Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adnan Rasheed | -- | 3846 | 2023-07-20 12:43:42 | | | |
| 2 | Dean Liu | -3 word(s) | 3843 | 2023-07-21 02:47:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rasheed, A.; Jie, H.; He, P.; Lv, X.; Ali, B.; Ma, Y.; Xing, H.; Almari, S.; Elnour, R.O.; Hassan, M.U.; et al. Improvement of Cd Stress Tolerance in Ramie Crop. Encyclopedia. Available online: https://encyclopedia.pub/entry/47042 (accessed on 07 February 2026).
Rasheed A, Jie H, He P, Lv X, Ali B, Ma Y, et al. Improvement of Cd Stress Tolerance in Ramie Crop. Encyclopedia. Available at: https://encyclopedia.pub/entry/47042. Accessed February 07, 2026.
Rasheed, Adnan, Hongdong Jie, Pengliang He, Xueying Lv, Basharat Ali, Yushen Ma, Hucheng Xing, Saad Almari, Rehab O. Elnour, Muhammad Umair Hassan, et al. "Improvement of Cd Stress Tolerance in Ramie Crop" Encyclopedia, https://encyclopedia.pub/entry/47042 (accessed February 07, 2026).
Rasheed, A., Jie, H., He, P., Lv, X., Ali, B., Ma, Y., Xing, H., Almari, S., Elnour, R.O., Hassan, M.U., Gillani, S.F.A., & Jie, Y. (2023, July 20). Improvement of Cd Stress Tolerance in Ramie Crop. In Encyclopedia. https://encyclopedia.pub/entry/47042
Rasheed, Adnan, et al. "Improvement of Cd Stress Tolerance in Ramie Crop." Encyclopedia. Web. 20 July, 2023.
Copy Citation
Cadmium (Cd) is a non-essential, highly phytotoxic metal and damages ramie plant growth and development even at low concentrations. Ramie is one of the most significant crops in China, with excellent fiber quality and immense industrial importance. Planting Cd-tolerant ramie cultivars can prevent yield loss on contaminated soil. Previously, significant efforts have been made to develop Cd tolerance in ramie.
ramie
Cd stress
tolerance
genes
marker-assisted selection
CRISPR/Cas9
1. Introduction
The significant rise in levels of heavy metal ions such as Cd, arsenic (As), and lead (Pb) in soils poses a constant threat to human and plant life [1][2][3][4]. These metals are naturally present in our environment in low concentrations [5][6]. Human-caused activities are a significant cause of the rising concentration of heavy metals in soil [7][8][9][10]. Cd stress is the most devastating abiotic stress that has led to the huge loss of crop growth and yields in large areas. Cd enters the food chain and causes an imbalance in food security [11][12]. Cd toxicity is a significant threat to crops. It is toxic even at low concentrations [13] and has no biological role in crop growth and development [14]. Anthropogenic activities include the atmospheric deposition of combustion emissions, mining, sewage sludge, and Cd-containing fertilizers [15]. Cd is ranked seventh in the top twenty toxic heavy metals list and is classified as a group 1 carcinogen [16]. Cd2+ is a divalent form of Cd in soils at concentrations typically ranging from 0.1–1.0 mg kg−1 [17]. Cd stress interferes with crops’ morphological, physiological, and biochemical traits. Cd stress mainly inhibits seed germination, reducing total plant weight, the number of leaves, and root length, ultimately leading to total plant death [18]. It encourages osmotic stress in plants by reducing leaf-relative water content, transpiration, and stomatal conductance [19][20]. Cd stress reduces the uptake of essential nutrients and leads to inappropriate plant growth [21][22]. Ramie is called China grass, and it is considered the most critical fiber crop next to cotton and is characterized by its rapid growth, shoot biomass, and excellent root system [23]. Ramie can be harvested three times a year. Ramie is mainly cultivated in China, India, and Pacific Rim countries. Ramie cultivation dates back to 5000 years ago in Southern China, with a high fiber yield next to cotton [23]. Toxic levels of Cd have led to a reduction in root length, shoot length, fiber yield, and enzymatic activities [12][24]. Meanwhile, it also reduces the chlorophyll contents, plant water relation, nutrients uptake, biomass, quality, protein contents, and damage to DNA [25]. Cd pollution is more terrible in the Hunan Province of China than in most parts of China [26]. China has small arable land per capita, but the food demand is too high and requires the effective and safe utilization of heavy metals-contaminated arable land to sustain food security. In recent years, numerous studies have focused on safe utilization, including soil improvement, selection, breeding varieties with low capacity for the absorption of heavy metals, and agronomic management [27].
