
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laurentiu Mihai Palade | -- | 2635 | 2023-07-22 14:41:57 | | | |
| 2 | Dean Liu | Meta information modification | 2635 | 2023-07-24 05:02:40 | | |
Video Upload Options
The chemical group comprising polycyclic aromatic hydrocarbons (PAHs) has received prolonged evaluation and scrutiny in the past several decades. PAHs are ubiquitous carcinogenic pollutants and pose a significant threat to human health through their environmental prevalence and distribution. Regardless of their origin, natural or anthropogenic, PAHs generally stem from the incomplete combustion of organic materials. Dietary intake, one of the main routes of human exposure to PAHs, is modulated by pre-existing food contamination (air, water, soil) and their formation and accumulation during food processing. To this end, processing techniques and cooking options entailing thermal treatment carry additional weight in determining the PAH levels in the final product.
1. Introduction
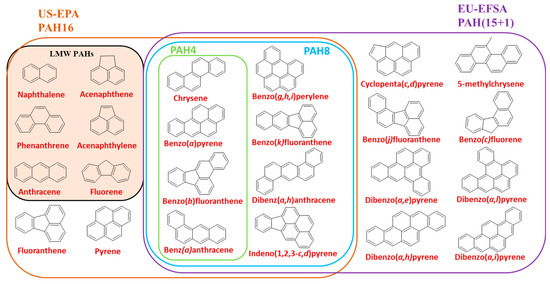
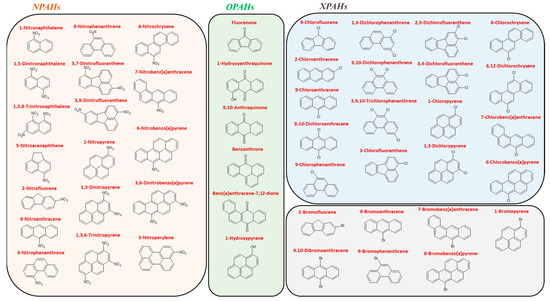
2. PAH Sample Pretreatment in Food and Quantitative Analysis
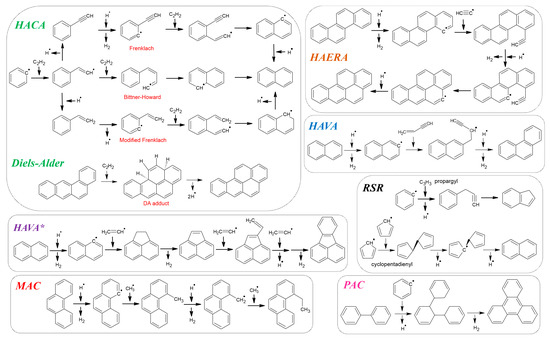
3. PAH Formation—Mechanistic Features
References
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781.
- Wu, S.; Gong, G.; Yan, K.; Sun, Y.; Zhang, L. Chapter Two—Polycyclic aromatic hydrocarbons in edible oils and fatty foods: Occurrence, formation, analysis, change and control. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 93, pp. 59–112. ISBN 1043-4526.
- Gachanja, A.N.; Maritim, P.K. Polycyclic Aromatic Hydrocarbons|Determination☆. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 328–340. ISBN 978-0-08-101984-9.
- Achten, C.; Andersson, J.T. Overview of Polycyclic Aromatic Compounds (PAC). Polycycl. Aromat. Compd. 2015, 35, 177–186.
- Li, G.; Wu, S.; Zeng, J.; Wang, L.; Yu, W. Effect of frying and aluminium on the levels and migration of parent and oxygenated PAHs in a popular Chinese fried bread youtiao. Food Chem. 2016, 209, 123–130.
- Sun, Y.; Wu, S.; Gong, G. Trends of research on polycyclic aromatic hydrocarbons in food: A 20-year perspective from 1997 to 2017. Trends Food Sci. Technol. 2019, 83, 86–98.
- Wang, Z.; Ng, K.; Warner, R.D.; Stockmann, R.; Fang, Z. Reduction strategies for polycyclic aromatic hydrocarbons in processed foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1598–1626.
- Andersson, J.T.; Achten, C. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycycl. Aromat. Compd. 2015, 35, 330–354.
