
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Reyad Dada | -- | 2806 | 2023-07-20 17:31:41 | | | |
| 2 | Jessie Wu | + 145 word(s) | 2951 | 2023-07-21 05:56:13 | | | | |
| 3 | Jessie Wu | + 6 word(s) | 2957 | 2023-07-21 07:06:46 | | | | |
| 4 | Jessie Wu | Meta information modification | 2957 | 2023-07-21 07:08:27 | | |
Video Upload Options
The treatment paradigms for patients with relapsed large B-cell lymphoma are expanding. Chimeric antigen receptor technology (CAR-T) has revolutionized the management of these patients. Novel bispecific antibodies and antibody–drug conjugates, used as chemotherapy-free single agents or in combination with other novel therapeutics, have been quickly introduced into the real-world setting. With such a paradigm shift, patients have an improved chance of better outcomes with unpredictable complete remission rates.
1. Introduction
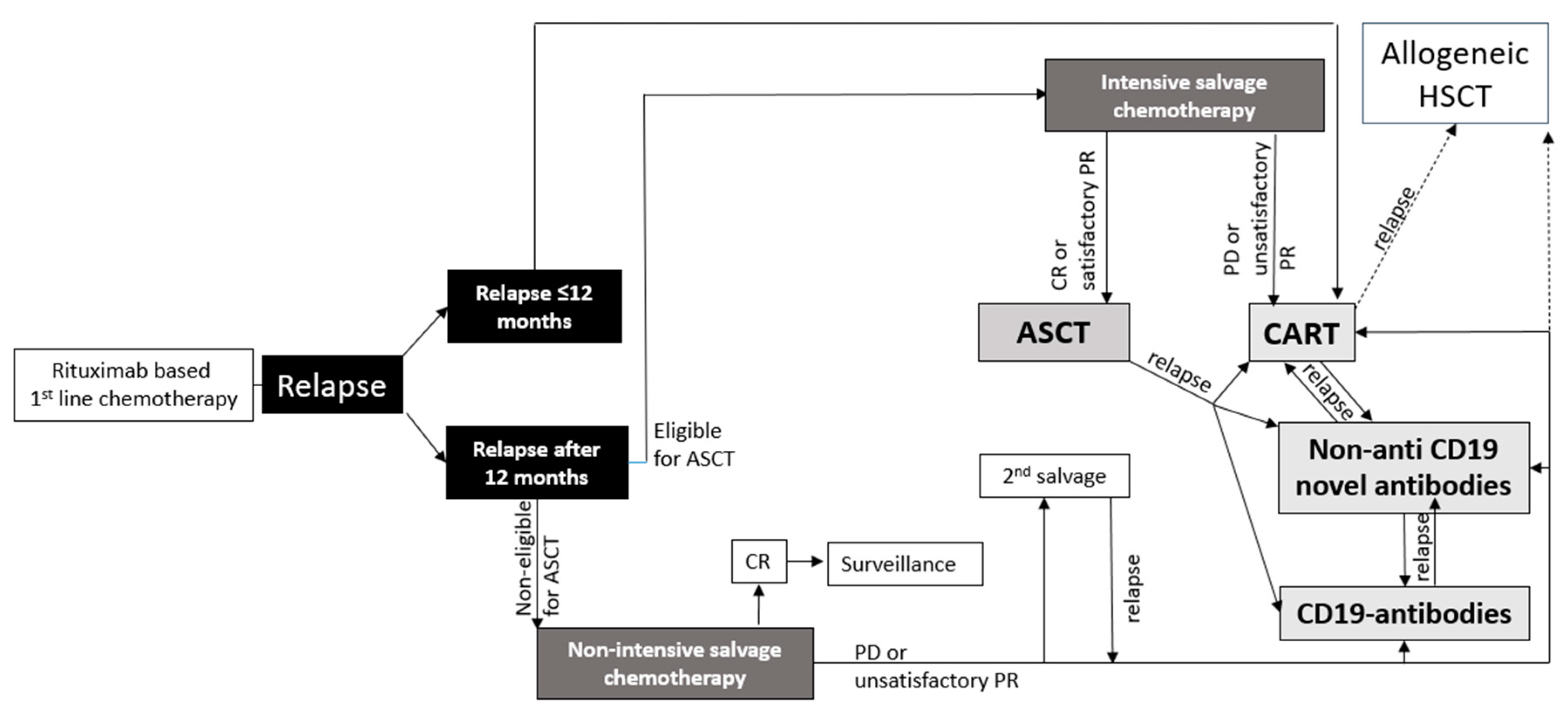