Previous studies have revealed that ramie crop has robust tolerance to Cd stress and can accumulate Cd ions from soil; hence, the use of ramie to prevent the Cd contamination of agricultural soils is of practical significance [27][28][29]. More significantly, ramie can accumulate a comparatively large number of heavy metals in its above-ground portion [30]. Ramie is a relatively tolerant crop against heavy metals stress, especially Cd stress, as it absorbs Cd from heavy metals-contaminated soils [27][30][31]. An earlier study showed that ramie has a certain degree of tolerance to soil Cd stress at a concentration of ≤20 mg/kg [32]. Ma et al. [33] treated the two ramie cultivars (Dazhuhuangbaima and Zhongzhu 1) with different Cd levels of 0, 25, and 75 mg kg−1 for 30 days and concluded that Zhongzhu 1 had a higher degree of tolerance to Cd stress, as indicated by Cd enrichment in all organs and Cd content was mainly observed in the cell wall of both varieties. In this regard, breeders have employed conventional breeding techniques to enhance the Cd tolerance in ramie [34]. Still, the complex genetic mechanism of Cd tolerance hindered the expansion of these techniques. On the other hand, hormonal applications significantly mediated ramie growth under Cd stress [35]. Previous research studies have shown that ramie has a potential tolerance against Cd stress, which can be exploited to develop Cd-tolerant cultivars; however, there is still limited information about the complete genetic mechanism of ramie.
2. Effects of Cd Stress and Genetic Mechanisms
In plants, Cd toxicity causes leaf chlorosis, reduced growth rates, respiration and photosynthesis, enhanced oxidative damage, and decreased nutrient uptake capability [36][37]. Ramie’s growth and development are badly affected by Cd stress. In previous studies, different ramie genotypes were evaluated to assess the effects of Cd stress. Cd stress affected the plant height, shoot dry weight, and root dry weight [35][38]. Ramie seedlings were subjected to Cd stress (3 and 7 mg1−1) for 10 days to investigate its effects on lipid membrane and chlorophyll synthesis. Cd stress-induced lipid peroxidation, as indicated by an enhanced malondialdehyde content (MDA) in leaves and roots. The prolonged exposure of ramie to Cd stress decreased antioxidant enzyme activity [24]. Zhu et al. [39] studied the effects of Cd stress on the growth parameters of different ramie cultivars. They concluded that Cd stress negatively affects the above-ground raw tissues and fiber yield [39]. Another ramie cultivar, Chuanzhu, was treated with Cd stress at a concentration of 50 mg/L, and it was noticed that plant height (PH), root length (RL), and biomass were reduced [40]. Higher Cd concentration reduces photosynthesis (Figure 1) and affects the reproductive stage in ramie [41]. Cd stress caused sugar degradation, the inactivation of antioxidant enzyme activity, protein oxidation, and growth inhibition in ramie [42]. Increasing Cd concentration affected the stem, leaves, and internal cell organs in ramie [43]. As mentioned earlier, Cd is highly toxic to ramie even at low concentrations. Some of the micro-regional studies showed that the ramie cultivar is significantly affected by Cd stress at 25 mg kg−1 concentration. Cd retained in stems and leaves greatly reduced the plant biomass [44]. Although several studies have indicated the toxic effects of Cd stress on ramie crops, there is still a need to expose the plant to extreme Cd stress to investigate the effects on biochemical and molecular functions. These studies would help researchers to adopt a better protective mechanism to counter the adverse effects of Cd stress through the screening and training of genotypes [45].
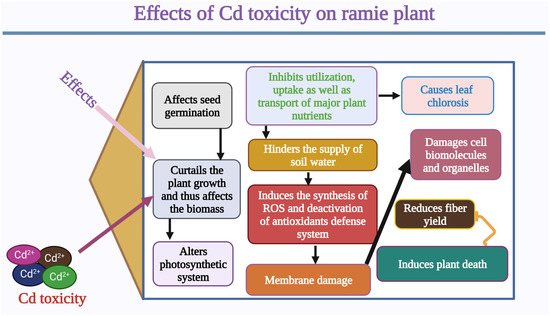
Figure 1. Effects of Cd toxicity on ramie growth and development. Cd toxicity leads to a reduction in seed germination, seedling growth, biomass, and photosynthesis. Cd toxicity reduces the uptake of nutrients and water. In the same way, Cd toxicity induces the production of reactive oxygen species (ROS) and reduces the activity of antioxidant enzymes. Cd toxicity causes damage to membranes and biomolecules. Cd toxicity reduces yield and causes plant death. This Figure is created with Biorender.com.
In the last decade, numerous studies have focused on the Cd tolerance mechanisms at the physiological, biochemical, and molecular levels [46][47]. Transcriptome and proteomics approaches have been adopted to understand the molecular factors involved in Cd tolerance [48][49]. Tolerance to Cd stress in ramie is a complex genetic mechanism controlled by many genes [45]. The Cd tolerance in ramie varies among the genotypes and germplasm. Ying et al. [50] evaluated the 269 ramie germplasm resources under the Cd stress condition. The results indicated a significant difference among the ramie germplasm regarding Cd accumulation and transport. The data showed the different genetic factors involved in ramie tolerance to Cd stress, which can be analyzed using molecular breeding tools rather than conventional tools [50]. Plants also have varied and complex defensive systems for resisting Cd stress [51].
