Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Supaporn Kulthinee | -- | 1303 | 2023-07-22 20:11:29 | | | |
| 2 | Sirius Huang | Meta information modification | 1303 | 2023-07-24 03:26:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kulthinee, S.; Tasanarong, A.; Franco, M.; Navar, L.G. Regulating Renal Afferent Arterioles in Angiotensin II-Dependent Hypertension. Encyclopedia. Available online: https://encyclopedia.pub/entry/47139 (accessed on 07 February 2026).
Kulthinee S, Tasanarong A, Franco M, Navar LG. Regulating Renal Afferent Arterioles in Angiotensin II-Dependent Hypertension. Encyclopedia. Available at: https://encyclopedia.pub/entry/47139. Accessed February 07, 2026.
Kulthinee, Supaporn, Adis Tasanarong, Martha Franco, Luis Gabriel Navar. "Regulating Renal Afferent Arterioles in Angiotensin II-Dependent Hypertension" Encyclopedia, https://encyclopedia.pub/entry/47139 (accessed February 07, 2026).
Kulthinee, S., Tasanarong, A., Franco, M., & Navar, L.G. (2023, July 22). Regulating Renal Afferent Arterioles in Angiotensin II-Dependent Hypertension. In Encyclopedia. https://encyclopedia.pub/entry/47139
Kulthinee, Supaporn, et al. "Regulating Renal Afferent Arterioles in Angiotensin II-Dependent Hypertension." Encyclopedia. Web. 22 July, 2023.
Copy Citation
In angiotensin II (Ang II)-dependent hypertension, Ang II activates angiotensin II type 1 receptors (AT1R) on renal vascular smooth muscle cells, leading to renal vasoconstriction with eventual glomerular and tubular injury and interstitial inflammation. While afferent arteriolar vasoconstriction is initiated by the increased intrarenal levels of Ang II activating AT1R, the progressive increases in arterial pressure stimulate the paracrine secretion of adenosine triphosphate (ATP), leading to the purinergic P2X receptor (P2XR)-mediated constriction of afferent arterioles.
hypertension
P2X receptors
AT1 receptors
afferent arteriole
angiotensin II infusions
1. Introduction
Angiotensin II (Ang II), the principal product of the renin angiotensin system, exerts a powerful role in the pathogenesis of hypertension and renal injury via the activation of angiotensin II type 1 receptors (AT1R), which are widely distributed in all regions of the kidneys [1][2][3]. Angiotensin II receptor blockers (ARBs) are recommended in the treatment of hypertension because they reduce blood pressure and the associated renal inflammation, fibrosis, and renal injury [4][5][6]. However, the pluripotent actions of Ang II involve interactions with many other vasoactive systems [7].
Extracellular nucleotides, particularly ATP, exert physiological and pathological actions via P2 purinergic receptors (P2XR and P2YR), which strongly influence renal vascular resistance, renal autoregulation, and tubular transport function [8][9][10][11][12]. However, the sustained overexpression and activation of purinergic receptors induces renal vasoconstriction and leads to the eventual development of glomerular and tubulointerstitial injury [13][14][15]. The participation of purinergic P2XR in the development and maintenance of hypertension-associated renal injury has progressively gained recognition [16]. In Ang II-dependent hypertension, P2X7R-mediated deleterious effects include the suppression of autoregulation and pressure natriuresis and the reduced oxygenation of the medulla [17]. Indeed, the P2X1R stimulation of afferent arteriolar vasoconstriction causes the reduction of glomerular blood flow and pressure [13]. Interestingly, in Ang II-dependent hypertension, the administration of P2X1R and P2X7R inhibitors restore the afferent arteriolar resistances back to normal values without reducing the blood pressure, which is maintained by the elevated systemic Ang II levels [18]. An important issue related to the influence of AT1 receptors under conditions of elevated intrarenal Ang II levels is the interaction between P2XR and AT1R in regulating afferent arteriolar resistance.
