Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adam Miodek | -- | 1048 | 2023-07-23 10:59:21 | | | |
| 2 | Dean Liu | -7 word(s) | 1041 | 2023-07-24 05:04:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kojs, P.; Miodek, A.; Miodek, A.P.; Włoch, W. Vascular Cambium. Encyclopedia. Available online: https://encyclopedia.pub/entry/47143 (accessed on 08 February 2026).
Kojs P, Miodek A, Miodek AP, Włoch W. Vascular Cambium. Encyclopedia. Available at: https://encyclopedia.pub/entry/47143. Accessed February 08, 2026.
Kojs, Paweł, Adam Miodek, Aldona Paulina Miodek, Wiesław Włoch. "Vascular Cambium" Encyclopedia, https://encyclopedia.pub/entry/47143 (accessed February 08, 2026).
Kojs, P., Miodek, A., Miodek, A.P., & Włoch, W. (2023, July 23). Vascular Cambium. In Encyclopedia. https://encyclopedia.pub/entry/47143
Kojs, Paweł, et al. "Vascular Cambium." Encyclopedia. Web. 23 July, 2023.
Copy Citation
The vascular cambium is the main lateral meristem responsible for the secondary growth of trees. There are a number of explicit and implicit assumptions behind this statement which allow questions to be raised about the mechanism underlying the radial growth of trees. Based on the hypothesis of the diurnal strains of plant organs, it is anticipated that the process of radial growth can be understood as an adaptation to the cyclically changing mechanical stress in the radial direction generated by the phloem during the 24 h day cycle.
diurnal
strains
radial
growth
xylem
phloem
cambium
1. Assumptions and Simplifications Used in the Osmo-Mechanical Hypothesis
Trees are among the most complex living systems and are subject to multi-level regulation. The hypothesis presents an element of the secondary growth mechanism of trees related to the radial expansion of the vascular cambium. Highlighting novelty of this framework for understanding secondary growth, this hypothesis provides insight into the sophisticated mechanisms that drive radial growth of the vascular cambium of trees. This theoretical perspective focuses on the diurnal activity of phloem, xylem and vascular cambium in deciduous trees and is particularly relevant during the equinoxes at the peak of the growth season. Research on such complex living systems as trees, subject to multi-level regulation, can benefit greatly from this fresh outlook. For the sake of simplicity, it can be assumed that this occurs in the equatorial zone and thus without any distinct seasonality. In this hypothesis, a description of the effect of the phloem–periderm interaction on phellogen was deliberately not included. It was considered that this would overcomplicate the basic description of the growth of the vascular cambium. The osmo-mechanical hypothesis of the radial growth of the vascular cambium presented describes one of the many very complex processes involved in the secondary growth of trees. It is important to point out that the model is as simple as possible, while maintaining its full factual correctness for the assumptions made. It therefore provides a starting point for explaining the radial growth-integrated processes and events of the vascular cambium, such as:
-
The mechanism of xylem and phloem formation;
-
The mechanism of early- and latewood formation;
-
The mechanism of ring-porous and diffuse-porous wood formation;
-
The mechanism of reaction wood formation;
-
The mechanism of xylem/phloem differentiation responses to injuries;
-
The mechanism of intrusive growth, including the growth of fibre tips, vessel elements, and cambial cell rearrangement.
The above cellular processes and events would require the inclusion of: the difference in the mechanism of phloem swelling between tree species that start radial growth before leaf development (ring-porous species) and those after leaf development (diffuse-porous species); the seasonal cycle; the explanation of the mechanism of formation of spaces between the tangential walls of neighbouring cells; and the explanation of the mechanism of summation and extinction of tensile and compressive stress acting in the radial direction, originating from various sources (diurnal cycle, wind, gravity, landslides, temperature, insect gradations, frost- and drought-induced embolism, wounding, etc.).
It should be emphasised that, in the simplified version presented, the hypothesis correctly describes the behaviour of a single radial row of fusiform cells of the vascular cambium during the diurnal cycle. It is assumed that individual cells grow symplastically and undergo equal periclinal divisions and longitudinal anticlinal divisions. The basis for this hypothesis is provided by studies on the diurnal relationships of the phloem (inner bark) and xylem [1][2][3][4][5][6][7]; the research on the mechanisms of cambial development [8][9][10][11][12][13][14][15][16][17]; and the works on the hormonal regulation of vascular cambium growth [18][19].
