
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Svetlana V. Guryanova | -- | 5825 | 2023-07-21 13:15:02 | | | |
| 2 | Dean Liu | + 229 word(s) | 6054 | 2023-07-24 03:57:45 | | | | |
| 3 | Dean Liu | Meta information modification | 6054 | 2023-07-24 04:09:02 | | |
Video Upload Options
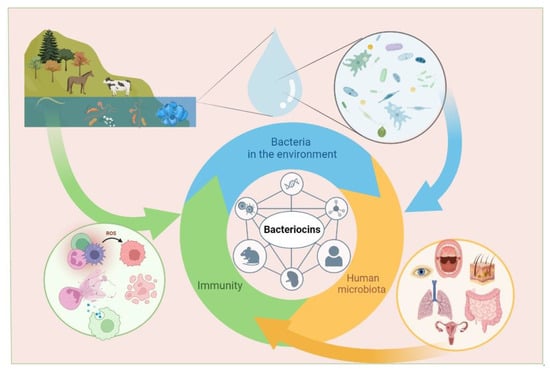
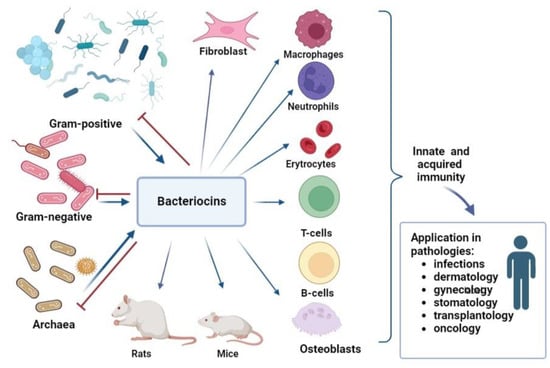
Bacteriocins can help in the treatment and prevention of infectious diseases. Moreover, bacteriocins can be obtained in prokaryotic organisms, and contribute s to their widespread use. While the use of bacteriocins is currently limited to the food industry (for example, nisin is used as a preservative, E234), a large number of studies on their microbicidal properties suggest that their use in medicine may increase in the foreseeable future. However, for the successful use of bacteriocins in medicine, it is necessary to understand their effect on the immune system, especially in cases where immunity is weakened due to infectious processes, oncological, allergic, or autoimmune diseases. Studies on the immuno-modulatory activity of bacteriocins in animal models and human cells have revealed their ability to induce both pro-inflammatory and anti-inflammatory factors involved in the implementation of innate immunity. The influence of bacteriocins on acquired immunity is revealed by an increase in the number of T-lymphocytes with a simultaneous decrease in B-lymphocyte levels, which makes them attractive substances for reducing inflammation. The widespread use of bacteriocins in the food industry, their low toxicity, and their broad and narrow specificity are reasons for researchers to pay attention to their immunomodulatory properties and explore their medical applications. Inflammation regulation by bacteriocins can be used in the treatment of various pathologies.
1. Introduction
2. Resources, Gene Organization and Biosynthesis of Bacteriocins
3. Mechanism of Action on Microorganisms
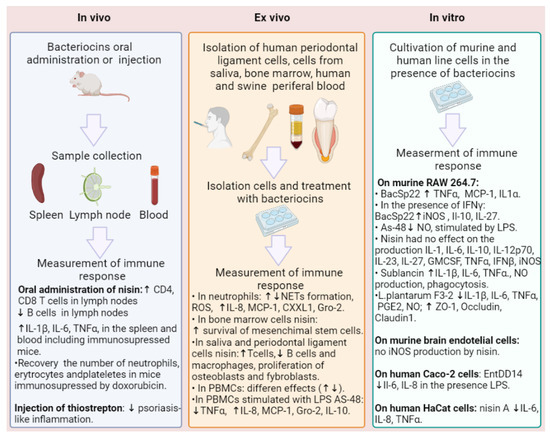
4. Immunomodulatory Activity of Bacteriocins
| Bacteriocin | Resource | Highlights | Ref. |
|---|---|---|---|
| Nisin Z | L. lactis | Inducing MCP-1, Gro-α, and IL-6 in human PBMC. In response to LPS, reducing TNF-α and inhibiting IL-6. | [99] |
| Nisin | L. lactis | Increasing T-cells, fibroblasts, and osteoblasts; increasing B cells. Increasing the expression of IL-10, FGF-2, and TGF-β genes and the synthesis of TGF-β and FGF-2. Decreasing phagocytosis and inhibiting the synthesis of IL-6. |
[100,101,102,105,106,107] |
| Nisin A | L. lactis | No influence on IL-1α, IL-1β, IL-6, IL-10, IL-12p70, IL-17A, IL-23, IL-27, GMCSF, IFN-β, TNF-α, iNOS, NO production, and NETs. | [108] |
| Inducing NETs formation and ROX in human neutrophils. | [111] | ||
| BacSp222 | Staphylococcus pseudintermedius 222 | Increasing NO, iNOS in P388.D1, and RAW 264.7; increasing IL-10 and IL-27 in combination with IFN-γ. In human neutrophils, upregulating IL-8; absence of ROS production or NETs formation. | [108] |
| AS-48 | Enterococcus spp. | Decreasing NO production induced by LPS on RAW cells; absence of pro-inflammatory effects. | [110] |
| Sublancin | Bacillus subtilis 168 | Increasing CXCL1 and MCP-1; decreasing TNF-α in murine peritoneal macrophages and neutrophils. Increasing IL-1β, IL-6, TNF-alpha, nitric oxide, and phagocytosis. Increasing T-cells in the mesenteric lymph nodes. Increasing IL-1β, IL-6, and TNF-α in the spleen of immunosuppressed BALB/c mice. | [112,113,114] |
| L. plantarum F3-2 | Lactiplantibacillus plantarum | Reducing elevated levels of IL1β, IL6, TNFα, nitric oxide, and prostaglandin E2 induced by LPS in RAW264.7 cells. Increasing the expression levels of ZO-1, Occludin, and Claudin 1. | [115] |
| Acidocin A | Lactobacillus acidophilus TK9201 | Increasing IL-6, TNFα, MIG/CXCL9, MCP-1/CCL2, MCP-3/CCL7, and MIP-1β in PBMCs. | [116] |
| Ent DD14 | Enterococcus faecalis 14 | Decreasing IL-6 and IL-8. | [117] |
| Thiostrepton | Streptomyces genus | Increasing HspA1A, Hsp70, Hsp90α, or Hsp105. | [127] |
5. Bioavailability and Safety of Bacteriocins
6. Conclusions
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655.