Moreover, antioxidant enzymes, ascorbate peroxidase (APX), superoxide dismutase (SOD), and catalase (CAT) are critical for scavenging ROS [52][53][54]. The complex genetic mechanism of Cd tolerance cannot be unfolded using conventional breeding methods because of their time-consuming and costly nature [55][56][57]. Despite all these efforts, the Cd-tolerant nature of ramie has not been thoroughly studied. The effects of Cd stress on fiber quality and genetic material should be further investigated to avoid potential loss. Hence, it would be better to make a complete genome sequence of ramie germplasm to characterize all possible genes and proteins involved in Cd tolerance in ramie and use them in Cd stress breeding programs. In addition to this, germplasm with Cd-tolerant ability can be fully exploited for Cd-tolerant genotype development. The activation of systematic resistance plays a crucial role in countering Cd stress in ramie and can be enhanced using Cd stress conditions. Investigations of the morphological, physiological, and biochemical mechanisms of Cd tolerance are not fully understood and need further studies to identify the potential traits involved in Cd tolerance in ramie.
3. Agronomic Approaches to Enhance Cd Tolerance in Ramie
Plant hormones or growth regulators play a crucial role in defense response against heavy metals (HMs) stress, especially Cd stress [58][59] (Figure 2). Each hormone’s mechanism of action may differ for each crop [60]. It has been determined that multiple hormones may regulate a single process, and simultaneously, different processes are controlled by a single hormone [61]. Plant hormones such as auxin, abscisic acid (ABA), cytokinin (CK), ethylene (ET), and jasmonic acid (JA) are essential plant hormones that are vital for plant growth and development, and are also involved in crosstalk with other hormones [62][63][64]. Hormones in very low concentrations control cell membrane permeability, secondary metabolites, and growth, as well as the reproduction of plants [65][66]. Cd stress retards plant growth and accumulates in below- and above-ground parts of the plant. Cd stress causes a delay in seed germination, and seeds may vary in their response [67]. Plants react to heavy Cd stress depending on the concentration of Cd stress. The role of these hormones has been investigated in many crops, including ramie, which has the ability of Cd phytoextraction [68].
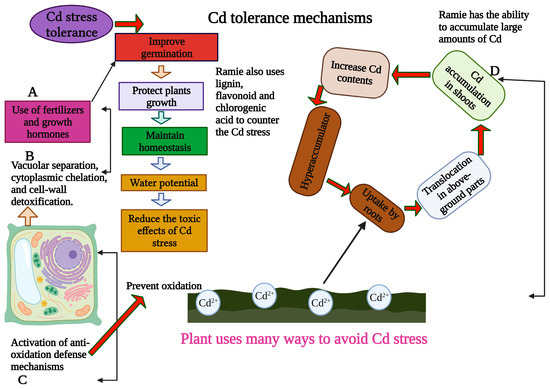
Figure 2. The ways to prevent Cd stress in ramie. The plant mainly prevents Cd stress by detoxification and chelation. The ramie plant also uses antioxidant defense mechanisms to avoid Cd stress. This Figure is created with Biorender.com.
ABA is a powerful hormone controlling plant tolerance to Cd stress in ramie. Chen et al. [69] conducted a study to investigate the effect of ABA on ramie under Cd stress. Directly adding ABA to the cultural solution promoted ramie growth and development under Cd stress, increased Cd enrichment, and indicated its regulatory role in enhancement or stress tolerance and as a potential agronomic agent [69]. Similarly, another research team led by He et al. [70] studied the effect of different growth regulators on the Cd content in ramie. The results showed that a foliar spray of hormones reduced the Cd content in underground parts of ramie, which showed the potential role of these growth regulators in increasing Cd tolerance [70].
Auxin is well known for its signaling function and plays a crucial role in life developments such as cell division, embryogenesis, meristem, and organ development [71][72]. Auxin plays a significant role in stress response, organ formation, and vascular development [73]. A recent study showed that Cd at a concentration of 500 mM had variable effects on hormonal function. The content of JA reduces, and ABA increases after exposure to Cd stress. Meanwhile, indole acetic acid (IAA) content decreases after increased Cd content in Koelreuteria paniculata [74]. Similarly, ABA content increases in soybean after exposure to Cd stress for 24 h and decreases after 140 h [75]. Chaca et al. [75] revealed that plants mainly produce JA and ABA hormones under Cd stress. Usually, phytohormones enhance plant tolerance to Cd due to their participation as signaling molecules and encourage plant growth by expressing different metabolites, genes, and pigments [76][77]. Earlier work has demonstrated that plant hormones regulate Cd tolerance in many crops, including rice [78], wheat [79], and Arabidopsis [80]. These crops respond to Cd stress by hormonal action. The priming of barley seed with GB3 reduces the toxic effects of Cd stress on coleoptile growth [81]. Gibberellin improves ramie growth, development, and metabolic processes [82][83]. The study indicated that brassinosteroids (BR) and gibberellin (GA) have different effects on the enrichment and transport of Cd in ramie. The results indicated that GA-1 enhanced the Cd content of the aboveground ramie to three times more than that of the control and decreased the Cd content of the underground ramie by 54.76%. Researchers offer an effective technique to advance the capability of ramie to adsorb heavy metals [70].