2. Phloem as a Tensile and Compressive Stress Generator in the Vascular Cambium
Between osmotic stress and mechanical stress, induced by diurnal and nocturnal water flux through the plant, time-varying patterns of mechanical stress emerge, which may have different developmental significance. The subject of this dial oscillation is primarily the phloem, the inner and outer parts of which behave oppositely during the day and night.
The whole process can be described as a cyclic generation and relaxation of tensile mechanical stress in the radial direction. The process can arbitrarily start with the opening of the stomata (1) at dawn (Figure 1) [20][21]. As a result of the loss of water, the xylem (2) and phloem (3) shrink. As the phloem shrinks to a much greater degree than the xylem [1][22], the vascular cambium, which after the night is stretched radially between the xylem and phloem (20), goes through successive phases of decreasing tensile stress values, first circumferentially (4), then radially (5), until the tensile stress relaxes in this direction (6). Further shrinkage of the phloem results in the compression of its inner part in the radial direction (7). Thus, the cambium (8) and then the xylem (9) is compressed in the radial direction. At the same time, the outer part of the phloem on the periderm side is stretched (10) in this direction. At night, the stomata close (11) [20][21]. The cessation of transpiration results in an increase in water potential in the tracheary system and the rehydration of the xylem. Its rehydration causes a slight swelling (12) [23], followed by a greater swelling of the phloem (13) [22][24]. The vascular cambium located between the xylem and phloem, which was under compression during the day (8), goes through the phase of a decrease in compressive stress value in the radial direction (14). Then an increase in the tension of the xylem in the radial and circumferential directions occurs (15), together with an increase in the tensile stress value of the vascular cambium in the circumferential direction (16) until the relaxation of the radial compressive stress in vascular cambium (17). Further swelling of the phloem causes its inner part to be in tension in the radial direction (18) and the compression of its outer part on the side of the periderm (19). According to the hypothesis presented here, cambium stretching is generated in the radial direction between the inner part of the phloem and the outer part of the xylem (20).
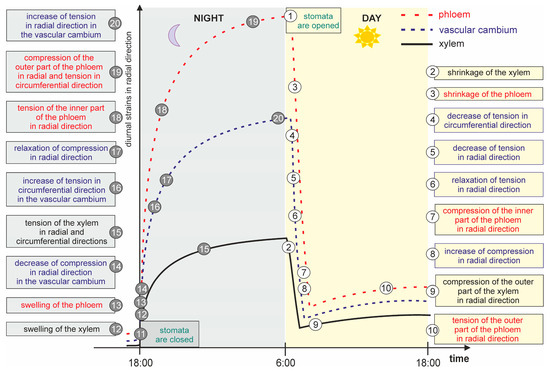
Figure 1. Generation of plant tissue strains in the tree trunk in the radial and circumferential directions due to the opening and closing of stomata in a diurnal rhythm. Tensile strains in the radial direction in the vascular cambium are generated by the swelling of the phloem at night, while compressive strains in the vascular cambium are caused by the shrinking of the phloem during the day (the scheme is an original development of the idea presented by Kojs and Rusin 2011 [25]).
References
- Alméras, T.; Yoshida, M.; Okuyama, T. Strains inside xylem and inner bark of a stem submitted to a change in hydrostatic pressure. Trees 2006, 20, 460–467.
- Zweifel, R.; Sterck, F.; Braun, S.; Buchmann, N.; Eugster, W.; Gessler, A.; Häni, M.; Peters, R.L.; Walthert, L.; Wilhelm, M.; et al. Why trees grow at night. New Phytol. 2021, 231, 2174–2185.
- Klepper, B.; Browning, V.D.; Taylor, H.M. Stem diameter in relation to plant water status. Plant Physiol. 1971, 48, 683–685.
- Alméras, T. Mechanical analysis of the strains generated by water tension in plant stems. Part II: Strains in wood and bark and apparent compliance. Tree Physiol. 2008, 28, 1513–1523.
- Alméras, T.; Gril, J. Mechanical analysis of the strains generated by water tension in plant stems. Part I: Stress transmission from the water to the cell walls. Tree Physiol. 2007, 27, 1505–1516.
- Molz, F.J.; Klepper, B. On the mechanism of water-stress-induced stem deformation. Agron. J. 1973, 65, 304–306.