- Kariuki, S.; Kering, K.; Wairimu, C.; Onsare, R.; Mbae, C. Antimicrobial Resistance Rates and Surveillance in Sub-Saharan Africa: Where Are We Now? Infect. Drug Resist. 2022, 15, 3589–3609.
- Shoaib, M.; Aqib, A.I.; Muzammil, I.; Majeed, N.; Bhutta, Z.A.; Kulyar, M.F.-E.; Fatima, M.; Zaheer, C.-N.F.; Muneer, A.; Murtaza, M.; et al. MRSA compendium of epidemiology, transmission, pathophysiology, treatment, and prevention within one health framework. Front. Microbiol. 2023, 13, 1067284.
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. 2022. Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 19 February 2023).
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184.
- Tiwari, A.; Kurittu, P.; Al-Mustapha, A.I.; Heljanko, V.; Johansson, V.; Thakali, O.; Mishra, S.K.; Lehto, K.-M.; Lipponen, A.; Oikarinen, S.; et al. Wastewater surveillance of antibiotic-resistant bacterial pathogens: A systematic review. Front. Microbiol. 2022, 13, 977106.
- Nguyen, A.Q.; Vu, H.P.; Nguyen, L.N.; Wang, Q.; Djordjevic, S.P.; Donner, E.; Yin, H.; Nghiem, L.D. Monitoring antibiotic resistance genes in wastewater treatment: Current strategies and future challenges. Sci. Total. Environ. 2021, 783, 146964.
- Alam, M.-U.; Ferdous, S.; Ercumen, A.; Lin, A.; Kamal, A.; Luies, S.K.; Sharior, F.; Khan, R.; Rahman, Z.; Parvez, S.M.; et al. Effective Treatment Strategies for the Removal of Antibiotic-Resistant Bacteria, Antibiotic-Resistance Genes, and Antibiotic Residues in the Effluent From Wastewater Treatment Plants Receiving Municipal, Hospital, and Domestic Wastewater: Protocol for a Systematic Review. JMIR Res. Protoc. 2021, 10, e33365.
- Meier, H.; Spinner, K.; Crump, L.; Kuenzli, E.; Schuepbach, G.; Zinsstag, J. State of Knowledge on the Acquisition, Diversity, Interspecies Attribution and Spread of Antimicrobial Resistance between Humans, Animals and the Environment: A Systematic Review. Antibiotics 2022, 12, 73.
- Xiao, R.; Huang, D.; Du, L.; Song, B.; Yin, L.; Chen, Y.; Gao, L.; Li, R.; Huang, H.; Zeng, G. Antibiotic resistance in soil-plant systems: A review of the source, dissemination, influence factors, and potential exposure risks. Sci. Total. Environ. 2023, 869, 161855.
- Kim, J.Y.; Suh, J.W.; Kang, J.S.; Kim, S.B.; Yoon, Y.K.; Sohn, J.W. Gram-Negative Bacteria’s Outer Membrane Vesicles. Infect. Chemother. 2023, 55, 557902.
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44.
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.; Cork, S.C.; Ronksley, P.E.; Barkema, H.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327.
- WHO. Stop Using Antibiotics in Healthy Animals to Prevent the Spread of Antibiotic Resistance. 7 November 2017. Available online: https://www.who.int/news/item/07-11-2017-stop-using-antibiotics-in-healthy-animals-to-prevent-the-spread-of-antibiotic-resistance (accessed on 19 February 2023).
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313.
- van Staa, T.P.; Palin, V.; Li, Y.; Welfare, W.; Felton, T.W.; Dark, P.; Ashcroft, D.M. The effectiveness of frequent antibiotic use in reducing the risk of infection-related hospital admissions: Results from two large population-based cohorts. BMC Med. 2020, 18, 40.
- Gupta, R.; Sharma, S. Role of alternatives to antibiotics in mitigating the antimicrobial resistance crisis. Indian J. Med. Res. 2022, 156, 464–477.
- Ye, J.; Chen, X. Current Promising Strategies against Antibiotic-Resistant Bacterial Infections. Antibiotics 2022, 12, 67.
- Guryanova, S.; Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of Russian Academy of Sciences; Khaitov, R.; National Research Center—Institute of Immunology of the Federal Medico-Biological Agency. Glucosaminylmuramyldipeptide—GMDP: Effect on mucosal immunity (on the issue of immunotherapy and immunoprophylaxis). Immunologiya 2020, 41, 174–183.