2. First Salvage Treatment Options in r/r Large B-cell Lymphoma
3. Novel Antibodies
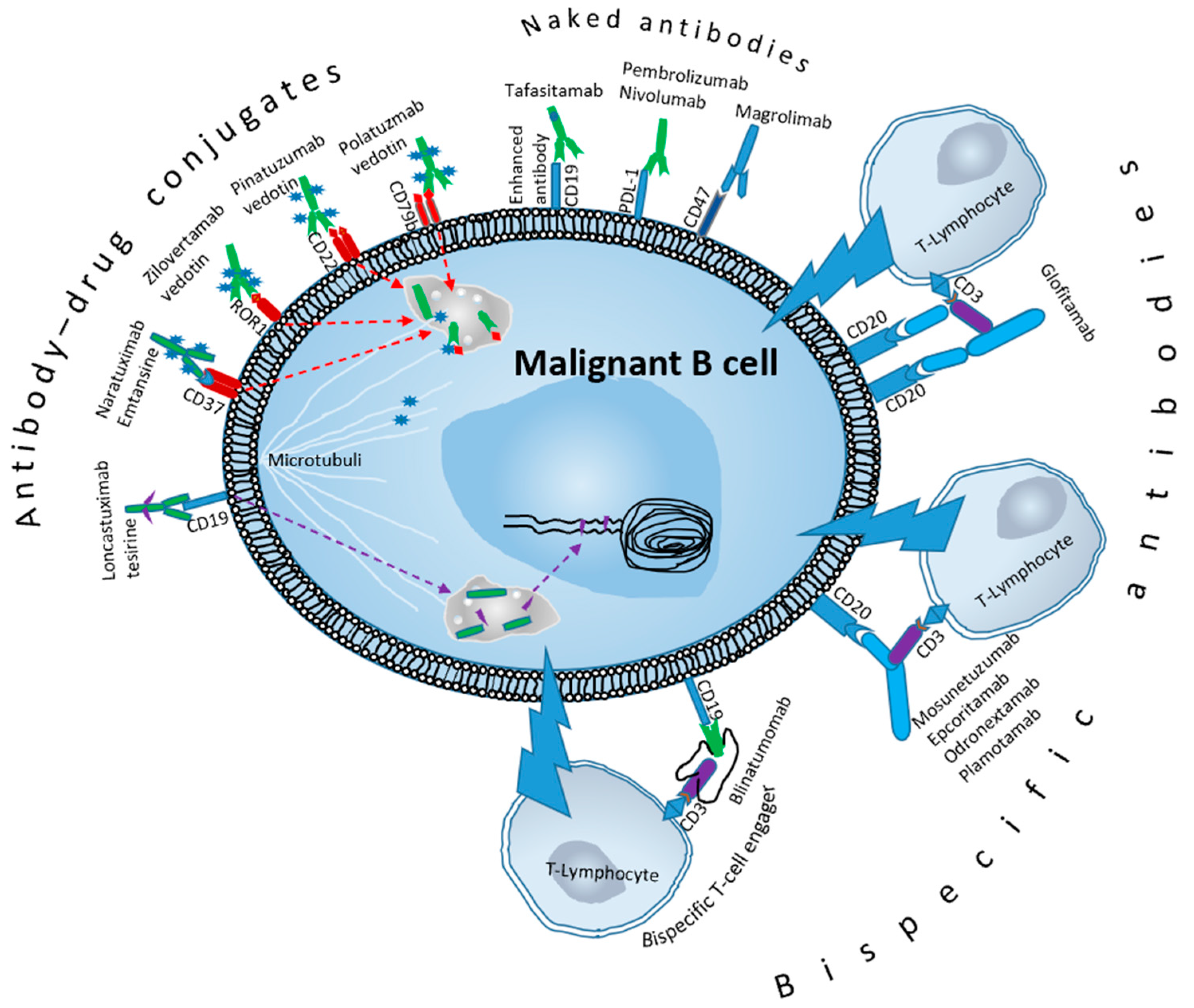
4. Tafasitamab
5. Checkpoint Inhibitors
6. Magrolimab
7. Antibody–Drug Conjugates
8. Polatuzumab Vedotin
9. Pinatuzumab Vedotin
10. Naratuximab Emtansine
11. Zilovertamab Vedotin
12. Loncastuximab Tesirine
13. Bispecific Antibodies (BsAbs) and Bispecific T-Cell Engagers (BiTE)
14. Glofitamab
15. Mosunetuzumab
16. Epcoritamab
17. Odronextamab
18. Blinatumomab
19. Resistance Mechanisms, Challenges, and Future Perspectives
To date, our understanding of the mechanisms underlying resistance to novel antibodies is limited due to the paucity of available research in this area. However, recent data, primarily consisting of those from preclinical studies, suggest that LBCL cells have the ability to evade the effects of novel antibodies including ADCs, BsAbs, and BiTEs through various mechanisms (Table 1).