2. Regulation of Renal Afferent Arterioles in Ang II-Dependent Hypertension
The renal afferent arteriole is unique in terms of its responses to two major feedback mechanisms responsible for renal autoregulation, namely the myogenic mechanism and tubuloglomerular feedback mechanism (TGF). The segments near the glomerulus are regulated mainly by the TGF [19]. The diameters and luminal pressure in the segments closest to their origin have greater wall tension. Increases in renal perfusion pressure lead to rapid myogenic-mediated vessel wall contraction, which increases preglomerular resistance. Collectively, the myogenic and TGF mechanisms protect against glomerular barotrauma in acute as well as sustained hypertension [20]. Although renal autoregulation is impaired in hypertension [19][21], renal microvascular responses are able to protect the glomerular vasculature resetting and elevating the vascular tone of renal afferent arterioles via interactions between AT1R and P2XR [20].
In angiotensin II-dependent hypertension, Ang II activates AT1R throughout the body to increase systemic vascular resistance, leading to elevation of blood pressure. In particular, renal afferent arteriolar responsiveness to Ang II is enhanced in this stage [22], and the increase in renal afferent arteriolar resistance contributes to an initial adaptation of renal function. Renal AT1R activation is of cardinal importance in the development of Ang II-dependent hypertension and when AT1R are selectively deleted from the kidneys, the extrarenal AT1R are not sufficient to induce hypertension [23]. Although total kidney AT1R mRNA levels and receptor protein were not significantly increased after 2 weeks of Ang II infusion, they were sufficient to cause hypertension [24].
Nishiyama et al. [25] demonstrated that renal interstitial fluid Ang II levels were increased in Ang II-infused rats. Ang II levels in Ang II-infused rats were higher in renal cortical endosomes than in control rats via an AT1 receptor-mediated mechanism [26]. Li et al. [27] demonstrated that in AT1a receptor-deficient mice, AT1 receptor-mediated increases in Ang II in the kidney were prevented. These studies indicate that AT1Rs contribute to the augmentation of intrarenal Ang II and provide the basis for sustained maintenance of hypertension. Chronic Ang II infusion elicits sustained renal afferent arteriolar vasoconstriction, as supported by the augmentation of intrarenal Ang II [28], and the restoration of normal blood pressures through the vasodilator responses to AT1R blockade [29]. These findings confirm that the AT1Rs are not desensitized during chronic Ang II infusion for two weeks and play an important role in Ang II-dependent hypertension.
Under physiological conditions, the increases in renal perfusion pressure result in the augmentation of renal interstitial fluid ATP levels. A study using microdialysis to collect renal interstitial fluid showed that ATP was increased in response to elevation in renal perfusion pressure within the autoregulatory range in anesthetized dogs [30]. Furthermore, a study using biosensors for assessment of ATP in the renal cortex in response to changes in renal perfusion pressure in anesthetized Sprague Dawley rats showed that increases in renal perfusion pressure are associated with elevated interstitial concentrations of ATP [31]. The mechanisms involved are shear stress-dependent ATP release from endothelial cells of the renal microvasculature and ATP from macula densa cells during tubuloglomerular feedback responses, which collectively augment Ca2+ influx into vascular smooth muscle via the P2X receptor [20][32][33].
Under normotensive conditions, P2X1 receptors are expressed on the renal afferent arterioles but not in efferent arterioles [34]. In contrast, P2X7R activity is very low under physiological conditions [18]. Interstitial ATP activates P2X1R and elicits afferent arteriolar vasoconstriction in response to increases in RPP [35]. Moreover, renal autoregulation is impaired in P2X1R knockout mice [36]. In the juxtamedullary nephron preparation, superfusion with ATP at normal pressures resulted in afferent vasoconstriction, which was abolished completely by a P2X1R inhibitor; however, there was no significant effect of P2X7R inhibition [37]. The results confirmed that the activation of P2X1R constricts renal afferent arterioles at normotensive pressures, whereas P2X7R expression is very low in normal rats. In contrast, both are overexpressed in Ang II-dependent hypertension [18][38], which indicates that both P2X1 and P2X7 receptors contribute to the renal adaptation to chronic Ang II infusion [39].
