect of triptolide in rheumatoid arthritis: From basic research towards clinical translation. Int. J. Mol. Sci. 2018, 19, 376.
- Xi, C.; Peng, S.; Wu, Z.; Zhou, Q.; Zhou, J. Toxicity of triptolide and the molecular mechanisms involved. Biomed. Pharmacother. 2017, 90, 531–541.
- Zhao, M.; Liang, G.-P.; Tang, M.-N.; Luo, S.-Y.; Zhang, J.; Cheng, W.-J.; Chan, T.-M.; Lu, Q.-J. Total glucosides of paeony induces regulatory CD4+ CD25+ T cells by increasing Foxp3 demethylation in lupus CD4+ T cells. Clin. Immunol. 2012, 143, 180–187.
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452.
- Chen, Y.; Wang, L.; Cao, Y.; Li, N. Total glucosides of Paeonia lactiflora for safely reducing disease activity in systemic lupus erythematosus: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 834947.
- Jun, L.; Junshan, L.; Aiwu, Z.; Minzhu, C.; Shuyun, X. Modulatory effects of total glucosides of paeony on B lymphocyte proliferation and interleukin 1 production. Chin. J. Pharmacol. Toxicol. 1994, 8, 53–55.
- Wang, X.W.; Chen, M.Z.; Xu, S.Y. The effects of total glucosides of paeony (TGP) on T lymphocyte subsets. Chin. Pharmacol. Bull. 1992, 8, 340–343.
- Wang, X.; Cheng, M.; Xu, S. Effects of total glucosides of paeony on immune system. Zhongguo Bing Li Sheng Li Za Zhi 1991, 7, 609–611.
- Wang, X.; Chen, M.; Xu, S. The effects of total glucosides’ of paeony (TGP) on T lymphocyte subsets. Zhongguo Yao Li Xue Tong Bao 1992, 8, 340–343.
- Zhang, H.; Xiao, W.; Hou, P. Clinical study of total glucosides of paeony in patients with systemic lupus erythematosus. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 2011, 31, 476–479.
- Ding, Z.-X.; Yang, S.-F.; Wu, Q.-F.; Lu, Y.; Chen, Y.-Y.; Nie, X.-L.; Jie, H.-Y.; Qi, J.-M.; Wang, F.-S. Therapeutic effect of total glucosides of paeony on lupus nephritis in MRL/lpr mice. J. South. Med. Univ. 2011, 31, 656–660.
- Zhao, M.; Liang, G.; Luo, S.; Lu, Q. Effect of total glucosides of peony on expression and DNA methylation status of ITGAL gene in CD4 (+) T cells of systemic lupus erythematosus. Zhong Nan Da Xue Xue Bao. Yi Xue Ban = J. Cent. South Univ. Med. Sci. 2012, 37, 463–468.
- Li, M.; Li, Y.; Xiang, L.; Li, L. Efficacy and safety of total glucosides of paeony as an add-on treatment in adolescents and adults with chronic urticaria: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 961371.
- Goh, E.; Tan, L.; Chow, S.; Teh, L.; Yeap, S. Use of complementary medicine in systemic lupus erythematosus patients in Malaysia. APLAR J. Rheumatol. 2003, 6, 21–25.
- Hamedi, A.; Sohrabpour, M.; Zarshenas, M.M.; Pasdaran, A. Phytochemical investigation and quantitative analysis of the fatty acids and sterol compounds of seven pharmaceutical valuable seeds. Curr. Pharm. Anal. 2018, 14, 475–482.
- Leiba, A.; Amital, H.; Gershwin, M.E.; Shoenfeld, Y. Diet and lupus. Lupus 2001, 10, 246–248.
- Maki, P.A.; Newberne, P.M. Dietary lipids and immune function. J. Nutr. 1992, 122, 610–614.
- Harbige, L.S. Fatty acids, the immune response, and autoimmunity: A question of n − 6 essentiality and the balance between n − 6 and n − 3. Lipids 2003, 38, 323–341.
- Ramessar, N.; Borad, A.; Schlesinger, N. The effect of Omega-3 fatty acid supplementation in systemic lupus erythematosus patients: A systematic review. Lupus 2022, 31, 287–296.
- Hejr, H.; Ghareghani, M.; Zibara, K.; Ghafari, M.; Sadri, F.; Salehpour, Z.; Hamedi, A.; Negintaji, K.; Azari, H.; Ghanbari, A. The ratio of 1/3 linoleic acid to alpha linolenic acid is optimal for oligodendrogenesis of embryonic neural stem cells. Neurosci. Lett. 2017, 651, 216–225.
- Wei, Y.; Meng, Y.; Li, N.; Wang, Q.; Chen, L. The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: A systematic review and meta-analysis. Food Funct. 2021, 12, 30–40.
- Duarte-Garcia, A.; Myasoedova, E.; Karmacharya, P.; Hocaoğlu, M.; Murad, M.H.; Warrington, K.J.; Crowson, C.S. Effect of omega-3 fatty acids on systemic lupus erythematosus disease activity: A systematic review and meta-analysis. Autoimmun. Rev. 2020, 19, 102688.
- Halade, G.V.; Rahman, M.M.; Bhattacharya, A.; Barnes, J.L.; Chandrasekar, B.; Fernandes, G. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J. Immunol. 2010, 184, 5280–5286.
- Borges, M.C.; Santos, F.d.M.M.; Telles, R.W.; Correia, M.I.T.D.; Lanna, C.C.D. Polyunsaturated omega-3 fatty acids and systemic lupus erythematosus: What do we know? Rev. Bras. Reumatol. 2014, 54, 459–466.
