Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Junsang Yoo | -- | 1673 | 2023-07-25 11:54:39 | | | |
| 2 | Lindsay Dong | + 3 word(s) | 1676 | 2023-07-26 05:35:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chang, Y.; Lee, S.; Kim, J.; Kim, C.; Shim, H.S.; Lee, S.E.; Park, H.J.; Kim, J.; Lee, S.; Lee, Y.K.; et al. Efficient Direct Lineage Reprogramming Technology for Neurological Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/47240 (accessed on 05 March 2026).
Chang Y, Lee S, Kim J, Kim C, Shim HS, Lee SE, et al. Efficient Direct Lineage Reprogramming Technology for Neurological Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/47240. Accessed March 05, 2026.
Chang, Yujung, Sungwoo Lee, Jieun Kim, Chunggoo Kim, Hyun Soo Shim, Seung Eun Lee, Hyeok Ju Park, Jeongwon Kim, Soohyun Lee, Yong Kyu Lee, et al. "Efficient Direct Lineage Reprogramming Technology for Neurological Diseases" Encyclopedia, https://encyclopedia.pub/entry/47240 (accessed March 05, 2026).
Chang, Y., Lee, S., Kim, J., Kim, C., Shim, H.S., Lee, S.E., Park, H.J., Kim, J., Lee, S., Lee, Y.K., Park, S., & Yoo, J. (2023, July 25). Efficient Direct Lineage Reprogramming Technology for Neurological Diseases. In Encyclopedia. https://encyclopedia.pub/entry/47240
Chang, Yujung, et al. "Efficient Direct Lineage Reprogramming Technology for Neurological Diseases." Encyclopedia. Web. 25 July, 2023.
Copy Citation
Gene therapy is an innovative approach in the field of regenerative medicine. This therapy entails the transfer of genetic material into a patient’s cells to treat diseases. Direct lineage reprogramming (DLR) enables the direct conversion of differentiated mature cells into various other cell types, without the need for an intermediate pluripotent state. This approach was inspired by the critical role played by transcription factors in the process of converting non-neuronal cells into neurons.
cell fate conversion
spinal cord injury
gene therapy
cell recuperating factors
1. Introduction
Cell fate conversion technology originated with the generation of induced pluripotent stem cells (iPSCs) via viral transduction of specific transcription factors, namely, octamer-binding transcription factor 4, SRY-Box transcription factor 2, KLF transcription factor 4, and MYC proto-oncogene, bHLH transcription factor (OSKM) [1]. This technology demonstrated that a fully differentiated somatic cell could be reprogrammed into a completely different cell type by introducing key transcriptional factors of starting cells. Based on this principle, numerous studies have shown the feasibility of direct lineage reprogramming (DLR) from fibroblasts to various cell types, such as neurons, cardiomyocytes, chondrocytes, and hepatocytes.
Specifically, for neuronal reprogramming, DLR technology can be used to reprogram fibroblasts into various neuronal types, including cholinergic motor, glutamatergic, GABAergic, and dopaminergic neurons [2][3][4][5][6]. In 2010, it was revealed that the ectopic expressions of achaete-scute homolog 1 (ASCL1), POU domain transcription factor (BRN2), and myelin transcription factor 1-like (MYT1L) (ABM) could induce DLR of fibroblasts into functional glutamatergic neurons, characterized by synaptic activity and the regulation of Na+/K+ currents. In 2014, Wernig et al. showed that ASCL1 alone could induce DLR of functional glutamatergic neurons, which demonstrated that ASCL1 is the key master regulator that drives neuronal lineage development, with the assistance of two other factors.