Collectively, two major mechanisms, Ang II via AT1R and ATP through P2X1R and P2X7R, (Figure 1) play critical roles in Ang II-dependent hypertension maintaining the elevated renal afferent arterial resistance. As described previously, treatment with AT1 receptor blockers reduced renal vascular resistance and decreased blood pressure back to normal levels indicating that AT1R activity is dominant and that the contribution of interstitial ATP associated with the reductions in arterial pressure is minimal. If the actions of the two systems were additive, the kidney’s vasculature would be under excessive vasoconstriction, which does not occur. One possible explanation that solves the conundrum is that P2XR and AT1R share intracellular signaling mechanisms to regulate renal vasoconstriction in Ang II-dependent hypertension. Two questions need to be answered to understand the mechanisms responsible for this regulation: (1) How do these two systems that regulate renal afferent arterioles interact via their respective receptors to share intracellular signaling mechanisms? and (2) Which system is dominant in regulating the renal afferent arterioles in sustained Ang II-dependent hypertension? Understanding how these mechanisms interact to reset myogenic tone and elevate renal vascular resistance could facilitate the design of therapeutic interventions that prevent the progression of renal injury in hypertension.
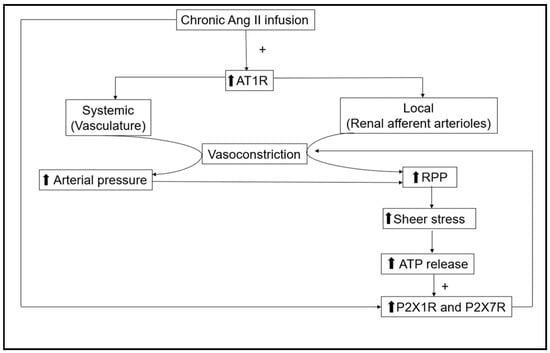
Figure 1. Actions of chronic angiotensin II (Ang II) infusion on systemic and kidney vasculature. Renal afferent arterioles are regulated via the direct effect of Ang II through AT1R and, secondarily, by P2X1 and P2X7 receptors due to a rise in ATP interstitial fluid concentrations caused by the increases in renal perfusion pressure (RPP). The increasing arrows represent the elevation of those factors.
References
- Harrison-Bernard, L.M.; Navar, L.G.; Ho, M.M.; Vinson, G.P.; el-Dahr, S.S. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am. J. Physiol. 1997, 273, F170–F177.
- Miyata, N.; Park, F.; Li, X.F.; Cowley, A.W., Jr. Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am. J. Physiol. 1999, 277, F437–F446.
- Wang, Z.Q.; Millatt, L.J.; Heiderstadt, N.T.; Siragy, H.M.; Johns, R.A.; Carey, R.M. Differential regulation of renal angiotensin subtype AT1A and AT2 receptor protein in rats with angiotensin-dependent hypertension. Hypertension 1999, 33, 96–101.
- Kasper, S.O.; Basso, N.; Kurnjek, M.L.; Paglia, N.; Ferrario, C.M.; Ferder, L.F.; Diz, D.I. Divergent regulation of circulating and intrarenal renin-angiotensin systems in response to long-term blockade. Am. J. Nephrol. 2005, 25, 335–341.
- Nagai, Y.; Yao, L.; Kobori, H.; Miyata, K.; Ozawa, Y.; Miyatake, A.; Yukimura, T.; Shokoji, T.; Kimura, S.; Kiyomoto, H.; et al. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J. Am. Soc. Nephrol. 2005, 16, 703–711.
- Navar, L.G.; Harrison-Bernard, L.M.; Imig, J.D.; Cervenka, L.; Mitchell, K.D. Renal responses to AT1 receptor blockade. Am. J. Hypertens. 2000, 13, 45s–54s.
- Eguchi, S.; Kawai, T.; Scalia, R.; Rizzo, V. Understanding Angiotensin II Type 1 Receptor Signaling in Vascular Pathophysiology. Hypertension 2018, 71, 804–810.
- Guan, Z.; Inscho, E.W. Role of adenosine 5′-triphosphate in regulating renal microvascular function and in hypertension. Hypertension 2011, 58, 333–340.
