
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniele Balducci | -- | 1807 | 2023-07-21 19:59:16 | | | |
| 2 | Lindsay Dong | Meta information modification | 1807 | 2023-07-24 04:30:28 | | |
Video Upload Options
Liver cancer is very frequent, and hepatocellular carcinoma (HCC) accounts for the majority of liver cancer cases. Its growing incidence has been greatly affected by the increasing prevalence of metabolic-associated fatty liver disease (MAFLD). The latter is a new epidemic in the era. In fact, HCC is often generated from noncirrhotic liver and its treatment benefits from surgical and nonsurgical approaches, potentially bridged by transjugular intrahepatic portosystemic shunt (TIPS) use. TIPS use is an effective treatment for portal hypertension complications, but its application in patients with HCC and clinically significant portal hypertension (CSPH) remains controversial due to concerns about tumor rupture, dissemination, and increased toxicity.
1. Introduction
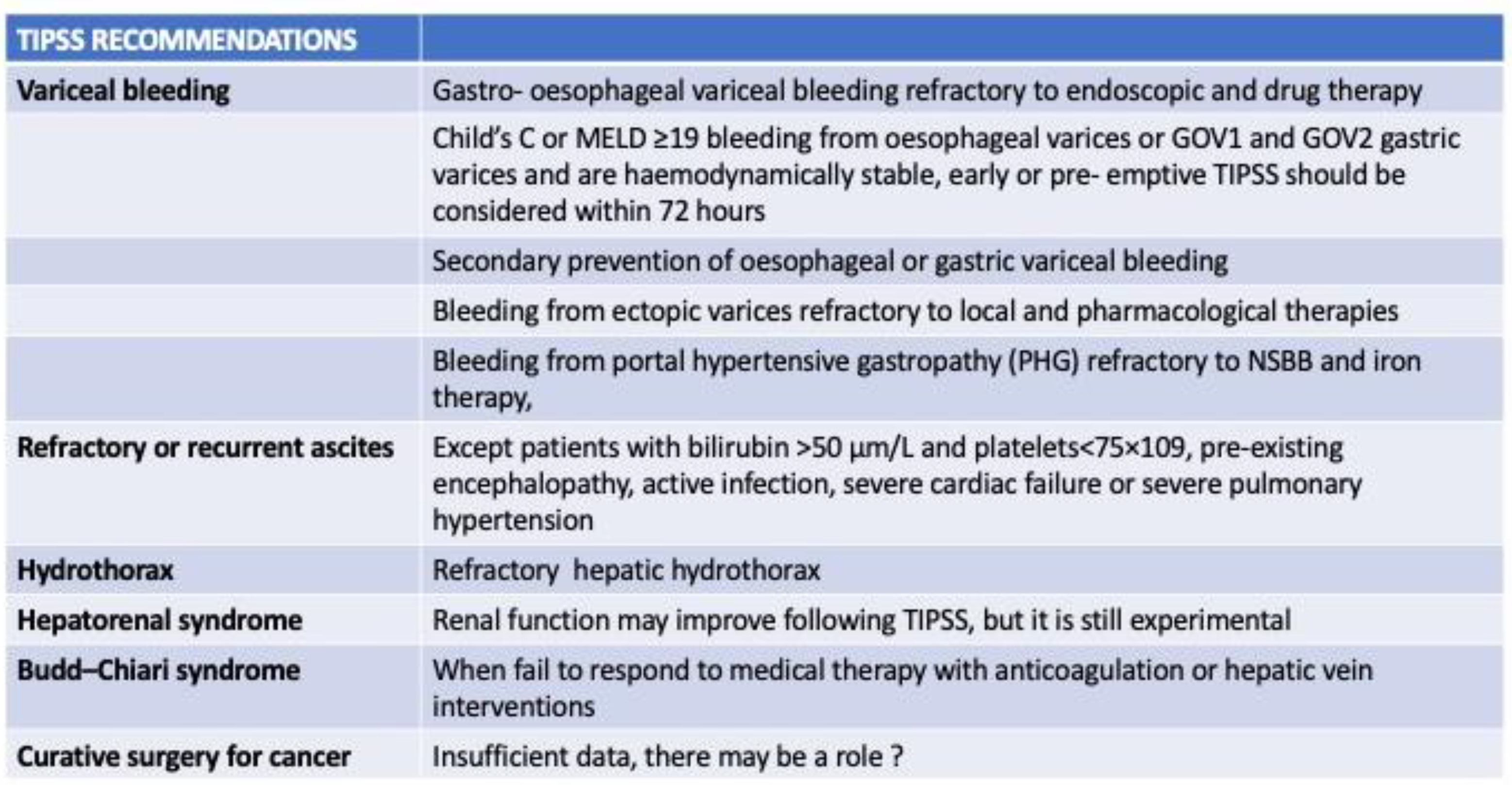
2. Technical Feasibility and Safety of TIPS Use in HCC Patients
3. TIPS and Locoregional Treatments: Transarterial Chemoembolization (TACE) and Transarterial Radioembolization (TARE)
4. TIPS Use as a Bridge to Systemic Therapy
5. Conclusions
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330.