- Nowakowski, M.; Rykowska, I.; Wolski, R.; Andrzejewski, P. Polycyclic Aromatic Hydrocarbons (PAHs) and their Derivatives (O-PAHs, N-PAHs, OH-PAHs): Determination in Suspended Particulate Matter (SPM)—A Review. Environ. Process. 2021, 9, 2.
- Xie, J.; Tao, L.; Wu, Q.; Lei, S.; Lin, T. Environmental profile, distributions and potential sources of halogenated polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2021, 419, 126164.
- Suzuki, S.; Kiuchi, S.; Kinoshita, K.; Takeda, Y.; Tanaka, K.; Oguma, M. Formation of Polycyclic Aromatic Hydrocarbons (PAHs) and Oxygenated PAHs in the Oxidation of Ethylene Using a Flow Reactor. Combust. Sci. Technol. 2022, 194, 464–490.
- Li, W.; Wu, S. Challenges of halogenated polycyclic aromatic hydrocarbons in foods: Occurrence, risk, and formation. Trends Food Sci. Technol. 2023, 131, 1–13.
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010.
- EFSA. Findings of the EFSA Data Collection on Polycyclic Aromatic Hydrocarbons in Food; EFSA: Parma, Italy, 2008; Volume 5.
- Onopiuk, A.; Kołodziejczak, K.; Szpicer, A.; Wojtasik-Kalinowska, I.; Wierzbicka, A.; Półtorak, A. Analysis of factors that influence the PAH profile and amount in meat products subjected to thermal processing. Trends Food Sci. Technol. 2021, 115, 366–379.
- Anyanwu, I.N.; Semple, K.T. Fate and behaviour of nitrogen-containing polycyclic aromatic hydrocarbons in soil. Environ. Technol. Innov. 2015, 3, 108–120.
- Hrdina, A.I.H.; Kohale, I.N.; Kaushal, S.; Kelly, J.; Selin, N.E.; Engelward, B.P.; Kroll, J.H. The Parallel Transformations of Polycyclic Aromatic Hydrocarbons in the Body and in the Atmosphere. Environ. Health Perspect. 2022, 130, 25004.
- Lammel, G.; Kitanovski, Z.; Kukučka, P.; Novák, J.; Arangio, A.M.; Codling, G.P.; Filippi, A.; Hovorka, J.; Kuta, J.; Leoni, C.; et al. Oxygenated and Nitrated Polycyclic Aromatic Hydrocarbons in Ambient Air—Levels, Phase Partitioning, Mass Size Distributions, and Inhalation Bioaccessibility. Environ. Sci. Technol. 2020, 54, 2615–2625.
- Qiao, M.; Qi, W.; Liu, H.; Qu, J. Oxygenated polycyclic aromatic hydrocarbons in the surface water environment: Occurrence, ecotoxicity, and sources. Environ. Int. 2022, 163, 107232.
- Haynes, J.P.; Miller, K.E.; Majestic, B.J. Investigation into Photoinduced Auto-Oxidation of Polycyclic Aromatic Hydrocarbons Resulting in Brown Carbon Production. Environ. Sci. Technol. 2019, 53, 682–691.
- Masuda, M.; Wang, Q.; Tokumura, M.; Miyake, Y.; Amagai, T. Simultaneous determination of polycyclic aromatic hydrocarbons and their chlorinated derivatives in grilled foods. Ecotoxicol. Environ. Saf. 2019, 178, 188–194.
- Duedahl-Olesen, L.; Ionas, A.C. Formation and mitigation of PAHs in barbecued meat—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3553–3568.
- Zelinkova, Z.; Wenzl, T. The Occurrence of 16 EPA PAHs in Food—A Review. Polycycl. Aromat. Compd. 2015, 35, 248–284.
- Lee, J.; Jeong, J.H.; Park, S.; Lee, K.G. Monitoring and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in processed foods and their raw materials. Food Control 2018, 92, 286–292.
- Zhang, Y.; Chen, X.; Zhang, Y. Analytical chemistry, formation, mitigation, and risk assessment of polycyclic aromatic hydrocarbons: From food processing to in vivo metabolic transformation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1422–1456.
- Duedahl-Olesen, L.; Iversen, N.M.; Kelmo, C.; Jensen, L.K. Validation of QuEChERS for screening of 4 marker polycyclic aromatic hydrocarbons in fish and malt. Food Control 2020, 108, 106434.
