Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Martin Rössle | -- | 2248 | 2023-07-21 21:03:48 | | | |
| 2 | Lindsay Dong | -327 word(s) | 1921 | 2023-07-24 04:41:44 | | | | |
| 3 | Lindsay Dong | + 4 word(s) | 1925 | 2023-07-24 04:42:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rössle, M. Interventional Treatment of Budd–Chiari Syndrome. Encyclopedia. Available online: https://encyclopedia.pub/entry/47130 (accessed on 07 February 2026).
Rössle M. Interventional Treatment of Budd–Chiari Syndrome. Encyclopedia. Available at: https://encyclopedia.pub/entry/47130. Accessed February 07, 2026.
Rössle, Martin. "Interventional Treatment of Budd–Chiari Syndrome" Encyclopedia, https://encyclopedia.pub/entry/47130 (accessed February 07, 2026).
Rössle, M. (2023, July 21). Interventional Treatment of Budd–Chiari Syndrome. In Encyclopedia. https://encyclopedia.pub/entry/47130
Rössle, Martin. "Interventional Treatment of Budd–Chiari Syndrome." Encyclopedia. Web. 21 July, 2023.
Copy Citation
Medical treatment is regarded as the primary course of action in patients with Budd–Chiari syndrome (BCS). Its efficacy, however, is limited, and most patients require interventional treatment during follow-up. Short-segment stenosis or the occlusion (the so-called web) of hepatic veins or the inferior vena cava are frequent in Asian countries. An angioplasty with or without stent implantation is the treatment of choice to restore hepatic and splanchnic blood flow. The long-segment thrombotic occlusion of hepatic veins, common in Western countries, is more severe and may require a portocaval shunting procedure to relieve hepatic and splanchnic congestion.
Budd–Chiari syndrome
TIPS
transjugular intrahepatic portosystemic shunt
angioplasty
interventional treatment
survival
1. Introduction
Budd–Chiari syndrome (BCS) occurs in many forms, ranging from asymptomatic or mild disease to severe liver failure. The variability in the presentation of the disease depends on the extent of thrombosis, the velocity of its formation, and the presence and capacity of collaterals [1][2][3]. In addition to the hepatic veins, the portal vein as well as the inferior vena cava (IVC) may develop thromboses, which aggravate the disease and influence outcomes. With increasing collateralization, the congestion of the liver and intestine decreases, and the disease may change to follow a chronical course. The congestive state may be abbreviated and the damage reduced by interventional treatment, such as angioplasty in case of a short-segment (web-like) BCS, or portosystemic side-to-side shunts, which allow the transition of the portal vein into an outflow, thereby facilitating hepatic arterial perfusion.
Based on a study published in 2006 [4] a step-up therapeutic algorithm was proposed starting with medication, followed by angioplasty in patients with web-like BCS amenable to angioplasty including stenting, transjugular intrahepatic portosystemic shunt (TIPS) intervention, and, last of all, liver transplantation. Treatment escalation depends on non-response, which is defined by clinical and biochemical factors [4][5]. Medical treatment is essential for preventing further thrombus growth and formation. It is, however, ineffective with respect to the recanalization of hepatic veins, and 80–90% of patients do not show a clear and durable clinical response [6].
2. Interventions
2.1. Angioplasty
2.1.1. Rationale
The recanalization of a short-segment occluded hepatic vein or the IVC may result in the restoration of the splanchnic and hepatic blood flow and lead to a clinical improvement. In the absence of cirrhosis, the procedure may be potentially curative [7].
2.1.2. Technique
A percutaneous transluminal balloon angioplasty with or without stent implantation can be performed by a transjugular, transfemoral, or percutaneous transhepatic access. The technical success rates are over 90% for both hepatic veins and IVC webs [8][9][10][11][12][13][14][15][16]. After an angioplasty is performed and the stenosis or occlusion is resolved, the hepatic venous pressure gradient (wedged minus free hepatic vein pressure) should be determined and a transjugular biopsy should be performed at the same time. Both of these measures are important to better predict progression and exclude the earlier extension of the BCS into small hepatic veins and the presence of advanced fibrosis.