- MacLean, C.H.; Mojica, W.A.; Morton, S.C.; Pencharz, J.; Garland, R.H.; Tu, W.; Newberry, S.J.; Jungvig, L.K.; Grossman, J.; Khanna, P. Effects of omega-3 fatty acids on lipids and glycemic control in type II diabetes and the metabolic syndrome and on inflammatory bowel disease, rheumatoid arthritis, renal disease, systemic lupus erythematosus, and osteoporosis: Summary. In AHRQ Evidence Report Summaries; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2004.
- Pestka, J.J. n-3 polyunsaturated fatty acids and autoimmune-mediated glomerulonephritis. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2010, 82, 251–258.
- Fassett, R.G.; Gobe, G.C.; Peake, J.M.; Coombes, J.S. Omega-3 polyunsaturated fatty acids in the treatment of kidney disease. Am. J. Kidney Dis. 2010, 56, 728–742.
- Fettouh, D.S.; Saif, D.S.; El Gazzar, S.F.; Sonbol, A.A. Study the relationship between vitamin A deficiency, T helper 17, regulatory T cells, and disease activity in patients with systemic lupus erythematosus. Egypt. Rheumatol. Rehabil. 2019, 46, 244–250.
- Handono, K.; Firdausi, S.N.; Pratama, M.Z.; Endharti, A.T.; Kalim, H. Vitamin A improve Th17 and Treg regulation in systemic lupus erythematosus. Clin. Rheumatol. 2016, 35, 631–638.
- Minami, Y.; Hirabayashi, Y.; Nagata, C.; Ishii, T.; Harigae, H.; Sasaki, T. Intakes of vitamin B6 and dietary fiber and clinical course of systemic lupus erythematosus: A prospective study of Japanese female patients. J. Epidemiol. 2011, 21, 246–254.
- Shah, M.; Adams-Huet, B.; Kavanaugh, A.; Coyle, Y.; Lipsky, P. Nutrient intake and diet quality in patients with systemic lupus erythematosus on a culturally sensitive cholesterol lowering dietary program. J. Rheumatol. 2004, 31, 71–75.
- Minami, Y.; Sasaki, T.; Arai, Y.; Kurisu, Y.; Hisamichi, S. Diet and systemic lupus erythematosus: A 4 year prospective study of Japanese patients. J. Rheumatol. 2003, 30, 747–754.
- Tam, L.S.; Li, E.K.; Leung, V.Y.; Griffith, J.F.; Benzie, I.F.; Lim, P.L.; Whitney, B.; Lee, V.W.; Lee, K.K.; Thomas, G.N. Effects of vitamins C and E on oxidative stress markers and endothelial function in patients with systemic lupus erythematosus: A double blind, placebo controlled pilot study. J. Rheumatol. 2005, 32, 275–282.
- Ben-Zvi, I.; Aranow, C.; Mackay, M.; Stanevsky, A.; Kamen, D.L.; Marinescu, L.M.; Collins, C.E.; Gilkeson, G.S.; Diamond, B.; Hardin, J.A. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS ONE 2010, 5, e9193.
- Antico, A.; Tampoia, M.; Tozzoli, R.; Bizzaro, N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun. Rev. 2012, 12, 127–136.
- Kamen, D.L.; Cooper, G.S.; Bouali, H.; Shaftman, S.R.; Hollis, B.W.; Gilkeson, G.S. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun. Rev. 2006, 5, 114–117.
- Solovastru, L.G.; Vâta, D.; Statescu, L.; Constantin, M.M.; Andrese, E. Skin cancer between myth and reality, yet ethically constrained. Rev. Romana Bioet. 2014, 12, 47–52.
- Klack, K.; Bonfa, E.; Borba Neto, E.F. Diet and nutritional aspects in systemic lupus erythematosus. Rev. Bras. Reumatol. 2012, 52, 395–408.
- Maeshima, E.; Liang, X.-M.; Goda, M.; Otani, H.; Mune, M. The efficacy of vitamin E against oxidative damage and autoantibody production in systemic lupus erythematosus: A preliminary study. Clin. Rheumatol. 2007, 26, 401–404.
- Leiter, L.M.; Reuhl, K.R.; Racis Jr, S.P.; Sherman, A.R. Iron status alters murine systemic lupus erythematosus. J. Nutr. 1995, 125, 474–484.
- Falcão, S.; Barros, R.; Mateus, M.; Nero, P.; De Matos, A.A.; Pimentão, J.B.; Ribeiro, I.; Weigert, A.; Branco, J. Lúpus eritematoso sistiémico e anemia. Acta Reumatol. Port. 2007, 32, 73–79.
- O’Dell, J.R.; McGivern, J.P.; Kay, H.; Klassen, L.W. Improved survival in murine lupus as the result of selenium supplementation. Clin. Exp. Immunol. 1988, 73, 322.
- Soni, C.; Sinha, I.; Fasnacht, M.J.; Olsen, N.J.; Rahman, Z.S.; Sinha, R. Selenium supplementation suppresses immunological and serological features of lupus in B6. Sle1b mice. Autoimmunity 2019, 52, 57–68.
- Sahebari, M.; Rezaieyazdi, Z.; Khodashahi, M. Selenium and autoimmune diseases: A review article. Curr. Rheumatol. Rev. 2019, 15, 123–134.
- Brown, A.C. Lupus erythematosus and nutrition: A review of the literature. J. Ren. Nutr. 2000, 10, 170–183.
- Selmi, C.; Tsuneyama, K. Nutrition, geoepidemiology, and autoimmunity. Autoimmun. Rev. 2010, 9, A267–A270.