Furthermore, the pretreatment of seeds of maize with SA and IAA enhances the growth of coleoptiles under Cd stress [84]. In another study, SA at a concentration of 500 mM increased the antioxidant defense system [85] and mitigation of the influences of Cd stress on plant growth and development in maize [86]. It has also been shown that SA use reduces the Cd stress in roots [87]. In barley plants, SA curbs the consequences of Cd stress on organic reserves, inhibits the impairment of plant growth due to Cd, and triggers Cd tolerance mechanisms [88]. Kalai et al. [88] described that SA at concentration of 600 mM enhanced growth and development in barley seedlings and decreased Cd stress. These reports showed very little information about using hormones to mitigate Cd stress in crops. It is strongly recommended to conduct more studies to investigate the regulatory role of plant growth regulators in alleviating Cd toxicity in ramie to develop a suitable approach for Cd-contaminated soils, which are the primary habitat of ramie.
Spermidine is a polyamine compound situated in ribosomes and living tissues and has numerous metabolic roles within plants [89][90][91]. Spermidine has been a significant polyamine in mitigating Cd stress in many crops [92]. Ramie was used to investigate the Cd tolerance mechanism with various treatment durations and the effect of the up-regulation of antioxidant capability by spermidine treatment. The outcomes exhibited that short-term (0–7 days) Cd stress caused the enrichment of pigment content, soluble sugars, and the activation of antioxidants, temporarily reducing the level of MDA, the primary oxidative stress marker. Spd treatment noticeably improved the soluble sugar content and condensed glutathione (GSH) in ramie leaves after short-duration Cd stress, while it showed no beneficial effects on other antioxidants. Long-term (0–15 days) Cd stress may lead to growth retardation related to Cd increment, protein sugar degradation, a rise in MDA, and the inactivation of antioxidants. Spd treatment reduced long-term Cd toxicity by reducing Cd content, stabilizing cellular macromolecules such as protein and sugar, and preventing the inactivation of catalase (CAT) and peroxidase (POD). These findings showed that Spd uses could be dynamic in the Cd tolerance’s rise by regulating different traits [42].
Another study used the combined exogenous application of Spd and calcium (Ca) to study the effects on Cd stress in ramie. The results exhibited that using 5 mM Ca meaningfully reduced Cd toxicity in ramie by reducing Cd content, discouraging H2O2 and MDA content, enhancing plants’ dry weight and chlorophyll contents, and changing the actions of total superoxide dismutase (SOD). Additionally, ramie accumulated Cd and was affected by more toxic effects of Cd stress by applying 1 mM Ca or Cd. Severe Cd toxicity could be reduced by adding exogenous Spd via a surge of plant growth and the decrease of the Cd-induced oxidative stress. Generally, the effects of 1 mM Ca and Spd seemed greater than other treatments in the ramie plants under Cd stress, with a more excellent Cd accumulation capability. They assessed Cd stress tolerance in ramie [93]. Sun et al. [34] studied the effects and mechanisms of IAA and Spd on Cd accumulation in ramie to broaden the understanding of Cd tolerance. The results showed that the application of Spd and IAA increased the activity of antioxidant enzymes. IAA and Spd application could meaningfully reduce the oxidative stress encouraged by Cd in ramie crop. Hence, the application of Spd, IAA, and GA can be combined to increase the Cd tolerance in ramie crops [34].