2.2. TIPS
2.2.1. Rationale
The rapid occlusion of the hepatic veins causes congestive liver damage due to reduced portal and arterial blood supply. In patients with a complete occlusion of all hepatic veins, hepatic blood flow depends on the development of intra- or extrahepatic collaterals which may discontinue disease progression and gradually change acute disease into chronical disease. If collateral formation is too slow or insufficient and does not increase blood flow adequately, the course of the disease may be progressive or deleterious, requiring immediate interventional or surgical treatment.
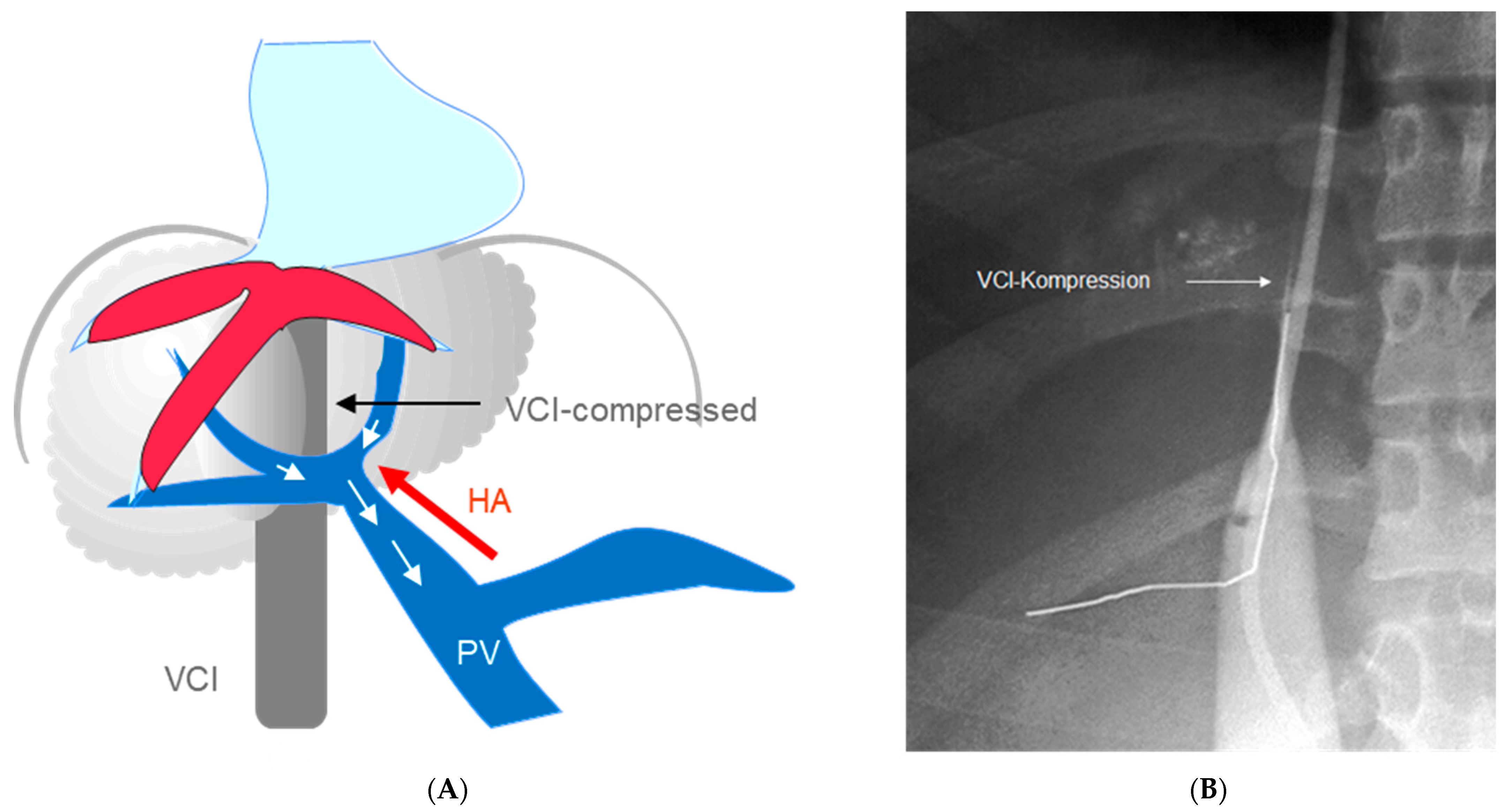
With respect to the enlargement of the caudate lobe, surgical porto-caval or meso-caval shunts alone were often ineffective and required an additional cavo-atrial anastomosis [17][18][19]. The TIPS, however, bypasses the stenosed IVC segment and operates with or without the enlargement of the caudate lobe (Figure 1).