- Menzies, R.I.; Tam, F.W.; Unwin, R.J.; Bailey, M.A. Purinergic signaling in kidney disease. Kidney Int. 2017, 91, 315–323.
- Nishiyama, A.; Navar, L.G. ATP mediates tubuloglomerular feedback. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R273–R275; discussion R278–R279.
- Nishiyama, A.; Jackson, K.E.; Majid, D.S.; Rahman, M.; Navar, L.G. Renal interstitial fluid ATP responses to arterial pressure and tubuloglomerular feedback activation during calcium channel blockade. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H772–H777.
- Zhang, Y.; Sands, J.M.; Kohan, D.E.; Nelson, R.D.; Martin, C.F.; Carlson, N.G.; Kamerath, C.D.; Ge, Y.; Klein, J.D.; Kishore, B.K. Potential role of purinergic signaling in urinary concentration in inner medulla: Insights from P2Y2 receptor gene knockout mice. Am. J. Physiol. Ren. Physiol. 2008, 295, F1715–F1724.
- Franco, M.; Bautista, R.; Tapia, E.; Soto, V.; Santamaria, J.; Osorio, H.; Pacheco, U.; Sanchez-Lozada, L.G.; Kobori, H.; Navar, L.G. Contribution of renal purinergic receptors to renal vasoconstriction in angiotensin II-induced hypertensive rats. Am. J. Physiol. Ren. Physiol. 2011, 300, F1301–F1309.
- Inscho, E.W.; Carmines, P.K.; Navar, L.G. Juxtamedullary afferent arteriolar responses to P1 and P2 purinergic stimulation. Hypertension 1991, 17, 1033–1037.
- Inscho, E.W.; Cook, A.K.; Clarke, A.; Zhang, S.; Guan, Z. P2X1 receptor-mediated vasoconstriction of afferent arterioles in angiotensin II-infused hypertensive rats fed a high-salt diet. Hypertension 2011, 57, 780–787.
- Menzies, R.I.; Unwin, R.J.; Bailey, M.A. Renal P2 receptors and hypertension. Acta Physiol. 2015, 213, 232–241.
- Menzies, R.I.; Howarth, A.R.; Unwin, R.J.; Tam, F.W.; Mullins, J.J.; Bailey, M.A. Inhibition of the purinergic P2X7 receptor improves renal perfusion in angiotensin-II-infused rats. Kidney Int. 2015, 88, 1079–1087.
- Franco, M.; Bautista-Perez, R.; Cano-Martinez, A.; Pacheco, U.; Santamaria, J.; Del Valle Mondragon, L.; Perez-Mendez, O.; Navar, L.G. Physiopathological implications of P2X1 and P2X7 receptors in regulation of glomerular hemodynamics in angiotensin II-induced hypertension. Am. J. Physiol. Ren. Physiol. 2017, 313, F9–F19.
- Hayashi, K.; Epstein, M.; Saruta, T. Altered myogenic responsiveness of the renal microvasculature in experimental hypertension. J. Hypertens. 1996, 14, 1387–1401.
- Navar, L.G.; Maddox, M.D.; Munger, K.A. The Renal Circulations and Glomerular Filtration. In Brenner & Rector’s the Kidney; Yu, A.S.L., Chertow, G.M., Luyckx, V.A., Marsden, P.A., Skorecki, K., Taal, M.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2020.
- Navar, L.G.; Kobori, H.; Prieto-Carrasquero, M. Intrarenal angiotensin II and hypertension. Curr. Hypertens. Rep. 2003, 5, 135–143.
- Imig, J.D. Afferent arteriolar reactivity to angiotensin II is enhanced during the early phase of angiotensin II hypertension. Am. J. Hypertens. 2000, 13, 810–818.
- Crowley, S.D.; Gurley, S.B.; Herrera, M.J.; Ruiz, P.; Griffiths, R.; Kumar, A.P.; Kim, H.S.; Smithies, O.; Le, T.H.; Coffman, T.M. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci. USA 2006, 103, 17985–17990.