- Ledesma, E.; Rendueles, M.; Díaz, M. Contamination of meat products during smoking by polycyclic aromatic hydrocarbons: Processes and prevention. Food Control 2016, 60, 64–87.
- Hokkanen, M. Polycyclic Aromatic Hydrocarbons in Foods and Their Mitigation, Food Mutagenicity and Children’s Dietary Exposure in Finland. Ph.D. Disertation, University of Helsinki, Finnish Food Authority (Ruokavirasto) Laboratory and Research Division Chemistry Unit, Helsinki, Finland, 2021.
- Lau, E.V.; Gan, S.; Ng, H.K. Extraction Techniques for Polycyclic Aromatic Hydrocarbons in Soils. Int. J. Anal. Chem. 2010, 2010, 398381.
- Jinadasa, B.K.K.K.; Monteau, F.; Morais, S. Critical review of micro-extraction techniques used in the determination of polycyclic aromatic hydrocarbons in biological, environmental and food samples. Food Addit. Contam. Part A 2020, 37, 1004–1026.
- Andreu, V.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. TrAC Trends Anal. Chem. 2019, 118, 709–721.
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431.
- Kim, L.; Lee, D.; Cho, H.-K.; Choi, S.-D. Review of the QuEChERS method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals. Trends Environ. Anal. Chem. 2019, 22, e00063.
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28.
- Anastassiades, M.; Maštovská, K.; Lehotay, S.J. Evaluation of analyte protectants to improve gas chromatographic analysis of pesticides. J. Chromatogr. A 2003, 1015, 163–184.
- BS EN 15662:2018; Foods of Plant Origin. Multimethod for the Determination of Pesticide Residues Using GC- and LC-Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE. Modular QuEChERS-method. British Standards Institution (BSI): London, UK, 2018.
- Lehotay, S.J. Determination of Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate: Collaborative Study. J. AOAC Int. 2007, 90, 485–520.
- Santana-Mayor, Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; Rodríguez-Delgado, M.Á. Current trends in QuEChERS method. A versatile procedure for food, environmental and biological analysis. TrAC Trends Anal. Chem. 2019, 116, 214–235.
- Zachara, A.; Gałkowska, D.; Juszczak, L. Contamination of smoked meat and fish products from Polish market with polycyclic aromatic hydrocarbons. Food Control 2017, 80, 45–51.
- ISO 15302:2017; Animal and Vegetable Fats and Oils—Determination of Benzopyrene—Reverse-Phase High Performance Liquid Chromatography Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- ISO 15753:2016; Animal and Vegetable Fats and Oils—Determination of Polycyclic Aromatic Hydrocarbons. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- ISO 22959:2009; Animal and Vegetable Fats and Oils—Determination of Polycyclic Aromatic Hydrocarbons by On-Line Donor-Acceptor Complex Chromatography and HPLC with Fluorescence Detection. International Organization for Standardization (ISO): Geneva, Switzerland, 2009.
- CEN/TS16621:2014; Food Analysis—Determination of Benzopyrene, benzAnthracene, Chrysene and benzoFluoranthene in Foodstuffs by High Performance Liquid Chromatography with Fluorescence Detection (HPLC-FD). British Standards Institution (BSI): London, UK, 2014.
- EN 16619:2015; Food Analysis—Determination of benzopyrene, benzanthracene, chrysene and benzofluoranthene in foodstuffs by gas chromatography mass spectrometry (GC-MS). European Standards: Brussels, Belgium, 2015.
- PD ISO/TR 24054:2019; Animal and Vegetable Fats and Oils—Determination of Polycyclic Aromatic Hydrocarbons (PAH)—Method Using Gas Chromatography/Mass Spectrometry (GC/MS). British Standards Institution (BSI): London, UK, 2019.
- Slámová, T.; Sadowska-Rociek, A.; Fraňková, A.; Surma, M.; Banout, J. Application of QuEChERS-EMR-Lipid-DLLME method for the determination of polycyclic aromatic hydrocarbons in smoked food of animal origin. J. Food Compos. Anal. 2020, 87, 103420.
- Reizer, E.; Viskolcz, B.; Fiser, B. Formation and growth mechanisms of polycyclic aromatic hydrocarbons: A mini-review. Chemosphere 2022, 291, 132793.