Figure 1. (A) Complete BCS. The portal vein is used as outflow tract. Enlarged caudate lobe leads to stenosis of the inferior caval vein; (B) Angiography of the inferior caval vein showing complete occlusion by the enlarged liver (VCI: inferior caval vein).
2.2.2. Technique of TIPS Implantation
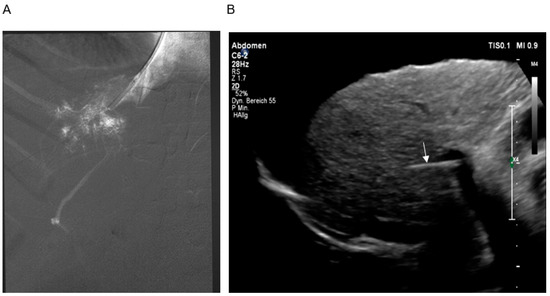
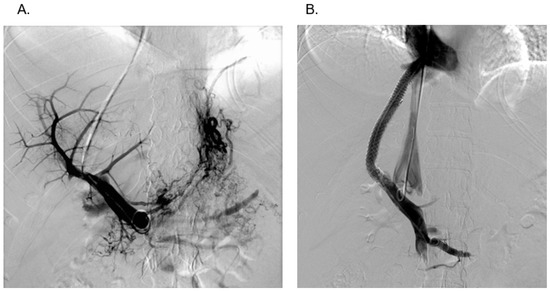
The intervention begins with a transjugular cavography to show or exclude IVC involvement (Figure 1B). By moving the needle catheter assembly along the lateral wall of the IVC, a hepatic vein stump may be discovered and can be used to enter the hepatic parenchyma. About half of patients do not show remnants of hepatic veins, and, therefore, a puncture through the wall of the IVC is performed [27]. After having advanced the needle through the right lateral wall of the IVC, a small amount of diluted contrast confirms the intrahepatic position of the needle tip (Figure 2A). The next step is the puncture of the intrahepatic right portal branch which should be guided by transcostal sonography (Figure 2B). Sonographic guidance is important to reach a high level of technical success which may otherwise be limited by very small intrahepatic portal branches, which are often dislocated by the enlarged caudate lobe. A guidewire and catheter are now used to carry out angiographies and pressure measurements. The patency of the portal vein and the presence of varices may be demonstrated (Figure 3A). After the dilatation of the needle track, a covered stent is introduced and dilatated with a 10 mm balloon. It is strongly recommended to create a shunt with a diameter of at least 10 mm to achieve sufficient shunt flow and to relieve hepatic and intestinal congestion. Finally, angiography and pressure measurements are performed to control shunt function (Figure 3B).
Figure 2. (A) Hand-injection of contrast into the parenchyma of the liver showing retrograde filling of a portal branch. (B) Sonographic guidance of the puncture. The location of the needle (arrow) and the portal vein is demonstrated.