Selenium (Se) is one of the most potent metalloids widely used to enhance crop tolerance to Cd stress. Se has also been used in ramie to mitigate the toxic effects of Cd stress. Ramie plants were grown in hydroponic conditions and were separately or instantaneously treated with Se spray, 1.2 μmol/L, and Cd stress 6 and 9 mg/L. At low Se levels, the activity of SOD was improved by 35.34% under 6 or 9 mg/L Cd stress, POD was improved by 12.45%, and the level of DNA methylation was reduced by 10.70%, correspondingly. The results established that low Se-level spray on ramie leaves could improve the action of POD and SOD, and control DNA methylation in ramie leaves. Hence, future studies must explore the effects of this hormone on functions of other antioxidants such as CAT and APX to improve the Cd tolerance in ramie [25]. In an earlier study, ethylenediaminedisuccinic acid (EDDS) and ethylenediaminetetraacetic acid (EDTA) were added to potted soil containing ramie plants. The chelants were found to decrease Cd content in roots by 15% and 12% in EDTA- and EDDS-treated plants. Hence, chelate-induced phytoremediation is an effective technique for the remediation of Cd-contaminated soils [94]. Similarly, in another study, the Cd accumulation and translocation mechanism in ramie was studied by adding ethylenediaminetetraacetic acid (EDTA) or nitrilotriacetic acid (NTA). The results demonstrated that EDTA and NTA could increase Cd Phyto-availability in soil, transport Cd from the roots to the aboveground tissues, and increase leaf Cd accumulation. Researchers highlight the control of Cd accumulation in ramie [95]. The above results indicate that more studies should be conducted to investigate the role of GA, CK, and ET in regulating Cd tolerance by enhancing the growth of ramie plants. These hormones can enhance the antioxidant defense system and prevent ramie growth under severe Cd stress conditions. Moreover, the hormonal application should be studied at different growth stages of ramie under Cd stress conditions.
4. Genetic Diversity for Cd Tolerance in Ramie
The breeding of genotypes tolerant to adverse growing environments is critical for ramie existence [96]. Hence, genetic diversity has been the foundation of plant breeding since the initial days of agriculture [97][98]. It helps plant breeders to develop new genotypes that can address grower requirements, acclimatize to climate variation, and meet the growing global food demand [99]. Researchers rely on dissimilarity in crop genetics, breeding methods, and approaches to integrate genetic assortment into cultivated genotypes [100]. Crop breeders exploit genetic diversity to breed new cultivars with improved traits such as resistance to biotic and abiotic stresses and to advance the nutritional value of foods for the world population [101][102]. Plant breeders have achieved the vital task of the planned incorporation of a new genetic assortment while protecting critical economic characteristics of crops [99]. Genetic diversity can be explained as a range of genetic traits in crop species. Genetic diversity can be measured by investigating changes in the DNA nucleotide sequences in a progeny of individuals [103][104][105]. Genetic diversity in crops gives rise to a population’s phenotypic differences and observable traits [106]. Crop genomes are naturally different, which causes more genetic and epigenetic variations in plants that help as the sources of a large volume of genetic and phenotypic diversity, even among the cultivars of the same species [107][108]. More considerable genetic diversity in crops gives plants an outstanding capability to acclimatize to sudden abiotic stresses [109][110].
The achievement of the crop improvement target depends on capably recognizing and integrating genetic diversity from numerous plant genetic sources comprising currently grown varieties [111], newly established varieties, wild and landraces of crops, and the collection of germplasm with elite plants [112]. Numerous genomic tools and breeding approaches have enhanced the competence and accuracy of integrating genetic diversity into cultivated crop varieties. However, plant breeding still remains a time- and resource-intensive procedure [113][114]. Ramie, a significant fiber crop [115], has genetic diversity, crucial in developing tolerant cultivars [116]. Genetic diversity is indispensable for breeding programs. Ramie has excellent potential for tolerance to Cd toxicity [50]. To assess genetic diversity for Cd tolerance, 269 ramie germplasm accessions were studied under Cd-contaminated soils and significant genetic diversity was observed among the studied germplasm [50]. Ramie has been best known for its phytoremediation capability for decades. The phytoremediation capability of ramie shows that it has considerable genetic diversity for Cd-tolerant genes, which can be exploited in breeding programs. Zhu et al. [117] showed that ramie could tolerate higher Cd concentrations (100 mg/kg), prevent significant economic loss, and safeguard crop security. Results showed that an extended period would be required to improve the soils affected by Cd toxicity. Other assortments and cultivations of Cd hyperaccumulator ramie are indispensable [117]. The first report on constitutional heavy metals tolerance was presented by Yang et al. [23]. They evaluated ramie in contaminated fields with various metals, including Cd, and revealed that ramie germplasm and genotypes performed better under contaminated conditions. These results determined that ramie holds a certain degree of constitutional Cd tolerance. This germplasm can be useful for increasing diversity and transforming tolerant genes to develop Cd-tolerant ramie cultivars [23].