Another example is the generation of induced dopaminergic neurons (iDNs). To generate iDNs, it is crucial to cotransduce dopaminergic lineage transcriptional factors, nuclear receptor 4A2 (NURR1), paired-like homeodomain 3 (PITX3), and LIM homeobox transcription factor 1 alpha (LMX1A) with ASCL1 (ANPL) [7]. By generating iDNs using DLR technology, researchers have provided evidence that Parkinson’s disease (PD) could be treated. PD is a progressive neurodegenerative disease that mainly affects motor function. It is characterized by the degeneration of dopaminergic neurons in the substantia nigra-to-striatum circuit, which leads to neurotransmitter imbalances and Parkinsonian symptoms. The cardinal symptoms of this disease include bradykinesia (slowness of movement), rigidity (stiffness and increased resistance to passive limb movements), tremor (involuntary and rhythmic shaking), and postural instability (impaired balance and coordination).
Spinal cord injury (SCI) is another major neurological disorder that can cause movement disabilities. SCI is a devastating condition that can lead to partial or complete loss of motor and sensory functions below the level of an injury. The pathophysiology of the disease involves complex interplay among secondary injury mechanisms, including oxidative stress, inflammation, cellular toxicity, and demyelination. Despite extensive research efforts, there is currently no effective therapy for SCI. Gene therapy is a promising approach as it allows targeted delivery of therapeutic genes to an injured spinal cord.
Motor neurons are specialized neurons that control the contraction of skeletal muscles, which permits movement and locomotion. In SCI, the motor neurons that innervate the muscles below the level of injury are often damaged or destroyed, which leads to paralysis and motor malfunction. Recently, the cotransduction of transcriptional factors, ASCL1, BRN2, MYT1L, and LIM homeobox 3 (Lhx3) has been reported to induce DLR of motor neurons from fibroblasts [8].