Figure 3. (A) Portography (DSA) after successful puncture of a very narrow right intrahepatic branch of the portal vein. (B) Simultaneous portography and cavography demonstrating good shunt function and perfect modelling of the two stents at both ends. This patient had only mild stenosis of the inferior caval vein by the enlarged caudate lobe.
2.2.3. Pre- and Post-Interventional Management
Anticoagulation is mandatory as soon as a diagnosis of BCS is made. Heparin should be avoided since about 30% of patients have heparin antibodies at the onset of the disease [6][20]. This is why low-molecular-weight heparin is preferred. A phlebotomy and acetylic salicylic acid (100 mg/day) should be applied in patients with polyglobulia or thrombocytosis, respectively. Unspecific medication consisting of albumin, diuretics, dopamine, and antibiotics should be given as required.
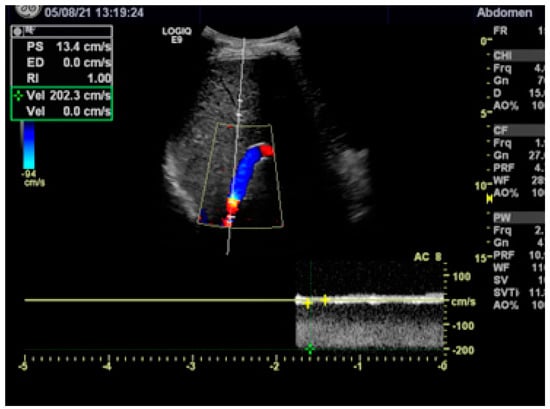
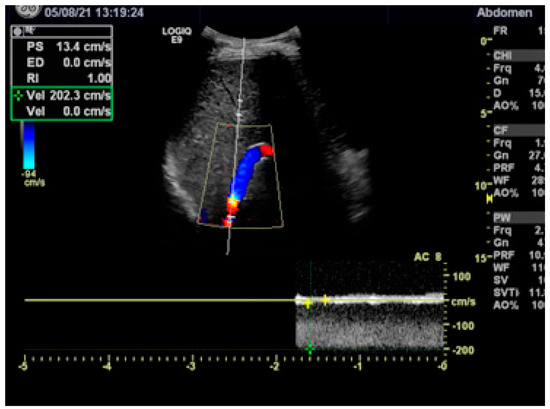
After a patient is discharged, anticoagulation has to be continued and a specific treatment of the hematological disease or coagulation disorder should be initiated. Duplex sonography before and every 3 to 6 months after a patient is discharged is recommended to assure the patency of the shunt and the portal vein. A reduced flow velocity of <60 cm/s or an increased flow velocity of >180 cm/s anywhere in the stent indicates shunt malfunction (Figure 4) [20][21]. In cases with a simple stenosis, the approximated Bernoulli equation (Δp = 4 v2) allows us to calculate the pressure gradient Δp in mmHg from the maximum flow velocity (in m/s) across the stenosis [21]. In the case presented in Figure 4, the maximum flow velocity of 2 m/s corresponds to an estimated pressure gradient of 16 mmHg, confirming the need for shunt revision. Shunt revision is also indicated when the flow velocity in the extrahepatic portal vein decreases to <30 cm/s. In addition to the duplex sonographic findings, any persistence of complications of portal hypertension (ascites, varices) should give rise to radiological shunt revision.

Figure 4. Duplex sonography showing stenosis in the proximal stent with a turbulent flow and a maximum flow velocity of 200 cm/s (2 m/s). According to the Bernouilli equation, the maximum flow velocity corresponds to a pressure gradient across the stenosis of 16 mmHg.
2.3. Pre- and Post-Interventional Management
Anticoagulation is mandatory as soon as a diagnosis of BCS is made. Heparin should be avoided since about 30% of patients have heparin antibodies at the onset of the disease [6,29]. This is why low-molecular-weight heparin is preferred. A phlebotomy and acetylic salicylic acid (100 mg/day) should be applied in patients with polyglobulia or thrombocytosis, respectively. Unspecific medication consisting of albumin, diuretics, dopamine, and antibiotics should be given as required.