| Challenge | Possible Future Management | |
|---|---|---|
| ADCs | Loss of CD79 b expression | BsAbs |
| Overexpression of efflux pumps | Drug efflux pump inhibitors | |
| Activation of compensatory signaling pathways | Novel payloads | |
| Downregulate immune checkpoint proteins or upregulate inhibitory proteins | Combination with checkpoint inhibitors | |
| Hypoxia and vascularization | HIF-1α inhibitors | |
| Neurological AES | Inhibitors of nicotinamide phosphoribosyl-transferase | |
| BsAbs and naked antibodies | Shedding of CD20 | Pretreatment with anti-CD20-directed therapy |
| Loss of CD20 | ADCs | |
| Hypoxia and vascularization | HIF-1α inhibitors | |
| P53 mutation | MDM2 inhibitor | |
| Downregulation of CD3 | Combination with checkpoint inhibitors | |
| ICANS | Lenzilumab | |
| CRS | Tocilizumab | |
| Complement activation | Avoid type IgG1 and 3 and use IgG4/IgM |
For instance, in vitro, downregulation, loss of expression, or mutation in CD79b may reduce the efficacy of polatuzumab [39]. However, other in vitro studies in animals and humans found no strong correlation between a high density of expression of CD79b or CD22 and the cytotoxic activity of ADCs, suggesting that there may be other factors than target overexpression that play a role in conferring sensitivity to ADCs [40][41]. Another way of escaping ADCs is the development of efflux pumps that can expel various toxic compounds. The overexpression of the multidrug resistance pump (MDR1) can reduce the effectiveness of payloads such as MMAE, resulting in resistance of lymphoma cells. This has led to investigations into several drug efflux pump inhibitors to address this issue [42]. Furthermore, activation of compensatory signaling pathways that bypass the targeted pathway reduces the effectiveness of ADCS. Some DLBCL cells may downregulate immune checkpoint proteins or upregulate inhibitory proteins, making them resistant to immune-mediated cytotoxicity induced by the novel antibody [25]. Resistance can also arise from the tumor microenvironment through processes such as hypoxia and vascularization, which can restrict the penetration and distribution of ADCs in lymphoma tissue.
References
- Li, W. The 5th Edition of the World Health Organization Classification of Hematolymphoid Tumors. In Leukemia; Chapter 1; Exon Publications: Brisbane, Australia, 2022.
- Morton, L.M.; Wang, S.S.; Devesa, S.S.; Hartge, P.; Weisenburger, D.D.; Linet, M.S. Lymphoma Incidence Patterns by WHO Subtype in the United States, 1992–2001. Blood 2006, 107, 265–276.
- Kanas, G.; Ge, W.; Quek, R.G.W.; Keeven, K.; Nersesyan, K.; Arnason, J.E.A. Epidemiology of Diffuse Large B-Cell Lymphoma (DLBCL) and Follicular Lymphoma (FL) in the United States and Western Europe: Population-Level Projections for 2020–2025. Leuk. Lymphoma 2022, 63, 54–63.
- Epperla, N.; Vaughn, J.L.; Othus, M.; Hallack, A.; Costa, L.J. CHOP-like Chemotherapy plus Rituximab versus CHOP-like Chemotherapy Alone in Young Patients with Good-Prognosis Diffuse Large-B-Cell Lymphoma: A Randomised Controlled Trial by the MabThera International Trial (MInT) Group. Cancer Med. 2020, 9, 5519–5525.
- Maddocks, K. Update on Mantle Cell Lymphoma. Blood 2018, 132, 1647–1656.