DLR exhibits several advantages over iPSC redifferentiation techniques because it does not pass through a pluripotent stage, which enables the production of stem cell-free cell sources (e.g., astrocytes and fibroblasts) [5]. Furthermore, DLR can address safety issues, such as teratoma formation, and it is more cost-effective for clinical applications. The OSKM-induced iPSCs possess a risk of differentiating into other types of pluripotent stem cells because the early phase of intermediate iPSCs is unstable. Moreover, the regeneration of iPSCs is less cost effective than DLR because multiple steps are involved in creating iPSCs; thus, dealing with iPSC reprogramming, differentiation, and transplantation to the target organs is difficult. However, in vivo DLR could be more successful for direct differentiation at precise locations in several organs. In vivo DLR is accomplished by manipulating the expression of certain genes in the targeted cells, leading to their conversion into a different cell type without the need for cell transplantation or external manipulation (Figure 1). In vivo DLR could be used to treat a wide range of diseases by converting the affected cells into healthy cells [9][10].
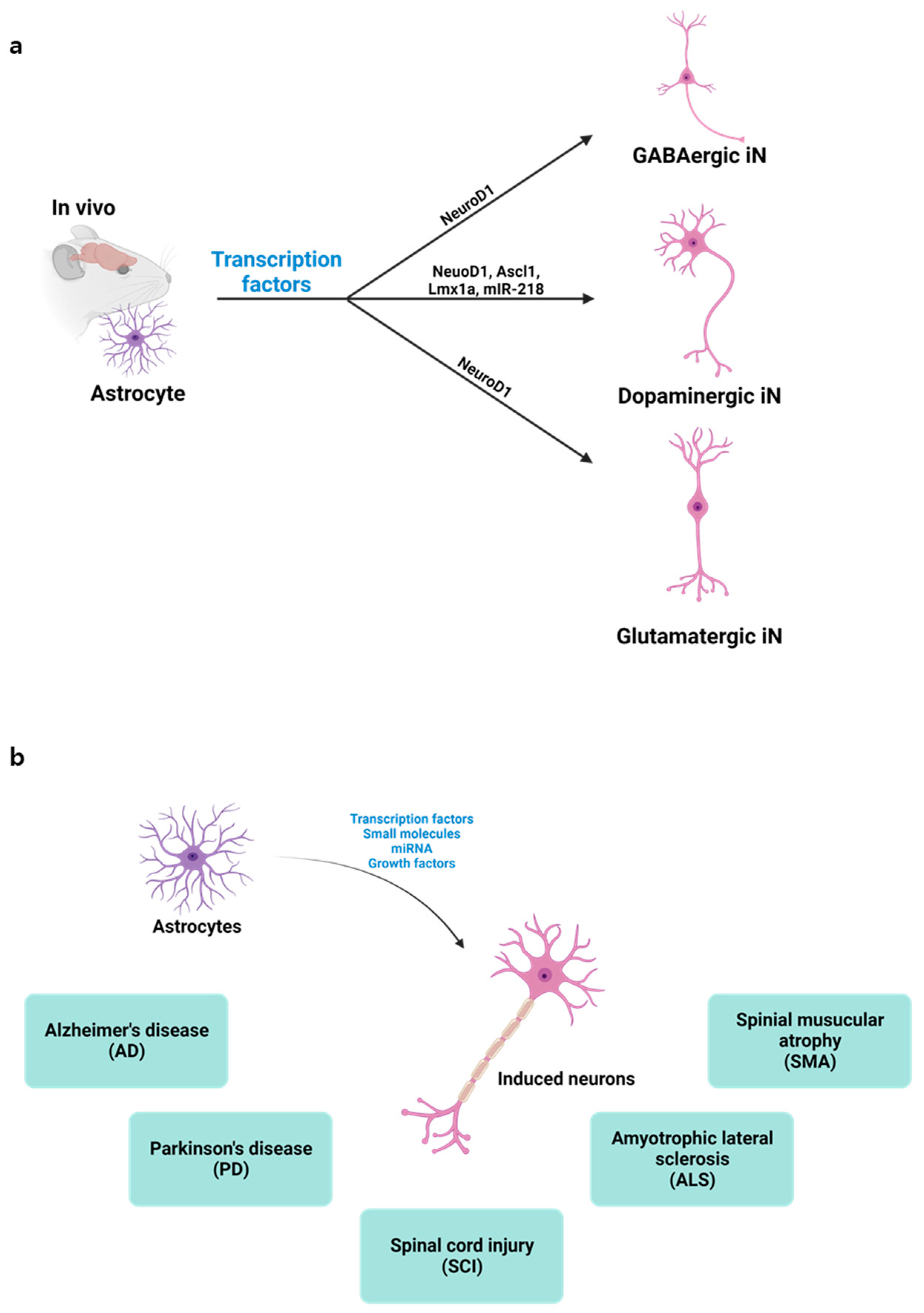
Figure 1. Direct conversion of astrocytes into induced neurons: (a) Depiction of in vivo astrocyte reprogramming into various neuron types, i.e., GABAergic, dopaminergic, or glutamatergic, achieved through the targeted expression of specific transcription factors; (b) Illustration showcasing the potential applications of in vivo direct neuronal reprogramming.
2. Generation of Induced Neurons via Cell Fate Conversion
2.1. Acceleration of the Direct Neuronal Reprogramming Process
Cell fate conversion techniques hold substantial promise within the realm of gene therapy for neurological diseases, and therefore, have garnered immense interest among researchers in the field. Consequently, numerous approaches aimed at expediting the process of cell fate conversion have been investigated [5][11][12][13]. Of particular note, cell DLR represents a cutting-edge technology within the field of regenerative medicine. A pioneering study discovered that the direct conversion of induced neurons could be achieved by transducing key transcriptional factors. The ectopic expression of ASCL1, BRN2, and MYT1L (ABM) induced cortical neuronal reprogramming; ASCL1, NURR1, PITX3, and LMX1A (ANPL) induced dopaminergic neuronal reprogramming; and ASCL1, BRN2, MYT1L, and Lhx3 produced motor neurons’ action potential, forming the neuromuscular junction [2][3][14]. Several studies have described various methodologies for promoting neuronal DLR.