- Nakai, T.; Abe, H.; Muramoto, T.; Nakao, T. The relationship between sap flow rate and diurnal change of tangential strain on inner bark in Cryptomeria japonica saplings. J. Wood Sci. 2005, 51, 441–447.
- Karczewska, D.; Karczewski, J.; Włoch, W.; Jura-Morawiec, J.; Kojs, P.; Iqbal, M.; Krawczyszyn, J. Mathematical modeling of intrusive growth of fusiform initials in relation to radial growth and expanding cambial circumference in Pinus sylvestris L. Acta Biotheor. 2009, 57, 331–348.
- Miodek, A.; Włoch, W.; Iqbal, M.; Gizińska, A.; Kojs, P. Controversy over the mode of growth of cambial cylinder. Bot. Rev. 2021, 87, 243–257.
- Miodek, A.; Gizińska, A.; Włoch, W.; Kojs, P. Intrusive growth of initials does not affect cambial circumference in Robinia pseudoacacia. Sci. Rep. 2022, 12, 7428.
- Kojs, P.; Włoch, W.; Rusin, A. Rearrangement of cells in storeyed cambium of Lonchocarpus sericeus (Poir.) DC connected with formation of interlocked grain in the xylem. Trees 2004, 18, 136–144.
- Kojs, P.; Rusin, A.; Iqbal, M.; Włoch, W.; Jura, J. Readjustments of cambial initials in Wisteria floribunda (Willd.) DC. for development of storeyed structure. New Phytol. 2004, 163, 287–297.
- Wilczek-Ponce, A.; Włoch, W.; Iqbal, M. How do trees grow in girth? Controversy on the role of cellular events in the vascular cambium. Acta Biotheor. 2021, 69, 643–670.
- Jura, J.; Kojs, P.; Iqbal, M.; Szymanowska-Pułka, J.; Włoch, W. Apical intrusive growth of cambial fusiform initials along the tangential walls of adjacent fusiform initials: Evidence for a new concept. Aust. J. Bot. 2006, 54, 493–504.
- Włoch, W.; Jura-Morawiec, J.; Kojs, P.; Iqbal, M.; Krawczyszyn, J. Does intrusive growth of fusiform initials really contribute to circumferential growth of vascular cambium? Botany 2009, 87, 154–163.
- Włoch, W.; Wilczek, A.; Jura-Morawiec, J.; Kojs, P.; Iqbal, M. Modelling for rearrangement of fusiform initials during radial growth of the vascular cambium in Pinus sylvestris L. Trees 2013, 27, 879–893.
- Wilczek, A.B.; Iqbal, M.; Włoch, W.; Klisz, M. Geometric analysis of intrusive growth of wood fibres in Robinia pseudoacacia. IAWA J. 2018, 39, 191–208.
- Wang, H. Regulation of vascular cambium activity. Plant Sci. 2020, 291, 110322.
- Fischer, U.; Kucukoglu, M.; Helariutta, Y.; Bhalerao, R.P. The Dynamics of Cambial Stem Cell Activity. Annu. Rev. Plant Biol. 2019, 70, 293–319.
- Wronski, E.B.; Holmes, J.W.; Turner, N.C. Phase and amplitude relations between transpiration, water potential and stem shrinkage. Plant Cell Environ. 1985, 8, 613–622.
- Caird, M.A.; Richards, J.H.; Donovan, L.A. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 2007, 143, 4–10.
- Sevanto, S.; Hölttä, T.; Holbrook, N.M. Effects of the hydraulic coupling between xylem and phloem on diurnal phloem diameter variation. Plant Cell Environ. 2011, 34, 690–703.
- Treydte, K.; Lehmann, M.M.; Wyczesany, T.; Pfautsch, S. Radial and axial water movement in adult trees recorded by stable isotope tracing. Tree Physiol. 2021, 41, 2248–2261.
- Mencuccini, M.; Hölttä, T.; Sevanto, S.; Nikinmaa, E. Concurrent measurements of change in the bark and xylem diameters of trees reveal a phloem-generated turgor signal. New Phytol. 2013, 198, 1143–1154.
- Kojs, P.; Rusin, T. Diurnal strains in plants. In Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec, J., Eds.; Springer: Berlin, Germany, 2011; pp. 220–224.
More
Information
Subjects:
Plant Sciences; Anatomy & Morphology; Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
24 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