- Rechkina, E.A.; Denisova, G.F.; Masalova, O.V.; Lideman, L.F.; Denisov, D.A.; Lesnova, E.I.; Ataullakhanov, R.I.; Gur’Ianova, S.V.; Kushch, A. Epitope mapping of antigenic determinants of hepatitis C virus proteins by phage display. Mol. Biologiia 2006, 40, 357–368. (In Russian)
- Konorev, M.R.; Guryanova, S.V.; Tyshevich, E.N.; Pavlyukov, R.A.; Borisova, O.Y. Advisable including glucosaminylmuramyldipeptide in Helicobacter pylori therapy: Experience of ten-year investigation. Rudn. J. Med. 2020, 24, 269–282.
- L’vov, V.L.; Gur’yanova, S.V.; Rodionov, A.V.; Gorshkova, R.P. Structure of the repeating unit of the O-specific polysaccha-ride of the lipopolysaccharide of yersinia kristensenii strain 490 (O:12,25). Carbohydr. Res. 1992, 228, 415–422. (In Russian)
- L’Vov, V.L.; Gur’Ianova, S.V.; Rodionov, A.V.; Dmitriev, B.A.; Shashkov, A.S.; Ignatenko, A.V.; Gorshkova, R.P.; Ovodov, I.S. The structure of a repetitive unit of the glycerolphosphate- containing O-specific polysaccharide chain from Yersinia kristensenii strain 103 (0:12,26) lipopolysaccharide. Bioorg. Khim. 1990, 16, 379–389.
- Tataurshchikova, N. OM-85: Personalized approach to the treatment of acute respiratory infections in children. Quest. Pract. Pediatr. 2020, 15, 61–68.
- Ayswaria, R.; Vijayan, J.; Nathan, V.K. Antimicrobial peptides derived from microalgae for combating antibiotic resistance: Current status and prospects. Cell Biochem. Funct. 2023, 41, 142–151.
- Cooper, I.I. A review of the potential for bacteriophages to effect antibiofilm activity, using selected examples. J. Appl. Microbiol. 2022, 134, lxac056.
- Tataurshchikova, N.S.; Sidorovich, I.G. The cytokine status as a criterion for the efficacy of intranasal aerosol therapy with the use of a cycloferon solution in the patients presenting with allergic rhinosinusitis. Vestn. Otorinolaringol. 2012, 3, 79–82.
- Al-Awsi, G.R.L.; Alameri, A.A.; Al-Dhalimy, A.M.B.; Gabr, G.A.; Kianfar, E. Application of nano-antibiotics in the diagnosis and treatment of infectious diseases. Braz. J. Biol. 2023, 84, e264946.
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and Allergenic Properties of Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 2499.
- Reeves, P. The Bacteriocins. Bacteriol. Rev. 1965, 29, 24–45.
- Gratia, A. “Sur un remarquable exemple d’antagonisme entre deux souches de coilbacille” . Compt. Rend. Soc. Biol. 1925, 93, 1040–1042. NAID 10027104803. (In French)
- Fredericq, P. Colicines et Autres Bacteriocines . Ergeb. Mikrobiol. Immun. Exp. Ther. 1963, 37, 114–161. PMID: 14324288. (In French)
- Cotter, P.D.; Ross, R.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Genet. 2012, 11, 95–105.
- Cotter, P.D.; Hill, C.; Ross, R.P. Food microbiology: Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777.
- Wilaipun, P.; Zendo, T.; Sangjindavong, M.; Nitisinprasert, S.; Leelawatcharamas, V.; Nakayama, J.; Sonomoto, K. The two-synergistic peptide bacteriocin produced by Enterococcus faecium NKR- 5-3 isolated from Thai fermented fish (Plara). Sci. Asia 2004, 30, 115–122.
- Fatima, D.; Mebrouk, K. Characterization and determination of the factors affecting anti-listerial bacteriocins from Lactobacillus plantarum and Pediococcus pentosaceus isolated from dairy milk products. Afr. J. Food Sci. 2013, 7, 35–44.
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28.
- Darbandi, A.; Asadi, A.; Ari, M.M.; Ohadi, E.; Talebi, M.; Zadeh, M.H.; Emamie, A.D.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2021, 36, e24093.
- Benech, R.-O.; Kheadr, E.E.; Lacroix, C.; Fliss, I. Antibacterial Activities of Nisin Z Encapsulated in Liposomes or Produced In Situ by Mixed Culture during Cheddar Cheese Ripening. Appl. Environ. Microbiol. 2002, 68, 5607–5619.
- Chandrakasan, G.; Rodríguez-Hernández, A.-I.; López-Cuellar, M.D.R.; Palma-Rodríguez, H.-M.; Chavarría-Hernández, N. Bacteriocin encapsulation for food and pharmaceutical applications: Advances in the past 20 years. Biotechnol. Lett. 2019, 41, 453–469.
- Habib, W.; Sakr, A. Development and human in vivo evaluation of a colonic drug delivery system. Pharm. Ind. 1999, 61, 1145–1149.
- Gough, R.; Cabrera Rubio, R.; O’Connor, P.M.; Crispie, F.; Brodkorb, A.; Miao, S.; Hill, C.; Ross, R.P.; Cotter, P.D.; Nilaweera, K.N.; et al. Oral delivery of nisin in resistant starch based matrices alter the gut micro- biota in mice. Front Microbiol. 2018, 9, 1186.
- Rollema, H.S.; Kuipers, O.P.; Both, P.; De Vos, W.M.; Siezen, R.J. Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl. Environ. Microbiol. 1995, 61, 2873–2878.
- Field, D.; Blake, T.; Mathur, H.; O’Connor, P.M.; Cotter, P.D.; Ross, R.P.; Hill, C. Bioengineering Nisin to overcome the Nisin Resistance Protein. Mol. Microbiol. 2019, 111, 717–731.