The use of an appropriate DNA carrier for reprogramming transcriptional factors is necessary for applying DLR technology in the field of gene therapy. Currently, there is significant interest among researchers in using viral vectors for gene therapy, owing to their ability to effectively deliver the necessary genetic material to the target cells. Globally, lentivirus, retrovirus, adenovirus, and adeno-associated virus (AAV) are commonly used viral vectors for ex vivo cell therapy and in vivo gene therapy [15][16][17][18].
2.2. The Role of AuNpRs in the Direct Neuronal Reprogramming Process
The other potential clinical method for gene therapy in the future is to apply biocompatible nanomaterials, particularly AuNpRs. Several studies have shown that specifically modified or functionalized nanoparticles exert crucial effects on biological processes, such as cell survival and differentiation. AuNPs exhibit various therapeutic characteristics, including biocompatibility, ROS scavenging effect, high surface reactivity, and plasmon resonance [19][20][21][22][23]. Not all AuNPs have these effects; however, specifically modified AuNPs or AuNPs integrated with other materials have a drastic impact on the DLR process. Recently, Yoo and Park et al. reported that AuNpRs played a key role as ROS scavengers, ameliorating Parkinsonian phenotypes (i.e., slowness, muscle rigidity, and loss of movement) [19]. They injected AuNpRs into the substantia nigra region in 6-OHDA-induced Parkinsonian mouse model with ASCL1, NURR1, PITX3, and LMX1A, which are dopaminergic neuron conversion transcriptional factors [19].
DA neurons are particularly susceptible to oxidative stress owing to several factors, which contribute to their vulnerability in PD. First, excessive ROS can negatively affect the enzymes involved in dopamine synthesis. TH is the rate-limiting enzyme in dopamine biosynthesis, which converts tyrosine to levodopa. ROS can inhibit TH activity by causing oxidative modification of the enzyme, such as the formation of disulfide bonds and carbonyl groups. This modification results in reduced dopamine production. Second, dopaminergic neurons store dopamine in vesicles via the action of vesicular monoamine transporter (VMAT2). Excessive ROS can impair VMAT2 function, which decreases the vesicular storage of dopamine. This process increases cytosolic dopamine levels, which, in turn, makes more dopamine available for auto-oxidation and subsequent ROS generation, thus, leading to a vicious cycle of oxidative stress and damage.
2.3. The Role of Biocompatible Materials as Delivery Cargo for DLR
Recently, several types of biocompatible materials have emerged as promising DNA carriers for gene therapy [24]. Nanoparticles, such as liposomes, polymeric nanoparticles, and inorganic nanoparticles, have been engineered to improve the efficiency, specificity, and safety of gene delivery to target cells [25][26][27]. Versatile carriers can protect the encapsulated DNA from degradation, enhance cellular uptake, and facilitate controlled release, and thereby, increase the possibility of successful gene delivery [26]. In the context of gene therapy for neurological diseases, the use of nanoparticles as DNA carriers can overcome the challenges associated with crossing the blood–brain barrier, thus, enabling more effective and targeted delivery of therapeutic genes [28]. By leveraging the advances in nanoparticle technology, DLR strategies can be further optimized to provide innovative and safer therapeutic options for patients with neurological disorders.
2.4. Gene Therapy for SCI Using DLR Technology
2.4.1. SCI: The Incurable Neurological Disorder
Over a decade ago, SCI was considered to lead to a lifetime of medical complications and reliance on a wheelchair, with extremely limited therapeutic options being available [29]. Patients with SCI often received frustrating and hopeless care. Since then, many researchers have been striving to identify the most effective treatment for patients with SCI. However, so far, no therapeutic methodology has been developed. Fortunately, recent advancements in neurosciences have provided new hope [30]. One promising avenue of research is gene therapy using AAV, which offers the potential for regeneration and functional restoration. Although therapeutic methodologies for SCI remain elusive, the prospects for successful treatment continue to improve with each new discovery [31].
