Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Derylo-Marczewska | -- | 1165 | 2023-07-25 14:27:35 | | | |
| 2 | Camila Xu | Meta information modification | 1165 | 2023-07-26 03:20:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Blachnio, M.; Kusmierek, K.; Swiatkowski, A.; Derylo-Marczewska, A. Phenoxy Carboxylic Acid Herbicides Physical and Chemical Properties. Encyclopedia. Available online: https://encyclopedia.pub/entry/47255 (accessed on 07 February 2026).
Blachnio M, Kusmierek K, Swiatkowski A, Derylo-Marczewska A. Phenoxy Carboxylic Acid Herbicides Physical and Chemical Properties. Encyclopedia. Available at: https://encyclopedia.pub/entry/47255. Accessed February 07, 2026.
Blachnio, Magdalena, Krzysztof Kusmierek, Andrzej Swiatkowski, Anna Derylo-Marczewska. "Phenoxy Carboxylic Acid Herbicides Physical and Chemical Properties" Encyclopedia, https://encyclopedia.pub/entry/47255 (accessed February 07, 2026).
Blachnio, M., Kusmierek, K., Swiatkowski, A., & Derylo-Marczewska, A. (2023, July 25). Phenoxy Carboxylic Acid Herbicides Physical and Chemical Properties. In Encyclopedia. https://encyclopedia.pub/entry/47255
Blachnio, Magdalena, et al. "Phenoxy Carboxylic Acid Herbicides Physical and Chemical Properties." Encyclopedia. Web. 25 July, 2023.
Copy Citation
Chlorophenoxy herbicides belong to the class of aryloxyalkanoic acids that are derivatives of 1–3 carbon hydroxyalkanoic acids with aromatic substituent attached to the alcoholic oxygen.
carbonaceous adsorbents
inorganic adsorbents
low-cost adsorbents
1. Introduction
The production of pesticides is an important branch of the chemical industry, and their use for crop protection and pest elimination is a common and necessary practice used throughout the world. The consumption of crop protection products has increased many times over the years. In 2020, their annual consumption in agriculture was estimated at 2.7 million tons, of which about 52% are herbicides, 23% are fungicides, 18% are insecticides, and 7% are other pesticides [1]. Today, the most commonly used herbicide is glyphosate, which is the main ingredient in the popular market preparation called Roundup. Apart from glyphosate, the most popular and widely used herbicides are those derived from phenoxyacetic acid, especially 2,4-dichlorophenoxyacetic acid (2,4-D) and 2-methyl-4-chlorophenoxyacetic acid (MCPA) are used on a mass scale. In 2014, the US Environmental Protection Agency (EPA) approved the combined use of glyphosate and 2,4-D, a mixture available under the trademark “Enlist Duo”. In January 2022, the EPA [2] renewed the limited-time registration of this product, which is expected to contribute to the even greater use of both herbicides. Phenoxyacetic herbicides act as synthetic auxins and growth regulators and are widely used to control broadleaf weeds in farmlands, pastures, lawns, and grassy rights of way. The widespread use of these herbicides is associated with their massive release into the surface and groundwater. Their presence in the aquatic environment is undesirable, especially since these compounds are characterized by quite high toxicity to living organisms [3][4][5]. Therefore, their degradation and removal from the aquatic environment and prevention of their entry into the ecosystem become a priority issue.
2. Physical, Chemical, and Biological Properties of Phenoxy Carboxylic Acid Herbicides
Chlorophenoxy herbicides belong to the class of aryloxyalkanoic acids that are derivatives of 1–3 carbon hydroxyalkanoic acids with aromatic substituent attached to the alcoholic oxygen. They were developed in the 1940s to synthesize analogues of the auxin, indole-3 acetic acid (IAA), playing the role of a natural plant growth regulator. The mechanism of action of this group of chemicals (also called as auxinic herbicides) is based on mimicking IAA at the molecular level, which controls cell enlargement, division, and plant growth through the plant life cycle [6].
Generally, it is assumed that chlorophenoxy herbicide interacts with auxin-binding proteins (ABPs) located in the cell membrane, endoplasmic reticulum, and cell nucleus, and causes similar physiological effects to a natural IAA [7]. However, as far as IAA concentration is regulated by synthesis, degradation, and conjugation to other molecule processes in a plant, in the case of auxin-like herbicide, its amount is too large to be controlled by the plant regulation system. Herbicide mobilizes metabolic reserves that are transported to the site of growth in meristematic tissue. Consequently, it comes down to twisting and elongation of leaves and stems, damaging repair mechanisms, and, finally, plant death.