- Duquesne, S.; Destoumieux-Garz’on, D.; Peduzzi, J.; Rebuffat, S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat. Prod. Rep. 2007, 24, 708–734.
- Drider, D.; Rebuffat, S. Prokaryotic Antimicrobial Peptides: From Genes to Applications; Springer Science & Business Media: Berlin/Heidelburg, Germany, 2011.
- Hammami, R.; Fernandez, B.; Lacroix, C.; Fliss, I. Anti-infective properties of bacteriocins: An update. Cell. Mol. Life Sci. 2013, 70, 2947–2967.
- Dobson, A.; Cotter, P.; Ross, R.; Hill, C. Bacteriocin Production: A Probiotic Trait? Appl. Environ. Microbiol. 2012, 78, 1–6.
- Lugtenberg, B.; Sc, Y.; Ch, L.; Ct, S.; Jy, F. Faculty Opinions recommendation of Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front Microbiol. 2014, 5, 241.
- Riley, M.A.; Wertz, J.E. Bacteriocins evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137.
- Shand, R.F.; Leyva, K.J. Archaeal antimicrobials: An undiscovered country. In Archaea: New Models for Prokaryotic Biology; Caister Academic Press: Norfolk, UK, 2008; 248p.
- Besse, A.; Peduzzi, J.; Rebuffat, S.; Carré-Mlouka, A. Antimicrobial peptides and proteins in the face of extremes: Lessons from archaeocins. Biochimie 2015, 118, 344–355.
- Oman, T.J.; van der Donk, W.A. Insights into the mode of action of the two-peptide lantibiotic haloduracin. ACS Chem. Biol. 2009, 4, 865–874.
- Hanchi, H.; Hammami, R.; Gingras, H.; Kourda, R.; Bergeron, M.G.; Ben Hamida, J.; Ouellette, M.; Fliss, I. Inhibition of MRSA and of Clostridium difficile by durancin 61A: Synergy with bacteriocins and antibiotics. Futur. Microbiol. 2017, 12, 205–212.
- Cui, Z.; Chen, Z.-H.; Zhang, Q.; Gribova, V.V.; Filaretov, V.F.; Huang, D.-S. RMSCNN: A Random Multi-Scale Convolutional Neural Network for Marine Microbial Bacteriocins Identification. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021, 19, 3663–3672.
- Zielińska, D.; Kolożyn-Krajewska, D. Food-Origin Lactic Acid Bacteria May Exhibit Probiotic Properties: Review. BioMed Res. Int. 2018, 2018, 5063185.
- Verso, L.L.; Lessard, M.; Talbot, G.; Fernandez, B.; Fliss, I. Isolation and Selection of Potential Probiotic Bacteria from the Pig Gastrointestinal Tract. Probiotics Antimicrob. Proteins 2018, 10, 299–312.
- Ryan, K.; Jayaraman, T.; Daly, P.; Canchaya, C.; Curran, S.; Fang, F.; Quigley, E.; O’toole, P. Isolation of lactobacilli with probiotic properties from the human stomach. Lett. Appl. Microbiol. 2008, 47, 269–274.
- Klaenhammer, T.R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85.
- Gautam, N.; Sharma, N. Purification and characterization of bacteriocin produced by strain of Lactobacillus brevis MTCC 7539. Indian J. Biochem. Biophys. 2009, 46, 337–341.
- Knorr, D. Technology aspects related to microorganisms in functional foods. Trends Food Sci. Technol. 1998, 9, 295–306.
- Karpinski, T.; Szkaradkiewicz, A.K. Characteristic of Bacteriocines and Their Application. Pol. J. Microbiol. 2013, 62, 223–235.
- Choi, S.; Baek, M.-G.; Chung, M.-J.; Lim, S.; Yi, H. Distribution of bacteriocin genes in the lineages of Lactiplantibacillus plantarum. Sci. Rep. 2021, 11, 20063.
- Collins, F.W.J.; O’Connor, P.M.; O’Sullivan, O.; Gómez-Sala, B.; Rea, M.C.; Hill, C.; Ross, R.P. Bacteriocin Gene-Trait matching across the complete Lactobacillus Pan-genome. Sci. Rep. 2017, 7, 3481.
- Chérier, D.; Patin, D.; Blanot, D.; Touzé, T.; Barreteau, H. The Biology of Colicin M and Its Orthologs. Antibiotics 2021, 10, 1109.
- De Jong, A.; Van Hijum, S.A.F.T.; Bijlsma, J.J.E.; Kok, J.; Kuipers, O.P. BAGEL: A web-based bacteriocin genome mining tool. Nucleic Acids Res. 2006, 34, W273–W279.
- Spangler, R.; Zhang, S.P.; Krueger, J.; Zubay, G. Colicin synthesis and cell death. J. Bacteriol. 1985, 163, 167–173.
- de Freire Bastos, M.d.C.; Coelho, M.L.V.; da Silva Santos, O.C. Resistance to bacteriocins produced by Gram-positive bacteria. Microbiol 2015, 161, 683–700.
- Bountra, K.; Hagelueken, G.; Choudhury, H.G.; Corradi, V.; El Omari, K.; Wagner, A.; Mathavan, I.; Zirah, S.; Wahlgren, W.Y.; Tieleman, D.P.; et al. Structural basis for antibacterial peptide selfimmunity by the bacterial ABC transporter McjD. EMBO J. 2017, 36, 3062–3079.
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255.
- Perez, R.H.; Zendo, T.; Sonomoto, K. Circular and Leaderless Bacteriocins: Biosynthesis, Mode of Action, Applications, and Prospects. Front. Microbiol. 2018, 9, 2085.