After a patient is discharged, anticoagulation has to be continued and a specific treatment of the hematological disease or coagulation disorder should be initiated. Duplex sonography before and every 3 to 6 months after a patient is discharged is recommended to assure the patency of the shunt and the portal vein. A reduced flow velocity of <60 cm/s or an increased flow velocity of >180 cm/s anywhere in the stent indicates shunt malfunction (Figure 4) [29,39]. In cases with a simple stenosis, the approximated Bernoulli equation (Δp = 4 v2) allows us to calculate the pressure gradient Δp in mmHg from the maximum flow velocity (in m/s) across the stenosis [39]. In the case presented in Figure 4, the maximum flow velocity of 2 m/s corresponds to an estimated pressure gradient of 16 mmHg, confirming the need for shunt revision. Shunt revision is also indicated when the flow velocity in the extrahepatic portal vein decreases to <30 cm/s. In addition to the duplex sonographic findings, any persistence of complications of portal hypertension (ascites, varices) should give rise to radiological shunt revision.
Figure 4. Duplex sonography showing stenosis in the proximal stent with a turbulent flow and a maximum flow velocity of 200 cm/s (2 m/s). According to the Bernouilli equation, the maximum flow velocity corresponds to a pressure gradient across the stenosis of 16 mmHg.
Shunt failure is seen in about 25% of patients when covered stents are used [20][22][23][24]. The problem can be solved by stent-in-stent implantation or by parallel stenting, achieving secondary long-term patency rates of 95% [20][25].
3. Outcomes
3.1. Hemodynamics
In a single-center study including 59 patients with acute (4 weeks from diagnosis, 15 patients), subacute (6 months from diagnosis, 26 patients), and chronic BCS (18 patients), the portal pressures were highest in the acute group of patients (40.4 ± 10.1 mmHg) and somewhat lower in the subacute (33.3 ± 7.5 mmHg) and chronic disease (32.2 ± 8.9 mmHg) groups [25]. Acute/fulminant disease may result in extremely high portal pressures of up to almost 60 mmHg. The TIPS reduced the pressure gradient to 10.8 ± 4.9 mmHg. The shunt resulted in an increase in the blood flow velocity in the portal vein from 12.7 ± 10.0 cm/s to 48.6 ± 16.9 cm/s [20][25]. This was accompanied by an increase in the retrograde blood flow in the intrahepatic portal branches from +2 ± 11 cm/s to −11 ± 13 cm/s, giving clear evidence that the hepatic congestion was reduced or eliminated by the TIPS [20].
3.2. Liver Function and Hepatic Encephalopathy (HE)
With respect to biochemical variables, the TIPS improved hepatic and renal test results, almost reaching normal values within 2 weeks. This was, in particular, true for patients with acute or fulminant disease [20][25]. As shown in our study and in other studies [23][26][27], the Child–Pugh score, the Clichy prognostic BCS index [28], and the Rotterdam prognostic BCS index [29] improved significantly after TIPS implantation. The effect was greatest in patients with acute disease.
3.3. Survival
Survival may depend on the type of the BCS. In patients with short-segment BCS without a cirrhosis angioplasty with or without stenting may have a physiological restitution of the hepatic blood flow, resulting in excellent survival [8][9][10][11][12][13][14][15][16]. However, more than half of the patients required TIPS implantation during follow-up [27][30].
Several studies have suggested that TIPS may improve survival [20][26][31][32][33][34][35][36][37], but comparable or randomized studies are lacking. A review on TIPS for BCS including 160 studies from 29 countries showed one-year and five-year survival rates of 80–100% and 74–78%, respectively [38]. The largest multicentre retrospective European study including 124 TIPS patients with long-term follow-ups [26] showed one-, five-, and ten-year OLT-free survival rates of 88%, 78%, and 70%, respectively. Survival cannot be predicted by the Rotterdam score [29] but it can be predicted by a new prognostic index (BCS PI TIPS), which includes age, bilirubin, and INR [26].