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691.
- Jinjuvadia, R.; Patel, S.; Liangpunsakul, S. The association between metabolic syndrome and hepatocellular carcinoma: Systemic review and meta-analysis. J. Clin. Gastroenterol. 2014, 48, 172–177.
- Tarao, K.; Nozaki, A.; Ikeda, T.; Sato, A.; Komatsu, H.; Komatsu, T.; Taguri, M.; Tanaka, K. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases—Meta-analytic assessment. Cancer Med. 2019, 8, 1054.
- Vizzutti, F.; Schepis, F.; Arena, U.; Fanelli, F.; Gitto, S.; Aspite, S.; Turco, L.; Dragoni, G.; Laffi, G.; Marra, F. Transjugular intrahepatic portosystemic shunt (TIPS): Current indications and strategies to improve the outcomes. Intern. Emerg. Med. 2020, 15, 37–48.
- Bai, M.; Qi, X.S.; Yang, Z.P.; Yang, M.; Fan, D.M.; Han, G.H. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: An updated meta-analysis. World J. Gastroenterol. 2014, 20, 2704–2714.
- Dariushnia, S.R.; Haskal, Z.J.; Midia, M.; Martin, L.G.; Gregory Walker, T.; Kalva, S.P.; Clark, T.W.I.; Ganguli, S.; Krishnamurthy, V.; Saiter, C.K.; et al. Quality Improvement Guidelines for Transjugular Intrahepatic Portosystemic Shunts. J. Vasc. Interv. Radiol. 2016, 27, 1–7.
- Wallace, M.; Swaim, M. Transjugular intrahepatic portosystemic shunts through hepatic neoplasms. J. Vasc. Interv. Radiol. 2003, 14, 501–507.
- Kohi, M.P.; Fidelman, N.; Naeger, D.M.; Laberge, J.M.; Gordon, R.L.; Kerlan, R.K. Hepatotoxicity after transarterial chemoembolization and transjugular intrahepatic portosystemic shunt: Do two rights make a wrong? J. Vasc. Interv. Radiol. 2013, 24, 68–73.
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693.
- Saad, W.E. Transjugular intrahepatic portosystemic shunt before and after liver transplantation. Semin. Interv. Radiol. 2014, 31, 243–247.
- Polacco, M.; Vitale, A.; Valmasoni, M.; D’Amico, F.; Gringeri, E.; Brolese, A.; Zanus, G.; Neri, D.; Carraro, A.; Pauletto, A.; et al. Liver Resection Associated With Mini Porto-Caval Shunt as Salvage Treatment in Patients With Progression of Hepatocellular Carcinoma Before Liver Transplantation: A Case Report. Transplant. Proc. 2010, 42, 1378–1380.
- Qiu, B.; Zhao, M.F.; Yue, Z.D.; Zhao, H.W.; Wang, L.; Fan, Z.H.; He, F.L.; Dai, S.; Yao, J.N.; Liu, F.Q. Combined transjugular intrahepatic portosystemic shunt and other interventions for hepatocellular carcinoma with portal hypertension. World J. Gastroenterol. 2015, 21, 12439.
- Liu, L.; Zhao, Y.; Qi, X.; Cai, G.; He, C.; Guo, W.; Yin, Z.; Chen, H.; Chen, X.; Fan, D.; et al. Transjugular intrahepatic portosystemic shunt for symptomatic portal hypertension in hepatocellular carcinoma with portal vein tumor thrombosis. Hepatol. Res. 2014, 44, 621–630.
- Chen, Z.X.; Qiu, Z.K.; Wang, G.B.; Wang, G.S.; Jiang, W.W.; Gao, F. Safety and effectiveness of transjugular intrahepatic portosystemic shunt in hepatocellular carcinoma patients with portal hypertension: A systematic review and meta-analysis. Clin. Radiol. 2023, 78, 209–218.
- Thabut, D.; Kudo, M. Treatment of portal hypertension in patients with HCC in the era of Baveno VII. J. Hepatol. 2023, 78, 658–662.