- Kislov, V.V.; Sadovnikov, A.I.; Mebel, A.M. Formation Mechanism of Polycyclic Aromatic Hydrocarbons beyond the Second Aromatic Ring. J. Phys. Chem. A 2013, 117, 4794–4816.
- Lemmens, A.K.; Rap, D.B.; Thunnissen, J.M.M.; Willemsen, B.; Rijs, A.M. Polycyclic aromatic hydrocarbon formation chemistry in a plasma jet revealed by IR-UV action spectroscopy. Nat. Commun. 2020, 11, 269.
- Frenklach, M.; Clary, D.W.; Gardiner, W.C.; Stein, S.E. Detailed kinetic modeling of soot formation in shock-tube pyrolysis of acetylene. Symp. Combust. 1985, 20, 887–901.
- Frenklach, M.; Wang, H. Detailed modeling of soot particle nucleation and growth. Symp. Combust. 1991, 23, 1559–1566.
- Frenklach, M. Reaction mechanism of soot formation in flames. Phys. Chem. Chem. Phys. 2002, 4, 2028–2037.
- Raj, A.; Prada, I.D.C.; Amer, A.A.; Chung, S.H. A reaction mechanism for gasoline surrogate fuels for large polycyclic aromatic hydrocarbons. Combust. Flame 2012, 159, 500–515.
- Bittner, J.D.; Howard, J.B. Composition profiles and reaction mechanisms in a near-sooting premixed benzene/oxygen/argon flame. Symp. Combust. 1981, 18, 1105–1116.
- Mebel, A.M.; Georgievskii, Y.; Jasper, A.W.; Klippenstein, S.J. Temperature- and pressure-dependent rate coefficients for the HACA pathways from benzene to naphthalene. Proc. Combust. Inst. 2017, 36, 919–926.
- Froese, R.D.J.; Coxon, J.M.; West, S.C.; Morokuma, K. Theoretical Studies of Diels−Alder Reactions of Acetylenic Compounds. J. Org. Chem. 1997, 62, 6991–6996.
- Siegmann, K.; Sattler, K. Formation mechanism for polycyclic aromatic hydrocarbons in methane flames. J. Chem. Phys. 2000, 112, 698–709.
- Kislov, V.V.; Islamova, N.I.; Kolker, A.M.; Lin, S.H.; Mebel, A.M. Hydrogen Abstraction Acetylene Addition and Diels−Alder Mechanisms of PAH Formation: A Detailed Study Using First Principles Calculations. J. Chem. Theory Comput. 2005, 1, 908–924.
- Mebel, A.M.; Landera, A.; Kaiser, R.I. Formation Mechanisms of Naphthalene and Indene: From the Interstellar Medium to Combustion Flames. J. Phys. Chem. A 2017, 121, 901–926.
- Yang, J.; Zhao, L.; Yuan, W.; Qi, F.; Li, Y. Experimental and kinetic modeling investigation on laminar premixed benzene flames with various equivalence ratios. Proc. Combust. Inst. 2015, 35, 855–862.
- Yang, X.J.; Glaser, R.; Li, A.; Zhong, J.X. The carriers of the unidentified infrared emission features: Clues from polycyclic aromatic hydrocarbons with aliphatic sidegroups. New Astron. Rev. 2017, 77, 1–22.
- Shi, X.; Wang, Q.; Violi, A. Reaction pathways for the formation of five-membered rings onto polyaromatic hydrocarbon framework. Fuel 2021, 283, 119023.
- Shukla, B.; Miyoshi, A.; Koshi, M. Role of Methyl Radicals in the Growth of PAHs. J. Am. Soc. Mass Spectrom. 2010, 21, 534–544.
- Georganta, E.; Rahman, R.K.; Raj, A.; Sinha, S. Growth of polycyclic aromatic hydrocarbons (PAHs) by methyl radicals: Pyrene formation from phenanthrene. Combust. Flame 2017, 185, 129–141.
- Jones, B.M.; Zhang, F.; Kaiser, R.I.; Jamal, A.; Mebel, A.M.; Cordiner, M.A.; Charnley, S.B. Formation of benzene in the interstellar medium. Proc. Natl. Acad. Sci. USA 2011, 108, 452–457.
- Mebel, A.M.; Kislov, V.V.; Kaiser, R.I. Photoinduced Mechanism of Formation and Growth of Polycyclic Aromatic Hydrocarbons in Low-Temperature Environments via Successive Ethynyl Radical Additions. J. Am. Chem. Soc. 2008, 130, 13618–13629.