2.4.2. The Potential Therapeutic Method for SCI: AAV Gene Therapy Using DLR Technology
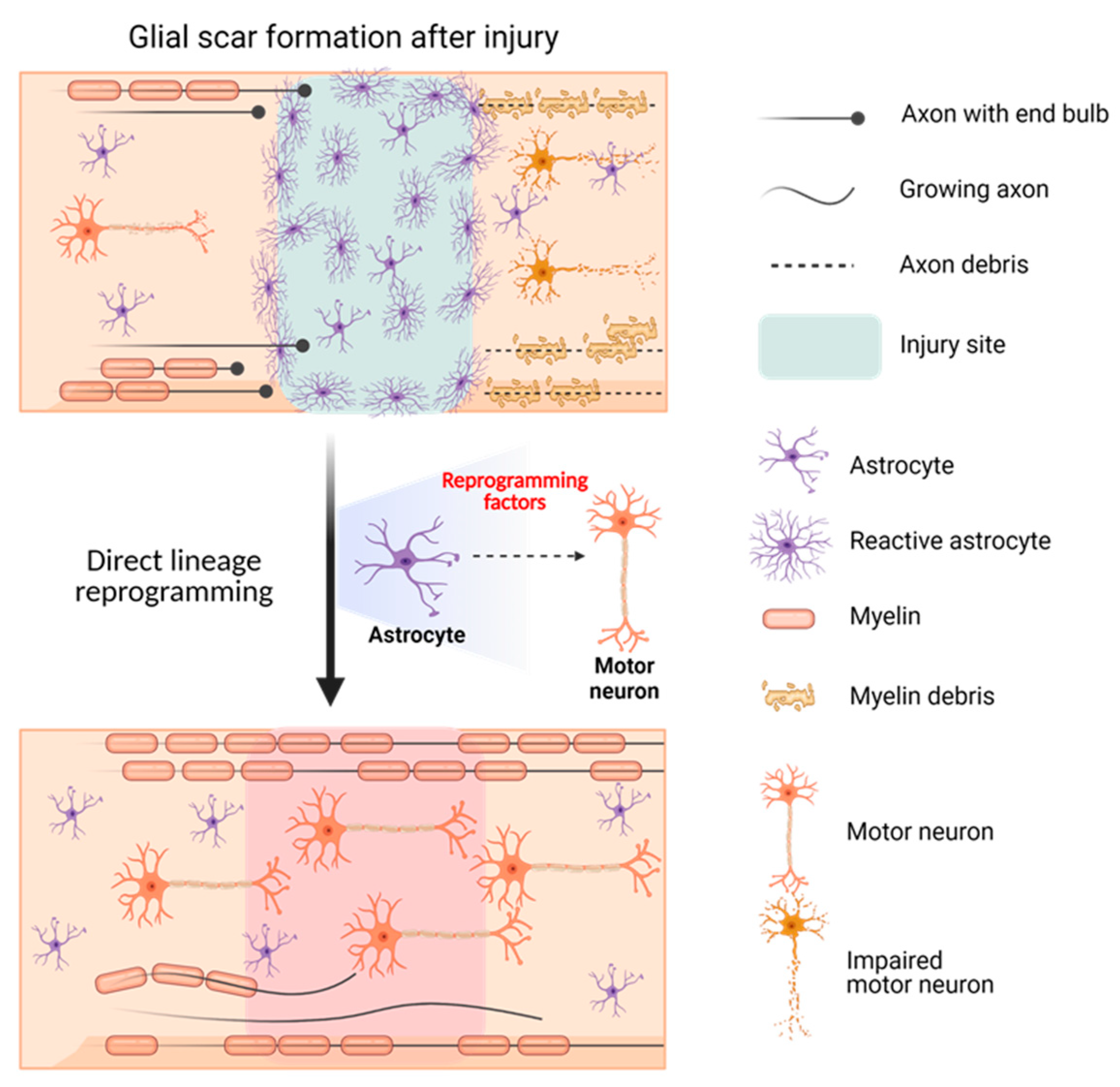
The possibility of using DLR to treat SCI has been studied based on the idea that reactive astrocytes that form glial scars at the injury site may hinder axonal regeneration and lead to neuronal impairment. In vivo DLR targeting of reactive astrocytes to convert them into motor neurons using appropriate transcriptional factors could provide a solution for paralysis in patients with SCI. Yoo et al. successfully identified factors, such as biocompatible materials or specific genetic molecules (cell recuperating factors), which could accelerate the DLR of motor neurons from fibroblasts or astrocytes. Moreover, converting GFAP-positive glial scars to synapsin-positive motor neurons could serve as a solution for the currently incurable SCI (Figure 2).