In terms of biochemistry, a plant treated with herbicide shows inhibition in respiration and photosynthesis processes due to the degradation of chlorophyll. The first symptoms of the herbicidal activity appear within a few hours from the moment of application. The effect of lethally abnormal growth is far less marked in grasses than in other species, hence chlorophenoxy acids are recognized as selective herbicides. Generally, the herbicidal activity of this group reveals towards dicotyledonous plant species while monocotyledonous ones remain relatively unaffected [8][9]. For this reason, auxinic herbicides are used to control broad-leaved annual and perennial weeds in agricultural and non-agricultural areas. Chlorophenoxy herbicides are usually applied post-emergence, alone, or combined with other herbicides. The last reports show [10][11][12] that chlorophenoxy group representatives—2,4-D and MCPA are at the top of the most commonly used pesticide-active ingredients in the US and the EU.
An active form of chlorophenoxy herbicides is acid but, commercially, these herbicides are formulated as esters, amines, choline salts, and salts with alkali metals. The type of formulation affects the method of application, the physicochemical properties of herbicide in the environment, and its ability to penetrate the plant. Because chlorophenoxy esters are soluble in organic solvents and oils while insoluble in water, they are used as aqueous emulsions with a suitable emulsifying agent. A great advantage of the ester formulation is resistance to washing from the leaves following rain. Nowadays, the production of methyl, ethyl, and isopropyl esters is limited because of their high vapor pressure and ability to volatilize, leading to the destruction of the surrounding crops. Ultimately, low molecular esters were replaced by ones with larger side chains that reduce their volatility [13]. The high solubility of esters in the plants’ cuticle affects more efficient absorption of them by plants, and after conversion to the corresponding acid, greater herbicidal activity is observed. For this reason, the ester formulation is recognized as a better weed controller than others. The herbicide’s residues that were not absorbed by the plant readily hydrolyze to their acid forms in the environmental conditions.
The amines and salts are readily soluble in water, which makes them vulnerable to being washed from leaves by rain. They are also characterized by low efficiency at moving from the leaf surface across the cuticle and into the plant. These forms of herbicides dissociate in water and form anions of the corresponding acids.
Because of the hydrolytic or dissociative abilities of the different formulations, the acid forms of chlorophenoxy herbicides are most often described in the studies on the mode of their action in living organisms. There are also many investigations on the chemical and physical processes they undergo in environmental systems. The pKa values of chlorophenoxyacetic acids are in the range of 2.56–3.36, so as weak acids, they dissociate into ions in water within the normal pH range of soils and environmental waters. They are slightly soluble in water and readily soluble in organic solvents, as indicated by the values of Kow, which depend on the chemical properties of the compound, i.e., quantity, type of functional groups, their position on an aromatic ring, and the length of hydrocarbon residue in a molecule. The solubility of weak acids depends on the solution’s pH. Both solubility and the weak-acid nature of herbicides are the main factors that influence their uptake by plants and translocation within their tissues.
The physical and chemical properties of phenoxyacetic acid herbicides (4-chlorophenoxy acetic acid, 4-CPA; 2,4-dichlorophenoxy acetic acid, 2,4-D; 2,4,5-trichlorophenoxy acetic acid, 2,4,5-T; 4-chloro-2-methylphenoxy acetic acid, MCPA) are presented in Table 1.
Table 1. Physical and chemical properties of phenoxyacetic acid herbicides.
| Parameter | 4-CPA | 2,4-D | 2,4,5-T | MCPA | Ref. |
|---|---|---|---|---|---|
| Molecular formula/ structure |
C8H7ClO3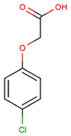 |
C8H6Cl2O3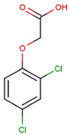 |
C8H5Cl3O3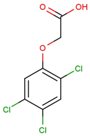 |
C9H9ClO3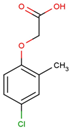 |
|
| Molecular weight, g mol−1 |
186.59 | 221.04 | 255.48 | 200.62 | |
| Ionization constant, (pKa) |
3.14 | 2.80 2.81 |
2.56 2.85 |
3.36 3.07 |
[14][15][16] |
| Solubility, g dm−3 at 20–25 °C |
0.848 0.957 |
0.682 0.900 |
0.268 0.278 0.281 0.280 |
0.825 0.734 |
[16][17][18][19] |
| Kow (logP) |
1.85 3.2 |
2.37 | 2.89 3.13 |
3.25 | [20][21] |
| Dmin/Dmax, Å |
4.33/9.38 | 4.88/9.49 | 6.22/8.88 | 5.47/9.52 | [22] |
| Melting/boiling point, °C | 157–158/160 | 136–140/160 | 157–158/ above 200 (decomposition) |
108–112/ 160 118–119 |
[17][18][19] |
| Vapour pressure, Pa at 20–25 °C |
9 × 10−2 | 1 × 10−5 2 × 10−5 |
0.7 × 10−6 | 2.3 × 10−5 2 × 10−4 |
[16][18][19] |
where: pKa—values based on partial charge distribution in a molecule; log D—octanol-water coefficient at a given pH; log P—partition coefficient of a compound between octanol/water; Dmin, Dmax—measure between the most distant molecule atoms.