References
- Mitchell, M.C.; Boitnott, J.K. Budd-Chiari syndrome: Etiology, diagnosis, and management. Medicine 1982, 61, 199–218.
- Janssen, H.L.; Garcia-Pagan, J.C. Budd-Chiari syndrome: A review by an expert panel. J. Hepatol. 2003, 38, 364–371.
- Valla, D. Primary Budd-Chiari syndrome. J. Hepatol. 2009, 50, 195–203.
- Plessier, A.; Sibert, A. Aiming at minimal invasiveness as a therapeutic strategy for Budd-Chiari syndrome. Hepatology 2006, 44, 1308–1316.
- Mancuso, A. Budd-Chiari management: Lights and shadows. World J. Hepatol. 2011, 3, 262–264.
- Valla, D. Budd-Chiari syndrome and veno-occlusive disease/sindusoidal obstruction syndrome. Gut 2008, 57, 1469–1478.
- Mukund, A.; Gamanagatti, S. Imaging and interventions in Budd-Chiari syndrome. World J. Radiol. 2011, 28, 69–177.
- Xu, K.; He, F.-X. Budd-Chiari syndrome caused by obstruction of the hepatic inferior vena cava: Immediate and 2-year treatment results of transluminal angioplasty and metallic stent placement. Cardiovasc. Interv. Radiol. 1996, 19, 32–36.
- Yang, X.-L.; Cheng, T.O. Successful treatment of percztaneous balloon angioplasty of Budd-Chiari syndrome caused by membranous obstruction of inferior vena cava: 8-year follow-up study. J. Am. Coll. Cardiol. 1996, 28, 1720–1724.
- Fisher, N.C.; McCafferty, I. Managing Budd-Chiari syndrome: A retrospective review of percutaneous hepatic vein angioplasty and surgical shunting. Gut 1999, 44, 568–574.
- Wu, T.; Wang, L. Percutaneous balloon angioplasty of inferior vena cava in Budd-Chiari syndrome-R1. Int. J. Cardiol. 2002, 83, 175–178.
- Zhang, C.Q.; Fu, L.N. Long-term effect of stent placement in 115 patients with Budd-Chiari syndrome. World J. Gastroenterol. 2003, 9, 2587–2591.
- Li, T.; Zhai, S. Feasibility and midterm outcomes of percutaneous transhepatic balloon angioplasty for symptomatic Budd-Chiari syndrome secondary to hepatic venous obstruction. J. Vasc. Surg. 2009, 50, 1079–1084.
- Han, G.; Qi, X.; Zhang, W.; He, C.; Yin, Z.; Wang, J.; Xia, J.; Xu, K.; Guo, W.; Niu, J.; et al. Percutaneous recanalization for Budd-Chiari syndrome: An 11-year retrospective study on patency and survival in 177 Chinese patients from a single centre. Radiology 2013, 266, 657–667.
- Ding, P.-X.; Zhang, S.-J. Long-term safety and outcome of percutaneous transhepatic venous balloon angioplasty for Budd-Chiari syndrome. J. Gastroenterol. Hepatol. 2015, 31, 222–228.
- Tripathi, D.; Sunderraj, L.; Vemala, V.; Mehrzad, H.; Zia, Z.; Mangat, K.; West, R.; Chen, F.; Elias, E.; Olliff, S.P. Long-term outcomes following percutaneous hepatic vein recanalization for Budd-Chiari syndrome. Liver Int. 2017, 37, 111–120.
- Henderson, J.M.; Warren, W.D. Surgical options, hematologic evaluation, and pathologic changes in Budd-Chiari syndrome. Am. J. Surg. 1990, 159, 41–50.
- Orloff, M.J. Budd-Chiari syndrome revisited: 38 years’ experience with surgical portal decompression. J. Gastrointest. Surg. 2012, 16, 286–300.