- Schuster, S.J.; Tam, C.S.; Borchmann, P.; Worel, N.; McGuirk, J.P.; Holte, H.; Waller, E.K.; Jaglowski, S.; Bishop, M.R.; Damon, L.E.; et al. Long-Term Clinical Outcomes of Tisagenlecleucel in Patients with Relapsed or Refractory Aggressive B-Cell Lymphomas (JULIET): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2021, 22, 1403–1415.
- Pfreundschuh, M.; Trümper, L.; Österborg, A.; Pettengell, R.; Trneny, M.; Imrie, K.; Ma, D.; Gill, D.; Walewski, J.; Zinzani, P.L.; et al. CHOP-like Chemotherapy plus Rituximab versus CHOP-like Chemotherapy Alone in Young Patients with Good-Prognosis Diffuse Large-B-Cell Lymphoma: A Randomised Controlled Trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006, 7, 379–391.
- Vercellino, L.; Di Blasi, R.; Kanoun, S.; Tessoulin, B.; Rossi, C.; D’Aveni-Piney, M.; Obéric, L.; Bodet-Milin, C.; Bories, P.; Olivier, P.; et al. Predictive Factors of Early Progression after CAR T-Cell Therapy in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood Adv. 2020, 4, 5607–5615.
- Wilson, W.H.; Young, R.M.; Schmitz, R.; Yang, Y.; Pittaluga, S.; Wright, G.; Lih, C.J.; Williams, P.M.; Shaffer, A.L.; Gerecitano, J.; et al. Targeting B Cell Receptor Signaling with Ibrutinib in Diffuse Large B Cell Lymphoma. Nat. Med. 2015, 21, 922–926.
- Zinzani, P.L.; Rigacci, L.; Cox, M.C.; Devizzi, L.; Fabbri, A.; Zaccaria, A.; Zaja, F.; Di Rocco, A.; Rossi, G.; Storti, S.; et al. Lenalidomide Monotherapy in Heavily Pretreated Patients with Non-Hodgkin Lymphoma: An Italian Observational Multicenter Retrospective Study in Daily Clinical Practice. Leuk. Lymphoma 2015, 56, 1671–1676.
- Goy, A.; Ramchandren, R.; Ghosh, N.; Munoz, J.; Morgan, D.S.; Dang, N.H.; Knapp, M.; Delioukina, M.; Kingsley, E.; Ping, J.; et al. Ibrutinib plus Lenalidomide and Rituximab Has Promising Activity in Relapsed/Refractory Non–Germinal Center B-Cell–like DLBCL. Blood 2019, 134, 1024–1036.
- Witzig, T.E.; Reeder, C.B.; Laplant, B.R.; Gupta, M.; Johnston, P.B.; Micallef, I.N.; Porrata, L.F.; Ansell, S.M.; Colgan, J.P.; Jacobsen, E.D.; et al. A Phase II Trial of the Oral MTOR Inhibitor Everolimus in Relapsed Aggressive Lymphoma. Leukemia 2011, 25, 341–347.
- Van Imhoff, G.W.; McMillan, A.; Matasar, M.J.; Radford, J.; Ardeshna, K.M.; Kuliczkowski, K.; Kim, W.S.; Hong, X.; Goerloev, J.S.; Davies, A.; et al. Ofatumumab versus Rituximab Salvage Chemoimmunotherapy in Relapsed or Refractory Diffuse Large B-Cell Lymphoma: The ORCHARRD Study. J. Clin. Oncol. 2017, 35, 544–551.
- Vitolo, U.; Trneny, M.; Belada, D.; Burke, J.M.; Carella, A.M.; Chua, N.; Abrisqueta, P.; Demeter, J.; Flinn, I.; Hong, X.; et al. Obinutuzumab or Rituximab plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large b-Cell Lymphoma. J. Clin. Oncol. 2017, 35, 3529–3537.
- Bailly, S.; Cartron, G.; Chaganti, S.; Córdoba, R.; Corradini, P.; Düll, J.; Ferrarini, I.; Osborne, W.; Rosenwald, A.; Sancho, J.M.; et al. Targeting CD19 in Diffuse Large B-Cell Lymphoma: An Expert Opinion Paper. Hematol. Oncol. 2022, 40, 505–517.