- Žgur-Bertok, D. Regulating colicin synthesis to cope with stress and lethality of colicin production. Biochem. Soc. Trans. 2012, 40, 1507–1511.
- Costa, S.S.; Moia, G.d.S.; Silva, A.; Baraúna, R.A.; Veras, A.A.d.O. BADASS: BActeriocin-Diversity ASsessment Software. BMC Bioinform. 2023, 24, 24.
- Hammami, R.; Zouhir, A.; Cetal, L.L. BACTIBASE second release: A database and tool platform for bacteriocin characteriza-tion. BMC Microbiol 2010, 10, 22.
- The sbv IMPROVER Project Team (in Alphabetical Order); Boué, S.; Fields, B.; Hoeng, J.; Park, J.; Peitsch, M.C.; Schlage, W.K.; Talikka, M.; Binenbaum, I.; Bondarenko, V.; et al. Enhancement of COPD biological networks using a web-based collaboration interface. F1000Research 2015, 4, 32.
- Guryanova, S.; Guryanova, A. sbv IMPROVER: Modern Approach to Systems Biology. Methods Mol. Biol. 2017, 1613, 21–29.
- Yan, J.; Cai, J.; Zhang, B.; Wang, Y.; Wong, D.F.; Siu, S.W.I. Recent Progress in the Discovery and Design of Antimicrobial Peptides Using Traditional Machine Learning and Deep Learning. Antibiotics 2022, 11, 1451.
- Andrès, E. Cationic antimicrobial peptides in clinical development, with special focus on thanatin and heliomicin. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 31, 881–888.
- Panina, I.S.; Balandin, S.V.; Tsarev, A.V.; Chugunov, A.O.; Tagaev, A.A.; Finkina, E.I.; Antoshina, D.V.; Sheremeteva, E.V.; Paramonov, A.S.; Rickmeyer, J.; et al. Specific Binding of the α-Component of the Lantibiotic Lichenicidin to the Peptidoglycan Precursor Lipid II Predetermines Its Antimicrobial Activity. Int. J. Mol. Sci. 2023, 24, 1332.
- Karpiński, T.M.; Szkaradkiewicz, A.K. Bacteriocins. In Encyclopedia of Food and Health; Caballero, B., Ed.; Elsivier Science: Amsterdam, The Netherlands, 2016; pp. 312–319.
- Cotter, P.D. An ‘Upp’-turn in bacteriocin receptor identification. Mol. Microbiol. 2014, 92, 1159–1163.
- Kouwen, T.R.H.M.; Trip, E.N.; Denham, E.L.; Sibbald, M.J.J.B.; Dubois, J.-Y.F.; van Dijl, J.M. The Large Mechanosensitive Channel MscL Determines Bacterial Susceptibility to the Bacteriocin Sublancin 168. Antimicrob. Agents Chemother. 2009, 53, 4702–4711.
- Kim, Y.C.; Tarr, A.W.; Penfold, C.N. Colicin import into E. coli cells: A model system for insights into the import mechanisms of bacteriocins. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2014, 1843, 1717–1731.
- Cohen-Khait, R.; Harmalkar, A.; Pham, P.; Webby, M.N.; Housden, N.G.; Elliston, E.; Hopper, J.T.S.; Mohammed, S.; Robinson, C.V.; Gray, J.J.; et al. Colicin-Mediated Transport of DNA through the Iron Transporter FepA. mBio 2021, 12, e0178721.
- Bieler, S.; Silva, F.; Soto, C.; Belin, D. Bactericidal Activity of both Secreted and Nonsecreted Microcin E492 Requires the Mannose Permease. J. Bacteriol. 2006, 188, 7049–7061.
- James, R.; Lazdunski, C.; Pattus, F. Bacteriocins, Microcins and Lantibiotics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 65.
- Chiumento, S.; Roblin, C.; Kieffer-Jaquinod, S.; Tachon, S.; Leprètre, C.; Basset, C.; Aditiyarini, D.; Olleik, H.; Nicoletti, C.; Bornet, O.; et al. Ruminococcin C, a promising antibiotic produced by a human gut symbiont. Sci. Adv. 2019, 5, eaaw9969.
- Hatakka, K.; Saxelin, M. Probiotics in intestinal and non-intestinal infectious diseases—Clinical evidence. Curr. Pharm. Design. 2008, 14, 1351–1367.
- Katla, T.; Naterstad, K.; Vancanneyt, M.; Swings, J.; Axelsson, L. Differences in Susceptibility of Listeria monocytogenes Strains to Sakacin P, Sakacin A, Pediocin PA-1, and Nisin. Appl. Environ. Microbiol. 2003, 69, 4431–4437.
- Collins, B.; Guinane, C.M.; Cotter, P.D.; Hill, C.; Ross, R.P. Assessing the Contributions of the LiaS Histidine Kinase to the Innate Resistance of Listeria monocytogenes to Nisin, Cephalosporins, and Disinfectants. Appl. Environ. Microbiol. 2012, 78, 2923–2929.
- Collins, B.; Curtis, N.; Cotter, P.D.; Hill, C.; Ross, R.P. The ABC Transporter AnrAB Contributes to the Innate Resistance of Listeria monocytogenes to Nisin, Bacitracin, and Various β-Lactam Antibiotics. Antimicrob. Agents Chemother. 2010, 54, 4416–4423.
- Rasch, M.; Knochel, S. Variations in tolerance of Listeria monocytogenes to nisin, pediocin PA-1 and bavaricin A. Lett. Appl. Microbiol. 1998, 27, 275–278.
- Nes, I.F.; Gabrielsen, C.; Brede, D.A.; Diep, D.B. Novel developments in bacteriocins from lactic acid bacteria. In Biotechnology of Lactic Acid Bacteria: Novel Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2015; pp. 80–99.