- Bannas, P.; Roldán-Alzate, A.; Johnson, K.M.; Woods, M.A.; Ozkan, O.; Motosugi, U.; Wieben, O.; Reeder, S.B.; Kramer, H. Longitudinal Monitoring of Hepatic Blood Flow before and after TIPS by Using 4D-Flow MR Imaging. Radiology 2016, 281, 574–582.
- Yan, H.; Wang, G.; Zhu, W.; Feng, K.; Zhu, W.; Wu, X.; Qiu, Z.; Chen, G.; Jiang, W.; Zhang, F.; et al. Feasibility and clinical value of TIPS combined with subsequent antitumor treatment in HCC patients with refractory ascites. Transl. Oncol. 2020, 13, 100864.
- Ruohoniemi, D.M.; Taslakian, B.; Aaltonen, E.A.; Hickey, R.; Patel, A.; Horn, J.C.; Chiarello, M.; McDermott, M. Comparative Analysis of Safety and Efficacy of Transarterial Chemoembolization for the Treatment of Hepatocellular Carcinoma in Patients with and without Pre-Existing Transjugular Intrahepatic Portosystemic Shunts. J. Vasc. Interv. Radiol. 2020, 31, 409–415.
- Kang, J.W.; Kim, J.H.; Ko, G.Y.; Gwon DIl Yoon, H.K.; Sung, K.B. Transarterial chemoembolization for hepatocellular carcinoma after transjugular intrahepatic portosystemic shunt. Acta Radiol. 2012, 53, 545–550.
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390.
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905.
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873.
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070.
- Sangro, B.; Gomez-Martin, C.; De La Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88.
- Merle, P.; Kudo, M.; Edeline, J.; Bouattour, M.; Cheng, A.-L.; Chan, S.L.; Yau, T.; Garrido, M.; Knox, J.; Daniele, B.; et al. Pembrolizumab as Second-Line Therapy for Advanced Hepatocellular Carcinoma: Longer Term Follow-Up from the Phase 3 KEYNOTE-240 Trial. Liver Cancer 2023, 1–12.
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952.
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90.
- Llovet, J.M.; Bustamante, J.; Castells, A.; Vilana, R.; Del Carmen Avuso, M.; Sala, M.; Brú, C.; Rodés, J.; Bruix, J. Natural history of untreated nonsurgical hepatocellular carcinoma: Rationale for the design and evaluation of therapeutic trials. Hepatology 1999, 29, 62–67.
- Qiu, Z.; Wang, G.; Yan, H.; Qi, H.; Zuo, M.; Wang, G.; Jiang, W.; Chen, Z.; Xue, J.; Lu, L.; et al. TIPS plus sequential systemic therapy of advanced HCC patients with tumour thrombus-related symptomatic portal hypertension. Eur. Radiol. 2022, 32, 6777–6787.
- Marasco, G.; Colecchia, A.; Colli, A.; Ravaioli, F.; Casazza, G.; Bacchi Reggiani, M.L.; Cucchetti, A.; Cescon, M.; Festi, D. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J. Hepatol. 2019, 70, 440–448.
- Takuma, Y.; Nouso, K.; Morimoto, Y.; Tomokuni, J.; Sahara, A.; Takabatake, H.; Matsueda, K.; Yamamoto, H. Portal Hypertension in Patients with Liver Cirrhosis: Diagnostic Accuracy of Spleen Stiffness. Radiology 2015, 279, 609–619.
- Allaire, M.; Rudler, M.; Thabut, D. Portal hypertension and hepatocellular carcinoma: Des liaisons dangereuses…. Liver Int. 2021, 41, 1734–1743.
- Glantzounis, G.K.; Karampa, A.; Peristeri, V.; Pappas-Gogos, G.; Tepelenis, K.; Tzimas, P.; Cyrochristos, D.J. Recent advances in the surgical management of hepatocellular carcinoma. Ann. Gastroenterol. 2021, 34, 453.
- Takemura, N.; Aoki, T.; Hasegawa, K.; Kaneko, J.; Arita, J.; Akamatsu, N.; Makuuchi, M.; Kokudo, N. Hepatectomy for hepatocellular carcinoma after perioperative management of portal hypertension. Br. J. Surg. 2019, 106, 1066–1074.
- Chalret Du Rieu, M.; Carrere, N.; Bureau, C.; Lagarde, S.; Otal, P.; Pradere, B. Dérivation portocave intrahépatique transjugulaire avant chirurgie hépatique en cas de cirrhose compliquée d’hypertension portale. J. Chir. 2009, 146, 191–194.
- Sliwinski, S.; Trojan, J.; Mader, C.; Vogl, T.; Bechstein, W. Liver resection after Transjugular Portosystemic Stent Shunt (TIPSS). Z. Gastroenterol. 2023, 61, 390–393.