- Bismuth, H.; Sherlock, D.J. Portasystemic shunting versus liver transplantation for the Budd-Chiari syndrome. Ann. Surg. 1991, 214, 581–589.
- Rössle, M.; Olschewski, M. The Budd-Chiari syndrome: Outcome after treatment with the transjugular intrahepatic portosystemic shunt. Surgery 2004, 135, 394–403.
- Rössle, M. TIPS: 25 years later. J. Hepatol. 2013, 59, 1081–1093.
- Hernandez-Guerra, M.; Turnes, J. PTFE-covered stents improve TIPS patency in Budd-Chiari syndrome. Hepatology 2004, 40, 1197–1202.
- Murad, S.D.; Luong, T.K. Long-term outcome of a covered versus uncovered transjugular intrahepatic portosystemic shunt in Budd-Chiari syndrome. Liver Int. 2008, 28, 249–256.
- Gandini, R.; Konda, D. Transjugular intrahepatic portosystemic shunt patency and clinical outcome in patients with Budd-Chiari syndrome: Covered versus uncovered stents. Radiology 2006, 241, 298–305.
- Kappenschneider, T. The Budd-Chiari Syndrome: Change in Treatment since 1990. Ph.D. Thesis, University Hospital Freiburg, Freiburg, Germany, 2010.
- Garcia-Pagan, J.C.; Heydtmann, M. TIPS for Budd-Chiari syndrome: Long-term results and prognostics factors in 124 patients. Gastroenterology 2008, 135, 808–815.
- Seijo, S.; Plessier, A. Good long-term outcome of Budd-Chiari syndrome with a step-wise management. Hepatology 2013, 57, 1962–1968.
- Zeitoun, G.; Escolano, S. Outcome of Budd-Chiari syndrome: A multivariate analysis of factors related to survival including surgical portosystemic shunting. Hepatology 1999, 30, 84–89.
- Murad, S.D.; Valla, D.C. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology 2004, 39, 500–508.
- Qi, X.; Guo, W. Transjugular intrahepatic portosystemic shunt for Budd-Chiari syndrome: Techniques, indications and results on 51 Chinese patients from a single centre. Liver Int. 2014, 34, 1164–1175.
- Blum, U.; Rössle, M. Budd-Chiari syndrome: Technical, hemodynamic, and clinical results of treatment with transjugular intrahepatic portosystemic shunt. Radiology 1995, 197, 805–811.
- Mancuso, A.; Fung, K. TIPS for acute and chronic Budd-Chiari syndrome: A single-centre experience. J. Hepatol. 2003, 38, 751–754.
- Eapen, C.E.; Velissaris, D. Favourable medium-term outcome following hepatic vein recanalisation and/or transjugular intrahepatic portosystemic shunt for Budd Chiari syndrome. Gut 2006, 55, 878–884.
- Panis, Y.; Belghiti, J. Portosystemic shunt in Budd-Chiari syndrome: Long-term survival and factors affecting shunt patency in 25 patients in Western countries. Surgery 1994, 115, 276–281.
- Rautou, P.E.; Moucari, R. Prognostic indices for Budd-Chiari syndrome: Valid for clinical studies but insufficient for individual management. Am. J. Gastroenterol. 2009, 104, 1140–1146.
- Perello, A.; Garcia-Pagan, J.C. TIPS is a useful long-term derivative therapy for patients with Budd-Chiari syndrome uncontrolled by medical therapy. Hepatology 2002, 35, 132–139.
- Ryu, R.K.; Durham, J.D. Role of TIPS as a bridge to hepatic transplantation in Budd-Chiari syndrome. J. Vasc. Interv. Radiol. 1999, 10, 799–805.
- Qi, X.; Yang, M. Transjugular intrahepatic portosystemic shunt in the treatment of Budd-Chiari syndrome: A critical review of literatures. Scand. J. Gastroeneterol. 2013, 48, 771–784.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
598
Revisions:
3 times
(View History)
Update Date:
24 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No