- Sedgley, C.M.; Clewell, D.B.; Flannagan, S.E. Plasmid pAMS1-Encoded, Bacteriocin-Related “Siblicide” in Enterococcus faecalis. J. Bacteriol. 2009, 191, 3183–3188.
- Martãnez, B.; Rodrãguez, A. Antimicrobial susceptibility of nisin resistant Listeria monocytogenes of dairy origin. FEMS Microbiol. Lett. 2005, 252, 67–72.
- Maaß, S.; Bartel, J.; Mücke, P.-A.; Schlüter, R.; Sura, T.; Zaschke-Kriesche, J.; Smits, S.H.J.; Becher, D. Proteomic Adaptation of Clostridioides difficile to Treatment with the Antimicrobial Peptide Nisin. Cells 2021, 10, 372.
- Lohans, C.T.; Vederas, J.C. Development of Class IIa Bacteriocins as Therapeutic Agents. Int. J. Microbiol. 2012, 2012, 386410.
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557.
- Kindrachuk, J.; Jenssen, H.; Elliott, M.; Nijnik, A.; Magrangeas-Janot, L.; Pasupuleti, M.; Thorson, L.; Ma, S.; Easton, D.M.; Bains, M.; et al. Manipulation of innate immunity by a bacterial secreted peptide: Lantibiotic nisin Z is selectively immunomodulatory. J. Endotoxin Res. 2013, 19, 315–327.
- Pablo, M.A.; Gaforio, J.J.; Gallego, A.M.; Ortega, E.; Gã¡Lvez, A.M.; Lã³Pez, G.A.d.C. Evaluation of immunomodulatory effects of nisin-containing diets on mice. FEMS Immunol. Med. Microbiol. 1999, 24, 35–42.
- Shin, J.M.; Ateia, I.; Paulus, J.R.; Liu, H.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Antimicrobial nisin acts against saliva derived multi-species biofilms without cytotoxicity to human oral cells. Front. Microbiol. 2015, 6, 617.
- Page, R.C.; Schroeder, H.E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab. Investig. 1976, 34, 235.
- Radaic, A.; Brody, H.; Contreras, F.; Hajfathalian, M.; Lucido, L.; Kamarajan, P.; Kapila, Y.L. Nisin and Nisin Probiotic Disrupt Oral Pathogenic Biofilms and Restore Their Microbiome Composition towards Healthy Control Levels in a Peri-Implantitis Setting. Microorganisms 2022, 10, 1336.
- Jia, Z.; He, M.; Wang, C.; Chen, A.; Zhang, X.; Xu, J.; Fu, H.; Liu, B. Nisin reduces uterine inflammation in rats by modulating concentrations of pro and antiinflammatory cytokines. Am. J. Reprod. Immunol. 2019, 81, e13096.
- Gao, L.; Kuraji, R.; Zhang, M.J.; Martinez, A.; Radaic, A.; Kamarajan, P.; Le, C.; Zhan, L.; Ye, C.; Rangé, H.; et al. Nisin probiotic prevents inflammatory bone loss while promoting reparative proliferation and a healthy microbiome. NPJ Biofilms Microbiomes 2022, 8, 45.
- Karimi, M.; Maghsoud, Z.; Halabian, R. Effect of Preconditioned Mesenchymal Stem Cells with Nisin Prebiotic on the Expression of Wound Healing Factors Such as TGF-β1, FGF-2, IL-1, IL-6, and IL-10. Regen. Eng. Transl. Med. 2021, 7, 30–40.
- Małaczewska, J.; Kaczorek-Łukowska, E.; Wójcik, R.; Rękawek, W.; Siwicki, A.K. In vitro immunomodulatory effect of nisin on porcine leucocytes. J. Anim. Physiol. Anim. Nutr. 2019, 103, 882–893.
- Śmiałek, J.; Bzowska, M.; Hinz, A.; Mężyk-Kopeć, R.; Sołtys, K.; Mak, P. Bacteriocin BacSp222 and Its Succinylated Forms Exhibit Proinflammatory Activities Toward Innate Immune Cells. J. Inflamm. Res. 2022, 15, 4601–4621.
- Śmiałek, J.; Nowakowski, M.; Bzowska, M.; Bocheńska, O.; Wlizło, A.; Kozik, A.; Dubin, G.; Mak, P. Structure, Biosynthesis, and Biological Activity of Succinylated Forms of Bacteriocin BacSp222. Int. J. Mol. Sci. 2021, 22, 6256.
- Cebrian, R.; Rodriguez-Cabezas, M.E.; Martín-Escolano, R.; Rubiño, S.; Barros, M.G.; Montalbán-López, M.; Rosales, M.J.; Sánchez-Moreno, M.; Valdivia, E.; Martínez-Bueno, M.; et al. Preclinical studies of toxicity and safety of the AS-48 bacteriocin. J. Adv. Res. 2019, 20, 129–139.
- Begde, D.; Bundale, S.; Mashitha, P.; Rudra, J.; Nashikkar, N.; Upadhyay, A. Immunomodulatory efficacy of nisin-a bacterial lantibiotic peptide. J. Pept. Sci. 2011, 17, 438–444.
- Li, J.; Chen, J.; Yang, G.; Tao, L. Sublancin protects against methicillin-resistant Staphylococcus aureus infection by the combined modulation of innate immune response and microbiota. Peptides 2021, 141, 170533.