The results presented significant variations in Cd tolerance; these germplasm resources can be exploited for other breeding programs [23]. Later, another hydroponic and field experiment was conducted to compare ramie’s superior genotypes and Cd tolerance indices. In the hydroponic culture experiment, considerable variances, and noteworthy genotypic differences in plant height (PH), leaf number per plant (LNP), shoot dry weight (SDW), and root dry weight (RDW) were observed under Cd treatments. The SDW was well associated with PH and RDW. The SDW was well associated with PH and bark thickness. This proposes that these features could be used as Cd tolerance indices in future studies [118]. She et al. [119] showed that Cd-tolerant ramie will be a great source of phytoremediation in Cd-contaminated soils. Cd contents in ramie were two to ten times higher compared to other plant species, as shown by the data collected from mining areas where the soil was heavily polluted with Cd and other metals. These ramie genotypes provide a more significant source of genetic diversity for Cd tolerance [119]. Zhu et al. [39] observed significant differences among the ramie varieties for Cd tolerance and accumulation. Results indicated that raw fiber yield and biological tissues of variety (Zhongzhu1) were higher than the other six varieties [39]. Several traits of ramie have been tested for Cd tolerance and accumulation. The significant difference in yield among the ramie cultivars showed more considerable genetic diversity and potential candidate genes for genetic engineering. The results obtained from a study indicated that the cultivar Zhongzhu No. 3 showed a high yield in Cd-contaminated soils. All varieties showed more than a 90% Cd removal ratio during the uncovering process of fibers [120]. The varieties such as Zhongzhu1 and Zhongzhu No. 3 should be used to develop new high-tolerant varieties for the phytoremediation of Cd-contaminated soils. The screening of all available germplasms of ramie to identify the potential Cd tolerant plant material for further breeding programs is critical.
Therefore, it is proposed that the screening of Cd-tolerant ramie genotypes would lead to the identification of tolerant genes for Cd-tolerant breeding programs. The screening ramie genotypes can be enhanced via breeding tools such as introduction, mass selection, and hybridization (Figure 3) to enhance Cd tolerance in the future when the crop faces more deadly threats of Cd toxicity. Molecular breeding tools are, however, more potent, and reliable in protecting the diversity and integrating the Cd-tolerant genes into elite cultivars. Researchers recommend collecting the ramie germplasm from different locations and screening it for its tolerance against Cd stress.
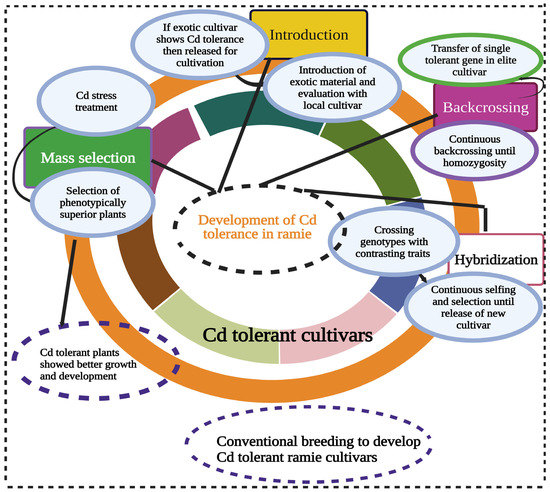
Figure 3. Developing Cd-tolerant ramie cultivar using conventional breeding methods such as introduction, mass selection, hybridization, and backcrossing. These breeding methods have great potential to develop Cd tolerance suitable for growth in Cd-contaminated soils. This Figure is created with Biorender.com.
References
- Liu, J.; Gai, L.; Zong, H. Foliage application of chitosan alleviates the adverse effects of cadmium stress in wheat seedlings (Triticum aestivum L.). Plant Physiol. Biochem. 2021, 164, 115–121.
- Guedes, F.R.C.M.; Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Alyemeni, M.N.; Ahmad, P.; da Silva Lobato, A.K. Exogenous 24-Epibrassinolide stimulates root protection, and leaf antioxidant enzymes in lead stressed rice plants: Central roles to minimize Pb content and oxidative stress. Environ. Pollut. 2021, 280, 116992.
- Neidhardt, H. Arbuscular mycorrhizal fungi alleviate negative effects of arsenic-induced stress on crop plants: A meta-analysis. Plants People Planet 2021, 3, 523–535.
- Zhao, Y.; Wang, J.; Huang, W.; Zhang, D.; Wu, J.; Li, B.; Li, M.; Liu, L.; Yan, M. Abscisic-Acid-Regulated Responses to Alleviate Cadmium Toxicity in Plants. Plants 2023, 12, 1023.
- Griffith, A.; Wise, P.; Gill, R.; Paukett, M.; Donofrio, N.; Seyfferth, A.L. Combined effects of arsenic and Magnaporthe oryzae on rice and alleviation by silicon. Sci. Total Environ. 2021, 750, 142209.
- Bhat, J.A.; Faizan, M.; Bhat, M.A.; Huang, F.; Yu, D.; Ahmad, A.; Bajguz, A.; Ahmad, P. Defense interplay of the zinc-oxide nanoparticles and melatonin in alleviating the arsenic stress in soybean (Glycine max L.). Chemosphere 2022, 288, 132471.
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth-Sci. Rev. 2017, 171, 621–645.
- Geng, N.; Wu, Y.; Zhang, M.; Tsang, D.C.; Rinklebe, J.; Xia, Y.; Lu, D.; Zhu, L.; Palansooriya, K.N.; Kim, K.-H. Bioaccumulation of potentially toxic elements by submerged plants and biofilms: A critical review. Environ. Int. 2019, 131, 105015.