References
- U.S. FAO Food and Agriculture Organization of the United Nations. Pesticides Use, Pesticides Trade and Pesticides Indicators. Global, Regional and Country Trends, 1990–2020. 2022. Available online: http://www.fao.org/faostat/en/#data/RP (accessed on 1 July 2023).
- U.S. EPA. Enlist One and Enlist Duo Decision; EPA-HQ-OPP-2021-0957; U.S. EPA: Washington, DC, USA, 2022.
- von Stackelberg, K. A systematic review of carcinogenic outcomes and potential mechanisms from exposure to 2,4-D and MCPA in the environment. J. Toxicol. 2013, 2013, 1–53.
- World Health Organization. WHO Guidelines for Drinking-Water Quality, 2,4-D in Drinking-Water, WHO/SDE/WSH/03.04/70; World Health Organization: Geneva, Switzerland, 2003.
- World Health Organization. WHO Guidelines for Drinking-Water Quality, MCPA in Drinking-Water, WHO/SDE/WSH/03.04/38; World Health Organization: Geneva, Switzerland, 2003.
- Mithila, J.; Hall, J.C.; Johnson, W.G.; Kelley, K.B.; Riechers, D.E. Evolution of resistance to auxinic herbicides: Historical perspectives, mechanisms of resistance, and implications for broadleaf weed management in agronomic crops. Weed Sci. 2011, 59, 445–457.
- Jiang, Z.; Li, J.; Qu, L.-J. 2-Auxins. In Hormone Metabolism and Signaling in Plants; Li, J., Li, C., Smith, S.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 39–76.
- Stenersen, J. Chemical Pesticides Mode of Action and Toxicology; CRC Press: Boca Raton, FL, USA, 2004.
- Benfeito, S.; Garrido, J.; Sottomayor, B.M.J.; Fernanda, G. Pesticides and cancer: Studies on the interaction of phenoxy acid herbicides with DNA. In Handbook on Herbicides; Kobayashi, D., Watanabe, E., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013.
- Atwood, D.; Paisley-Jones, C. Pesticides Industry Sales and Usage 2008–2012 Market Estimates. Available online: https://www.epa.gov/sites/production/files/2017-01/documents/pesticides-industry-sales-usage-2016_0.pdf (accessed on 1 January 2023).
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446.
- Morton, P.A.; Fennell, C.; Cassidy, R.; Doody, D.; Fenton, O.; Mellander, P.E.; Jordan, P. A review of the pesticide MCPA in the land-water environment and emerging research needs. Wiley Interdiscip. Rev. Water 2020, 7, e1402.
- Tu, M.; Hurd, C.; Randall, J.M. Weed Control Methods Handbook: Tools & Techniques for Use in Natural Areas; The Nature Conservancy: Harrisburg, PA, USA, 2001.
- Derylo-Marczewska, A.; Blachnio, M.; Marczewski, A.W.; Seczkowska, M.; Tarasiuk, B. Phenoxyacid pesticide adsorption on activated carbon–Equilibrium and kinetics. Chemosphere 2019, 214, 349–360.
- Hornsby, A.G.; Herner, A.E.; Wauchope, R.D. Pesticide Properties in the Environment; Springer: New York, NY, USA, 1996.
- Roberts, T.R.; Hutson, D.H.; Lee, P.W.; Nicholls, P.H. Metabolic Pathways of Agrochemicals. Part 1: Herbicides and Plant Growth Regulators; Royal Society of Chemistry: Cambridge, UK, 1998.
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data; CRC Press Library of Congress: Boca Raton, FL, USA, 2010.
- Khan, A.A.; Akhtar, T. Adsorption thermodynamics studies of 2,4,5-trichlorophenoxy acetic acid on poly-o-toluidine Zr(IV) phosphate, a nano-composite used as pesticide sensitive membrane electrode. Desalination 2011, 272, 259–264.
- Kennepohl, E.; Munro, I.C.; Bus, J.S. Phenoxy herbicides (2,4-D). In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Krieger, R., Ed.; Academic Press: New York, NY, USA, 2010; pp. 1829–1847.
- Tűrker, L. AM1 treatment of some phenoxyacetic acid herbicides. Turk. J. Biol. 2000, 24, 291–298.
- Birnbaum, L.S. The role of structure in the disposition of halogenated aromatic xenobiotics. Environ. Health Perspect. 1985, 61, 11–20.
- Marvin 14.8.25.0 Suite Program; ChemAxon Ltd.: Budapest, Hungary, 2017.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
26 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