- Wang, S.; Ye, Q.; Wang, K.; Zeng, X.; Huang, S.; Yu, H.; Ge, Q.; Qi, D.; Qiao, S. Enhancement of Macrophage Function by the Antimicrobial Peptide Sublancin Protects Mice from Methicillin-Resistant Staphylococcus aureus. J. Immunol. Res. 2019, 2019, 3979352.
- Wang, S.; Huang, S.; Ye, Q.; Zeng, X.; Yu, H.; Qi, D.; Qiao, S. Prevention of Cyclophosphamide-Induced Immunosuppression in Mice with the Antimicrobial Peptide Sublancin. J. Immunol. Res. 2018, 2018, 4353580.
- Bu, Y.; Liu, Y.; Liu, Y.; Wang, S.; Liu, Q.; Hao, H.; Yi, H. Screening and Probiotic Potential Evaluation of Bacteriocin-Producing Lactiplantibacillus plantarum In Vitro. Foods 2022, 11, 1575.
- Antoshina, D.V.; Balandin, S.V.; Bogdanov, I.V.; Vershinina, M.A.; Sheremeteva, E.V.; Toropygin, I.Y.; Finkina, E.I.; Ovchinnikova, T.V. Antimicrobial Activity and Immunomodulatory Properties of Acidocin A, the Pediocin-like Bacteriocin with the Non-Canonical Structure. Membranes 2022, 12, 1253.
- Teiar, R.; Pérez-Ramos, A.; Zgheib, H.; Cudennec, B.; Belguesmia, Y.; Drider, D. Anti-adhesion and Anti-inflammatory Potential of the Leaderless Class IIb Bacteriocin Enterocin DD14. Probiotics Antimicrob. Proteins 2022, 14, 613–619.
- Donovick, R.; Pagano, J.F.; Stout, H.A.; Weinstein, M.J. Thiostrepton, a new antibi-otic. I. In vitro studies. Antibiot Annu. 1955, 3, 554–559.
- Chiu, M.L.; Folcher, M.; Griffin, P.; Holt, T.; Klatt, T.; Thompson, C.J. Characterization of the covalent binding of thiostrepton to a thiostrepton-induced protein from Streptomyces lividans. Biochemistry 1996, 35, 2332–2341.
- Halasi, M.; Zhao, H.; Dahari, H.; Bhat, U.G.; Gonzalez, E.B.; Lyubimo, A.V.; Tonetti, D.A.; Gartel, A.L. Thiazole antibiotics against breast cancer. Cell Cycle 2010, 9, 1214–1217.
- Qiao, S.; Lamore, S.D.; Cabello, C.M.; Lesson, J.L.; Munoz-Rodriguez, J.L.; Wondrak, G.T. Thiostrepton is an inducer of oxidative and proteotoxic stress that impairs viability of human melanoma cells but not primary melanocytes. Biochem. Pharmacol. 2012, 83, 1229–1240.
- Newick, K.; Cunniff, B.; Preston, K.; Held, P.; Arbiser, J.; Pass, H.; Mossman, B.; Shukla, A.; Heintz, N. Peroxiredoxin 3 is a re-dox-dependent target of thiostrepton in malignant mesothelioma cells. PLoS ONE 2012, 7, e39404.
- Hegde, N.S.; Sanders, D.A.; Rodriguez, R.; Balasubramanian, S. The transcription factor FOXM1 is a cellular target of the natural product thiostrepton. Nat. Chem. 2011, 3, 725–731.
- Bhat, U.G.; Halasi, M.; Gartel, A.L. FoxM1 is a general target for proteasome in-hibitors. PLoS ONE 2009, 4, e6593.
- Bailly, C. The bacterial thiopeptide thiostrepton. An update of its mode of action, pharmacological properties and applications. Eur. J. Pharmacol. 2022, 914, 174661.
- Sandu, C.; Wetie, A.G.N.; Darie, C.C.; Steller, H. Thiostrepton, a Natural Compound That Triggers Heat Shock Response and Apoptosis in Human Cancer Cells: A Proteomics Investigation. Adv. Exp. Med. Biol. 2014, 806, 443–451.
- Lai, C.-Y.; Yeh, D.-W.; Lu, C.-H.; Liu, Y.-L.; Huang, L.-R.; Kao, C.-Y.; Chen, H.-Y.; Huang, C.-Y.F.; Chang, C.-H.; Luo, Y.; et al. Identification of Thiostrepton as a Novel Inhibitor for Psoriasis-like Inflammation Induced by TLR7–9. J. Immunol. 2015, 195, 3912–3921.
- Salvador, P.B.U.; Dalmacio, L.M.M.; Kim, S.H.; Kang, D.-K.; Balolong, M.P. Immunomodulatory potential of four candidate probiotic Lactobacillus strains from plant and animal origin using comparative genomic analysis. Access Microbiol. 2021, 3, 000299.
- Paiva, A.D.; de Oliveira, M.D.; de Paula, S.O.; Baracat-Pereira, M.C.; Breukink, E.; Mantovani, H.C. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology 2012, 158, 2851–2858.
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20.
- De Vuyst, L.; Leroy, F. Bacteriocins from Lactic Acid Bacteria: Production, Purification, and Food Applications. Microb. Physiol. 2007, 13, 194–199.
- Fernandez, B.; Le Lay, C.; Jean, J.; Fliss, I. Growth, acid production and bacteriocin production by probiotic candidates under simulated colonic conditions. J. Appl. Microbiol. 2013, 114, 877–885.
- Kheadr, E.; Zihler, A.; Dabour, N.; Lacroix, C.; Le Blay, G.; Fliss, I. Study of the physicochemical and biological stability of pediocin PA-1 in the upper gastrointestinal tract conditions using a dynamic in vitro model. J. Appl. Microbiol. 2010, 109, 54–64.