- Harrison-Bernard, L.M.; El-Dahr, S.S.; O’Leary, D.F.; Navar, L.G. Regulation of angiotensin II type 1 receptor mRNA and protein in angiotensin II-induced hypertension. Hypertension 1999, 33, 340–346.
- Nishiyama, A.; Seth, D.M.; Navar, L.G. Angiotensin II type 1 receptor-mediated augmentation of renal interstitial fluid angiotensin II in angiotensin II-induced hypertension. J. Hypertens. 2003, 21, 1897–1903.
- Zhuo, J.L.; Imig, J.D.; Hammond, T.G.; Orengo, S.; Benes, E.; Navar, L.G. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: Role of AT(1) receptor. Hypertension 2002, 39, 116–121.
- Li, X.C.; Navar, L.G.; Shao, Y.; Zhuo, J.L. Genetic deletion of AT1a receptors attenuates intracellular accumulation of ANG II in the kidney of AT1a receptor-deficient mice. Am. J. Physiol. Ren. Physiol. 2007, 293, F586–F593.
- Wang, C.T.; Navar, L.G.; Mitchell, K.D. Proximal tubular fluid angiotensin II levels in angiotensin II-induced hypertensive rats. J. Hypertens. 2003, 21, 353–360.
- Wang, C.T.; Zou, L.X.; Navar, L.G. Renal responses to AT1 blockade in angiotensin II-induced hypertensive rats. J. Am. Soc. Nephrol. 1997, 8, 535–542.
- Nishiyama, A.; Majid, D.S.; Taher, K.A.; Miyatake, A.; Navar, L.G. Relation between renal interstitial ATP concentrations and autoregulation-mediated changes in renal vascular resistance. Circ. Res. 2000, 86, 656–662.
- Palygin, O.; Evans, L.C.; Cowley, A.W., Jr.; Staruschenko, A. Acute In Vivo Analysis of ATP Release in Rat Kidneys in Response to Changes of Renal Perfusion Pressure. J. Am. Heart Assoc. 2017, 6, e006658.
- Yamamoto, K.; Imamura, H.; Ando, J. Shear stress augments mitochondrial ATP generation that triggers ATP release and Ca2+ signaling in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1477–H1485.
- Bell, P.D.; Lapointe, J.Y.; Sabirov, R.; Hayashi, S.; Peti-Peterdi, J.; Manabe, K.; Kovacs, G.; Okada, Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc. Natl. Acad. Sci. USA 2003, 100, 4322–4327.
- Burnstock, G.; Knight, G.E. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Cytol. 2004, 240, 31–304.
- Inscho, E.W.; Cook, A.K.; Imig, J.D.; Vial, C.; Evans, R.J. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J. Clin. Investig. 2003, 112, 1895–1905.
- Inscho, E.W.; Cook, A.K.; Imig, J.D.; Vial, C.; Evans, R.J. Renal autoregulation in P2X1 knockout mice. Acta Physiol. Scand. 2004, 181, 445–453.
- Kulthinee, S.; Shao, W.; Franco, M.; Navar, L.G. Purinergic P2X1 receptor, purinergic P2X7 receptor, and angiotensin II type 1 receptor interactions in the regulation of renal afferent arterioles in angiotensin II-dependent hypertension. Am. J. Physiol. Ren. Physiol. 2020, 318, F1400–F1408.
- Bautista-Perez, R.; Perez-Mendez, O.; Cano-Martinez, A.; Pacheco, U.; Santamaria, J.; Rodriguez-Iturbe, F.R.B.; Navar, L.G.; Franco, M. The Role of P2X7 Purinergic Receptors in the Renal Inflammation Associated with Angiotensin II-induced Hypertension. Int. J. Mol. Sci. 2020, 21, 4041.
- Graciano, M.L.; Nishiyama, A.; Jackson, K.; Seth, D.M.; Ortiz, R.M.; Prieto-Carrasquero, M.C.; Kobori, H.; Navar, L.G. Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am. J. Physiol. Ren. Physiol. 2008, 294, F161–F169.
More
Information
Subjects:
Urology & Nephrology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
590
Revisions:
2 times
(View History)
Update Date:
24 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