Figure 2. Schematic illustration showing the effect of motor neuron DLR on spinal cord injury.
References
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676.
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Südhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041.
- Son, E.Y.; Ichida, J.K.; Wainger, B.J.; Toma, J.S.; Rafuse, V.F.; Woolf, C.J.; Eggan, K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 2011, 9, 205–218.
- Victor, M.B.; Richner, M.; Olsen, H.E.; Lee, S.W.; Monteys, A.M.; Ma, C.; Huh, C.J.; Zhang, B.; Davidson, B.L.; Yang, X.W. Striatal neurons directly converted from Huntington’s disease patient fibroblasts recapitulate age-associated disease phenotypes. Nat. Neurosci. 2018, 21, 341–352.
- Yoo, J.; Lee, E.; Kim, H.Y.; Youn, D.-H.; Jung, J.; Kim, H.; Chang, Y.; Lee, W.; Shin, J.; Baek, S. Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson’s disease therapy. Nat. Nanotechnol. 2017, 12, 1006–1014.
- Colasante, G.; Lignani, G.; Rubio, A.; Medrihan, L.; Yekhlef, L.; Sessa, A.; Massimino, L.; Giannelli, S.G.; Sacchetti, S.; Caiazzo, M.; et al. Rapid Conversion of Fibroblasts into Functional Forebrain GABAergic Interneurons by Direct Genetic Reprogramming. Cell Stem Cell 2015, 17, 719–734.
- Hong, S.; Chung, S.; Leung, K.; Hwang, I.; Moon, J.; Kim, K.S. Functional roles of Nurr1, Pitx3, and Lmx1a in neurogenesis and phenotype specification of dopamine neurons during in vitro differentiation of embryonic stem cells. Stem Cells Dev. 2014, 23, 477–487.
- Colasante, G.; Rubio, A.; Massimino, L.; Broccoli, V. Direct Neuronal Reprogramming Reveals Unknown Functions for Known Transcription Factors. Front. Neurosci. 2019, 13, 283.
- Vignoles, R.; Lentini, C.; D’Orange, M.; Heinrich, C. Direct Lineage Reprogramming for Brain Repair: Breakthroughs and Challenges. Trends Mol. Med. 2019, 25, 897–914.
- Zhang, Y.; Xie, X.; Hu, J.; Afreen, K.S.; Zhang, C.L.; Zhuge, Q.; Yang, J. Prospects of Directly Reprogrammed Adult Human Neurons for Neurodegenerative Disease Modeling and Drug Discovery: iN vs. IPSCs Models. Front. Neurosci. 2020, 14, 546484.
- Chang, Y.; Yoo, J.; Kim, J.; Hwang, Y.; Shim, G.; Oh, Y.K.; Kim, J. Electromagnetized Graphene Facilitates Direct Lineage Reprogramming into Dopaminergic Neurons. Adv. Funct. Mater. 2021, 31, 2105346.
- Ma, X.; Kong, L.; Zhu, S. Reprogramming cell fates by small molecules. Protein Cell 2017, 8, 328–348.
- Kim, Y.; Jeong, J.; Choi, D. Small-molecule-mediated reprogramming: A silver lining for regenerative medicine. Exp. Mol. Med. 2020, 52, 213–226.