- Cooper, L.J.N.; Al-Kadhimi, Z.; DiGiusto, D.; Kalos, M.; Colcher, D.; Raubitschek, A.; Forman, S.J.; Jensen, M.C. Development and Application of CD19-Specific T Cells for Adoptive Immunotherapy of B Cell Malignancies. Blood Cells Mol. Dis. 2004, 33, 83–89.
- Pytlik, R.; Polgarova, K.; Karolova, J.; Klener, P. Current Immunotherapy Approaches in Non-Hodgkin Lymphomas. Vaccines 2020, 8, 708.
- Smith, S.D.; Fromm, J.R.; Fang, M.; Till, B.G.; Shadman, M.; Lynch, R.C.; Cowan, A.J.; Wu, Q.V.; Voutsinas, J.; Rasmussen, H.A.; et al. Pembrolizumab with R-CHOP in Previously Untreated Diffuse Large B-Cell Lymphoma: Long Term Follow up and Analysis of the Mechanism of Pdl-1 Tumor Expression. Blood 2020, 136, 13–14.
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.G.M.; Reddy, N.; et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J. Clin. Oncol. 2019, 37, 481–489.
- Frigault, M.J.; Armand, P.; Redd, R.A.; Jeter, E.; Merryman, R.W.; Coleman, K.C.; Herrera, A.F.; Dahi, P.; Nieto, Y.; LaCasce, A.S.; et al. PD-1 Blockade for Diffuse Large B-Cell Lymphoma after Autologous Stem Cell Transplantation. Blood Adv. 2020, 4, 122–126.
- Armand, P.; Rodig, S.; Melnichenko, V.; Thieblemont, C.; Bouabdallah, K.; Tumyan, G.; Özcan, M.; Portino, S.; Fogliatto, L.; Caballero, M.D.; et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma. Proc. J. Clin. Oncol. 2019, 37, 3291.
- Zinzani, P.L.L.; Thieblemont, C.; Melnichenko, V.; Bouabdallah, K.; Waleswski, J.; Majlis, A.; Fogliatto, L.; Martin Garcia-Sancho, A.; Christian, B.; Gulbas, Z.; et al. Final Analysis of Keynote-170: Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma (PMBCL). Blood 2021, 138, 306.
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713.
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.; van Rooijen, N.; Weissman, I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 2009, 138, 286–299.
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal. Transduct. Target. Ther. 2022, 7, 93.
- Morschhauser, F.; Flinn, I.W.; Advani, R.; Sehn, L.H.; Diefenbach, C.; Kolibaba, K.; Press, O.W.; Salles, G.; Tilly, H.; Chen, A.I.; et al. Polatuzumab Vedotin or Pinatuzumab Vedotin plus Rituximab in Patients with Relapsed or Refractory Non-Hodgkin Lymphoma: Final Results from a Phase 2 Randomised Study (ROMULUS). Lancet Haematol. 2019, 6, e254–e265.
- Advani, R.H.; Lebovic, D.; Chen, A.; Brunvand, M.; Goy, A.; Chang, J.E.; Hochberg, E.; Yalamanchili, S.; Kahn, R.; Lu, D.; et al. Phase i Study of the Anti-CD22 Antibody-Drug Conjugate Pinatuzumab Vedotin with/without Rituximab in Patients with Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma. Clin. Cancer Res. 2017, 23, 1167–1176.
- Levy, M.Y.; Grudeva-Popova, Z.; Trneny, M.; Jurczak, W.; Pylypenko, H.; Jagadeesh, D.; Andre, M.; Nasta, S.; Rechavi-Robinson, D.; Toffanin, S.; et al. Safety and Efficacy of CD37-Targeting Naratuximab Emtansine Plus Rituximab in Diffuse Large B-Cell Lymphoma and Other Non-Hodgkin’s B-Cell Lymphomas—A Phase 2 Study. Hematol. Oncol. 2021, 39.
- Wang, M.; Mei, M.; Barr, P.M.; Barrientos, J.; de Vos, S.; Furman, R.; Patel, K.; Thompson, P.A.; Choi, M.; Kallam, A.; et al. Phase 1 Dose Escalation and Cohort Expansion Study of the Anti-ROR1 Antibody-Drug Conjugate Zilovertamab Vedotin (MK-2140) for the Treatment of Non-Hodgkin Lymphoma. Blood 2021, 138, 528.