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046.
- Kučerová, D.; Vivodová, Z.; Kollárová, K. Silicon alleviates the negative effects of arsenic in poplar callus in relation to its nutrient concentrations. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 145, 275–289.
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.; Sebastian, A.; Prasad, M.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2020, 52, 675–726.
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913.
- Liu, Z.; Wu, X.; Hou, L.; Ji, S.; Zhang, Y.; Fan, W.; Li, T.; Zhang, L.; Liu, P.; Yang, L. Effects of cadmium on transcription, physiology, and ultrastructure of two tobacco cultivars. Sci. Total Environ. 2023, 869, 161751.
- Manzoor, M.; Gul, I.; Kallerhoff, J.; Arshad, M. Fungi-assisted phytoextraction of lead: Tolerance, plant growth–promoting activities and phytoavailability. Environ. Sci. Pollut. Res. 2019, 26, 23788–23797.
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. App. Geochem. 2019, 108, 104388.
- Zulfiqar, U.; Ayub, A.; Hussain, S.; Waraich, E.A.; El-Esawi, M.A.; Ishfaq, M.; Ahmad, M.; Ali, N.; Maqsood, M.F. Cadmium toxicity in plants: Recent progress on morpho-physiological effects and remediation strategies. J. Soil Sci. Plant Nutrit. 2021, 22, 212–269.
- Smolders, E.; Brans, K.; Földi, A.; Merckx, R. Cadmium fixation in soils measured by isotopic dilution. Soil Sci. Soc. Amer. J. 1999, 63, 78–85.
- Astolfi, S.; Ortolani, M.R.; Catarcione, G.; Paolacci, A.R.; Cesco, S.; Pinton, R.; Ciaffi, M. Cadmium exposure affects iron acquisition in barley (Hordeum vulgare) seedlings. Physiol. Plant. 2014, 152, 646–659.
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53.
- Hussain, B.; Ashraf, M.N.; Abbas, A.; Li, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188.
- Chen, Q.; Lu, X.; Guo, X.; Pan, Y.; Yu, B.; Tang, Z.; Guo, Q. Differential responses to Cd stress induced by exogenous application of Cu, Zn or Ca in the medicinal plant Catharanthus roseus. Ecotoxicol. Environ. Saf. 2018, 157, 266–275.
- Liu, J.; Liang, J.; Li, K.; Zhang, Z.; Yu, B.; Lu, X.; Yang, J.; Zhu, Q. Correlations between cadmium and mineral nutrients in absorption and accumulation in various genotypes of rice under cadmium stress. Chemosphere 2003, 52, 1467–1473.
- Yang, B.; Zhou, M.; Shu, W.; Lan, C.; Ye, Z.; Qiu, R.; Jie, Y.; Cui, G.; Wong, M.H. Constitutional tolerance to heavy metals of a fiber crop, ramie (Boehmeria nivea), and its potential usage. Environ. Pollut. 2010, 158, 551–558.
- Liu, Y.; Wang, X.; Zeng, G.; Qu, D.; Gu, J.; Zhou, M.; Chai, L. Cadmium-induced oxidative stress and response of the ascorbate–glutathione cycle in Bechmeria nivea (L.) Gaud. Chemosphere 2007, 69, 99–107.
- Wang, C.-L.; Liu, Y.-G.; Zeng, G.-M.; Hu, X.-J.; Ying, Y.-C.; Xi, H.; Lu, Z.; Wang, Y.-Q.; Li, H.-Y. Mechanism of exogenous selenium alleviates cadmium induced toxicity in Bechmeria nivea (L.) Gaud (Ramie). Transact. Nonferrous Met. Soc. China 2014, 24, 3964–3970.
- Du, Y.; Hu, X.F.; Wu, X.H.; Shu, Y.; Jiang, Y.; Yan, X.J. Affects of mining activities on Cd pollution to the paddy soils and rice grain in Hunan province, Central South China. Environ. Monit. Assess. 2013, 185, 9843–9856.
- An, X.; Wei, J.; Liu, Q.; Ying, J.; Zhou, H.; Luo, X.; Li, W.; Liu, T.; Zou, L.; Zhu, G. Progress in the Study of the absorption, accumulation, and tolerance of ramie to the heavy metal cadmium. Mol. Plant Breed. 2023, 14, 3.
- Lei, M.; Yue, Q.; Chen, T.; Huang, Z.; Liao, X.; Liu, Y.; Zheng, G.; Chang, Q. Heavy metal concentrations in soils and plants around Shizhuyuan mining area of Hunan Province. Acta Ecol. Sin. 2005, 25, 1146–1151.