- Birri, D.J.; Brede, D.A.; Nes, I.F. Salivaricin D, a Novel Intrinsically Trypsin-Resistant Lantibiotic from Streptococcus salivarius 5M6c Isolated from a Healthy Infant. Appl. Environ. Microbiol. 2012, 78, 402–410.
- Johnson, E.M.; Jung, Y.-G.; Jin, Y.-Y.; Jayabalan, R.; Yang, S.H.; Suh, J.W. Bacteriocins as food preservatives: Challenges and emerging horizons. Crit. Rev. Food Sci. Nutr. 2018, 58, 2743–2767.
- Jarvis, B.; Mahoney, R. Inactivation of Nisin by Alpha-Chymotrypsin. J. Dairy Sci. 1969, 52, 1448–1450.
- Gough, R.; O’Connor, P.M.; Rea, M.C.; Gómez-Sala, B.; Miao, S.; Hill, C.; Brodkorb, A. Simulated gastrointestinal digestion of nisin and interaction between nisin and bile. LWT 2017, 86, 530–537.
- Pomares, M.F.; Salomón, R.A.; Pavlova, O.; Severinov, K.; Farías, R.; Vincent, P.A. Potential Applicability of Chymotrypsin-Susceptible Microcin J25 Derivatives to Food Preservation. Appl. Environ. Microbiol. 2009, 75, 5734–5738.
- Naimi, S.; Zirah, S.; Hammami, R.; Fernandez, B.; Rebuffat, S.; Fliss, I. Fate and Biological Activity of the Antimicrobial Lasso Peptide Microcin J25 under Gastrointestinal Tract Conditions. Front. Microbiol. 2018, 9, 1764.
- Soleimanpour, M.; Mirhaji, S.S.; Jafari, S.; Derakhshankhah, H.; Mamashli, F.; Nedaei, H.; Karimi, M.R.; Motasadizadeh, H.; Fatahi, Y.; Ghasemi, A.; et al. Designing a new alginate-fibrinogen biomaterial composite hydrogel for wound healing. Sci. Rep. 2022, 12, 7213.
- Pourhajibagher, M.; Pourakbari, B.; Bahador, A. Contribution of antimicrobial photo-sonodynamic therapy in wound healing: An in vivo effect of curcumin-nisin-based poly (L-lactic acid) nanoparticle on Acinetobacter baumannii biofilms. BMC Microbiol. 2022, 22, 28.
- Cunha, E.; Tavares, L.; Oliveira, M. Revisiting Periodontal Disease in Dogs: How to Manage This New Old Problem? Antibiotics 2022, 11, 1729.
- Lallukka, M.; Gamna, F.; Gobbo, V.A.; Prato, M.; Najmi, Z.; Cochis, A.; Rimondini, L.; Ferraris, S.; Spriano, S. Surface Functionalization of Ti6Al4V-ELI Alloy with Antimicrobial Peptide Nisin. Nanomaterials 2022, 12, 4332.
- Azmi, M.F.; Al Khateeb, A.; Ab Rahim, S.; Froemming, G.R.A.; Omar, E. Effect of different solvents on nisin ZP potential as anticancer agent against MG-63 osteosarcoma cells. Asia Pac. J. Mol. Biol. Biotechnol. 2022, 30, 43–54.
- Shin, J.; Gwak, J.; Kamarajan, P.; Fenno, J.; Rickard, A.; Kapila, Y. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465.
- Thanjavur, N.; Sangubotla, R.; Lakshmi, B.A.; Rayi, R.; Mekala, C.D.; Reddy, A.S.; Viswanath, B. Evaluating the antimicrobial and apoptogenic properties of bacteriocin (nisin) produced by Lactococcus lactis. Process. Biochem. 2022, 122, 76–86.
- Ortiz-Rodríguez, T.; Mendoza-Acosta, F.; Martínez-Zavala, S.A.; Salcedo-Hernández, R.; Casados-Vázquez, L.E.; Bideshi, D.K.; Barboza-Corona, J.E. Thurincin H Is a Nonhemolytic Bacteriocin of Bacillus thuringiensis with Potential for Applied Use. Probiotics Antimicrob. Proteins 2022, 6, 77–84.
- Cox, P.S.C.A.M.S.G.C.R.; Coburn, P.S.; Gilmore, M.S. Enterococcal Cytolysin: A Novel Two Component Peptide System that Serves as a Bacterial Defense against Eukaryotic and Prokaryotic Cells. Curr. Protein Pept. Sci. 2005, 6, 77–84.
- Taggar, R.; Jangra, M.; Dwivedi, A.; Bansal, K.; Patil, P.B.; Bhattacharyya, M.S.; Nandanwar, H.; Sahoo, D.K. Bacteriocin isolated from the natural inhabitant of Allium cepa against Staphylococcus aureus. World J. Microbiol. Biotechnol. 2021, 37, 20.
- Aguilar-Pérez, C.; Gracia, B.; Rodrigues, L.; Vitoria, A.; Cebrián, R.; Deboosère, N.; Song, O.-R.; Brodin, P.; Maqueda, M.; Aínsa, J.A. Synergy between Circular Bacteriocin AS-48 and Ethambutol against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e00359-18.
- Behrens, H.M.; Six, A.; Walker, D.; Kleanthous, C. The therapeutic potential of bacteriocins as protein antibiotics. Emerg. Top. Life Sci. 2017, 1, 65–74.
- Behrens, H.M.; Six, A.; Walker, D.; Kleanthous, C. The therapeutic potential of bacteriocins as protein antibiotics. Emerg. Top. Life Sci. 2017, 1, 65–74.