- Caimi, P.F.; Ai, W.; Alderuccio, J.P.; Ardeshna, K.M.; Hamadani, M.; Hess, B.; Kahl, B.S.; Radford, J.; Solh, M.; Stathis, A.; et al. Loncastuximab Tesirine in Relapsed or Refractory Diffuse Large B-Cell Lymphoma (LOTIS-2): A Multicentre, Open-Label, Single-Arm, Phase 2 Trial. Lancet Oncol. 2021, 22, 790–800.
- Hartley, J.A.; Flynn, M.J.; Bingham, J.P.; Corbett, S.; Reinert, H.; Tiberghien, A.; Masterson, L.A.; Antonow, D.; Adams, L.; Chowdhury, S.; et al. Pre-Clinical Pharmacology and Mechanism of Action of SG3199, the Pyrrolobenzodiazepine (PBD) Dimer Warhead Component of Antibody-Drug Conjugate (ADC) Payload Tesirine. Sci. Rep. 2018, 8, 10479.
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N. Engl. J. Med. 2011, 365, 725–733.
- Thakur, A.; Huang, M.; Lum, L.G. Bispecific Antibody Based Therapeutics: Strengths and Challenges. Blood Rev. 2018, 32, 339–347.
- Bröske, A.M.E.; Korfi, K.; Belousov, A.; Wilson, S.; Ooi, C.H.; Bolen, C.R.; Canamero, M.; Alcaide, E.G.; James, I.; Piccione, E.C.; et al. Pharmacodynamics and Molecular Correlates of Response to Glofitamab in Relapsed/Refractory Non-Hodgkin Lymphoma. Blood Adv. 2022, 6, 1025–1037.
- Kaplon, H.; Crescioli, S.; Chenoweth, A.; Visweswaraiah, J.; Reichert, J.M. Antibodies to Watch in 2023. MAbs 2023, 15, 2153410.
- Salvaris, R.; Ong, J.; Gregory, G.P. Bispecific Antibodies: A Review of Development, Clinical Efficacy and Toxicity in B-Cell Lymphomas. J. Pers. Med. 2021, 11, 355.
- Phillips, T.; Thieblemont, C.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.A.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab Monotherapy Provides Deep and Durable Responses Including Minimal Residual Disease (MRD) Negativity: Novel Subgroup Analyses in Patients with Relapsed/Refractory (R/R) Large B-Cell Lymphoma (LBCL). Blood 2022, 140, 9443–9445.
- Einsele, H.; Borghaei, H.; Orlowski, R.Z.; Subklewe, M.; Roboz, G.J.; Zugmaier, G.; Kufer, P.; Iskander, K.; Kantarjian, H.M. The BiTE (Bispecific T-Cell Engager) Platform: Development and Future Potential of a Targeted Immuno-Oncology Therapy across Tumor Types. Cancer 2020, 126, 3192–3201.
- Dornan, D.; Bennett, F.; Chen, Y.; Dennis, M.; Eaton, D.; Elkins, K.; French, D.; Go, M.A.T.; Jack, A.; Junutula, J.R.; et al. Therapeutic Potential of an Anti-CD79b Antibody-Drug Conjugate, Anti-CD79b-vc-MMAE, for the Treatment of Non-Hodgkin Lymphoma. Blood 2009, 114, 2721–2729.
- Li, D.; Poon, K.A.; Yu, S.F.; Dere, R.; Go, M.A.; Lau, J.; Zheng, B.; Elkins, K.; Danilenko, D.; Kozak, K.R.; et al. DCDT2980S, an Anti-CD22-Monomethyl Auristatin E Antibody-Drug Conjugate, Is a Potential Treatment for Non-Hodgkin Lymphoma. Mol. Cancer Ther. 2013, 12, 1255–1265.
- Pfeifer, M.; Zheng, B.; Erdmann, T.; Koeppen, H.; Mccord, R.; Grau, M.; Staiger, A.; Chai, A.; Sandmann, T.; Madle, H.; et al. Anti-CD22 and Anti-CD79B Antibody Drug Conjugates Are Active in Different Molecular Diffuse Large B-Cell Lymphoma Subtypes. Leukemia 2015, 29, 1578–1586.
- Cheema, Y.; Kiani, Y.S.; Linton, K.J.; Jabeen, I. Identification and Empiric Evaluation of New Inhibitors of the Multidrug Transporter P-Glycoprotein (ABCB1). Int. J. Mol. Sci. 2023, 24, 5298.