- Kim, J.; Su, S.C.; Wang, H.; Cheng, A.W.; Cassady, J.P.; Lodato, M.A.; Lengner, C.J.; Chung, C.-Y.; Dawlaty, M.M.; Tsai, L.-H. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell 2011, 9, 413–419.
- Colella, P.; Ronzitti, G.; Mingozzi, F. Emerging issues in AAV-mediated in vivo gene therapy. Mol. Ther. Methods Clin. Dev. 2018, 8, 87–104.
- Chen, Y.; Zhi, S.; Liu, W.; Wen, J.; Hu, S.; Cao, T.; Sun, H.; Li, Y.; Huang, L.; Liu, Y. Development of Highly Efficient Dual-AAV Split Adenosine Base Editor for In Vivo Gene Therapy. Small Methods 2020, 4, 2000309.
- Flotte, T.R.; Cataltepe, O.; Puri, A.; Batista, A.R.; Moser, R.; McKenna-Yasek, D.; Douthwright, C.; Gernoux, G.; Blackwood, M.; Mueller, C. AAV gene therapy for Tay-Sachs disease. Nat. Med. 2022, 28, 251–259.
- Yu, T.W.; Bodamer, O. A solid start for gene therapy in Tay–Sachs disease. Nat. Med. 2022, 28, 236–237.
- Lee, S.; Shim, H.S.; Park, H.J.; Chang, Y.; Han, Y.-E.; Oh, S.-J.; Lee, W.; Im, H.; Seol, Y.; Ryu, H. Elongated nanoporous Au networks improve somatic cell direct conversion into induced dopaminergic neurons for Parkinson’s disease therapy. Acta Biomater. 2022, 151, 561–575.
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the art biocompatible gold nanoparticles for cancer theragnosis. Pharmaceutics 2020, 12, 701.
- Tournebize, J.; Boudier, A.; Sapin-Minet, A.; Maincent, P.; Leroy, P.; Schneider, R.l. Role of gold nanoparticles capping density on stability and surface reactivity to design drug delivery platforms. ACS Appl. Mater. Interfaces 2012, 4, 5790–5799.
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter. 2017, 29, 203002.
- Shin, J.; Lee, S.; Yoo, S.; Jung, I.; Lee, S.; Kim, J.; Son, J.; Kim, J.-E.; Kim, J.-M.; Nam, J.-M. Enormous Enhancement in Single-Particle Surface-Enhanced Raman Scattering with Size-Controllable Au Double Nanorings. Chem. Mater. 2022, 34, 2197–2205.
- Douglas, K.L.; Tabrizian, M. Effect of experimental parameters on the formation of alginate–chitosan nanoparticles and evaluation of their potential application as DNA carrier. J. Biomater. Sci. Polym. Ed. 2005, 16, 43–56.
- Lee, Y.S.; Kim, S.W. Bioreducible polymers for therapeutic gene delivery. J. Control. Release 2014, 190, 424–439.
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555.
- Gao, X.; Huang, L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem. Biophys. Res. Commun. 1991, 179, 280–285.
- Thomsen, L.B.; Larsen, A.B.; Lichota, J.; Moos, T. Nanoparticle-derived non-viral genetic transfection at the blood-brain barrier to enable neuronal growth factor delivery by secretion from brain endothelium. Curr. Med. Chem. 2011, 18, 3330–3334.
- Sarhan, F.; Saif, D.; Saif, A. An overview of traumatic spinal cord injury: Part 1. aetiology and pathophysiology. Br. J. Neurosci. Nurs. 2012, 8, 319–325.
- Mothe, A.J.; Tator, C.H. Advances in stem cell therapy for spinal cord injury. J. Clin. Investig. 2012, 122, 3824–3834.
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S. Current clinical applications of in vivo gene therapy with AAVs. Mol. Ther. 2021, 29, 464–488.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
724
Revisions:
2 times
(View History)
Update Date:
26 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