- Reizer, E.; Csizmadia, I.G.; Nehéz, K.; Viskolcz, B.; Fiser, B. Theoretical investigation of benzo(a)pyrene formation. Chem. Phys. Lett. 2021, 772, 138564.
- Shukla, B.; Koshi, M. A novel route for PAH growth in HACA based mechanisms. Combust. Flame 2012, 159, 3589–3596.
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123.
- Shukla, B.; Susa, A.; Miyoshi, A.; Koshi, M. Role of Phenyl Radicals in the Growth of Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. A 2008, 112, 2362–2369.
- Raj, A.; Man, P.L.W.; Totton, T.S.; Sander, M.; Shirley, R.A.; Kraft, M. New polycyclic aromatic hydrocarbon (PAH) surface processes to improve the model prediction of the composition of combustion-generated PAHs and soot. Carbon N. Y. 2010, 48, 319–332.
- Shukla, B.; Koshi, M. Comparative study on the growth mechanisms of PAHs. Combust. Flame 2011, 158, 369–375.
- Zhao, L.; Prendergast, M.B.; Kaiser, R.I.; Xu, B.; Ablikim, U.; Ahmed, M.; Sun, B.-J.; Chen, Y.-L.; Chang, A.H.H.; Mohamed, R.K.; et al. Synthesis of Polycyclic Aromatic Hydrocarbons by Phenyl Addition–Dehydrocyclization: The Third Way. Angew. Chem. Int. Ed. 2019, 58, 17442–17450.
- Raj, A.; Al Rashidi, M.J.; Chung, S.H.; Sarathy, S.M. PAH Growth Initiated by Propargyl Addition: Mechanism Development and Computational Kinetics. J. Phys. Chem. A 2014, 118, 2865–2885.
- Matsugi, A.; Miyoshi, A. Modeling of two- and three-ring aromatics formation in the pyrolysis of toluene. Proc. Combust. Inst. 2013, 34, 269–277.
- Zhao, L.; Lu, W.; Ahmed, M.; Zagidullin, M.V.; Azyazov, V.N.; Morozov, A.N.; Mebel, A.M.; Kaiser, R.I. Gas-phase synthesis of benzene via the propargyl radical self-reaction. Sci. Adv. 2023, 7, eabf0360.
- Zhu, L.; Shi, X.; Sun, Y.; Zhang, Q.; Wang, W. The growth mechanism of polycyclic aromatic hydrocarbons from the reactions of anthracene and phenanthrene with cyclopentadienyl and indenyl. Chemosphere 2017, 189, 265–276.
- Long, A.E.; Merchant, S.S.; Vandeputte, A.G.; Carstensen, H.-H.; Vervust, A.J.; Marin, G.B.; Van Geem, K.M.; Green, W.H. Pressure dependent kinetic analysis of pathways to naphthalene from cyclopentadienyl recombination. Combust. Flame 2018, 187, 247–256.
- Robinson, R.K.; Lindstedt, R.P. On the chemical kinetics of cyclopentadiene oxidation. Combust. Flame 2011, 158, 666–686.
- Sharma, S.; Green, W.H. Computed Rate Coefficients and Product Yields for c-C5H5 + CH3 → Products. J. Phys. Chem. A 2009, 113, 8871–8882.
- Ghildina, A.R.; Porfiriev, D.P.; Azyazov, V.N.; Mebel, A.M. The mechanism and rate constants for oxidation of indenyl radical C9H7 with molecular oxygen O2: A theoretical study. Phys. Chem. Chem. Phys. 2019, 21, 8915–8924.
- Ghildina, A.R.; Porfiriev, D.P.; Azyazov, V.N.; Mebel, A.M. Scission of the Five-Membered Ring in 1-H-Inden-1-one C9H6O and Indenyl C9H7 in the Reactions with H and O Atoms. J. Phys. Chem. A 2019, 123, 5741–5752.
- Sinha, S.; Rahman, R.K.; Raj, A. On the role of resonantly stabilized radicals in polycyclic aromatic hydrocarbon (PAH) formation: Pyrene and fluoranthene formation from benzyl–indenyl addition. Phys. Chem. Chem. Phys. 2017, 19, 19262–19278.