- She, W.; Jie, Y.-C.; Xing, H.-C.; Lu, Y.-W.; Kang, W.-L.; Wang, D. Heavy metal concentrations and bioaccumulation of ramie (Boehmeria nivea) growing on 3 mining areas in Shimen, Lengshuijiang and Liuyang of Hunan Province. Shengtai Xuebao/Acta Ecol. Sin. 2010, 31, 874–881.
- Feng, X.; Abubakar, A.; Chen, K.; Yu, C.; Zhu, A.; Chen, J.; Gao, G.; Wang, X.; Mou, P.; Chen, P. Genome-wide analysis of R2R3-MYB transcription factors in Boehmeria nivea (L.) Gaudich revealed potential cadmium tolerance and anthocyanin biosynthesis genes. Front. Genet. 2023, 14, 1080909.
- Satya, P.; Mitra, S.; Ray, D.P. Ramie (Boehmeria nivea L. Gaud) genetic improvement. In Advances in Plant Breeding Strategies: Industrial and Food Crops; Springer: Berlin/Heidelberg, Germany, 2019; pp. 115–150.
- Zhu, Q.; Huang, D.; Liu, S.; Luo, Z.; Rao, Z.; Cao, X.; Ren, X. Accumulation and subcellular distribution of cadmium in ramie (Boehmeria nivea L. Gaud.) planted on elevated soil cadmium contents. Plant Soil Environ. 2013, 59, 57–61.
- Ma, Y.; Jie, H.; Tang, Y.; Xing, H.; Jie, Y. The Role of hemicellulose in cadmium tolerance in ramie (Boehmeria nivea (L.) Gaud.). Plants 2022, 11, 1941.
- Sun, Z.; Liu, Y.; Huang, Y.; Zeng, G.; Wang, Y.; Hu, X.; Zhou, L. Effects of indole-3-acetic, kinetin and spermidine assisted with EDDS on metal accumulation and tolerance mechanisms in ramie (Boehmeria nivea (L.) Gaud.). Ecol. Engin. 2014, 71, 108–112.
- Tang, H.; Liu, Y.; Gong, X.; Zeng, G.; Zheng, B.; Wang, D.; Sun, Z.; Zhou, L.; Zeng, X. Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress. Environ. Sci. Pollut. Res. 2015, 22, 9999–10008.
- Shanying, H.; Xiaoe, Y.; Zhenli, H.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438.
- Baliardini, C.; Meyer, C.-L.; Salis, P.; Saumitou-Laprade, P.; Verbruggen, N. CATION EXCHANGER1 cosegregates with cadmium tolerance in the metal hyperaccumulator Arabidopsis halleri and plays a role in limiting oxidative stress in Arabidopsis spp. Plant Physiol. 2015, 169, 549–559.
- She, W.; Jie, Y.-C.; Xing, H.-C.; Lu, Y.-W.; Huang, M.; Kang, W.-L.; Wang, D. Tolerance to cadmium in ramie (Boehmeria nivea) genotypes and its evaluation indicators. Acta Agron. Sinica 2011, 37, 348–353.
- Zhu, S.; Shi, W.; Zhang, J. Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils. Open Chem. 2022, 20, 444–454.
- Quan, R.; Chen, J.; Zhang, L.; Xu, M.; Yang, R.; She, W.; Cui, G. Responses of ramie to antioxidant enzymes and plant chelating peptides to Cd stress. Chin. J. Trop. Crops 2022, 43, 1023.
- She, W.; Cui, G.; Li, X.; Su, X.; Jie, Y.; Yang, R. Characterization of cadmium concentration and translocation among ramie cultivars as affected by zinc and iron deficiency. Acta Physiol. Plant. 2018, 40, 104.
- Zhou, L.; Liu, Y.; Hu, X.; Zeng, G.; Wang, Y.; Hu, X.; Zhou, Y.; Tan, X.; Jiang, L.; Zeng, X. Time-dependent antioxidative responses of ramie (Boehmeria nivea (L.) Gaudich) to moderate cadmium stress and its up-regulation mechanism by spermidine antioxidant. RSC Adv. 2015, 5, 76141–76149.
- Wang, X.; Liu, Y.; Zeng, G.; Chai, L.; Song, X.; Min, Z.; Xiao, X. Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ. Expe. Bot. 2008, 62, 389–395.
- She, W.; Jie, Y.-C.; Xing, H.-C.; Luo, Z.-Q.; Kang, W.-L.; Huang, M.; Zhu, S.-J. Absorption and accumulation of cadmium by ramie (Boehmeria nivea) cultivars: A field study. Acta Agricul. Scand. Section B-Soil Plant Sci. 2011, 61, 641–647.
- Zhao, X.; Luan, M.; Qiu, C.; Guo, Y.; Long, S.; Wang, Y.; Qiu, H. Analysis of the potential of 165 ramie germplasms to be used for cadmium-contamination remediation. Indust. Crops Prod. 2021, 171, 113841.
- Djemal, R.; Khoudi, H. The ethylene-responsive transcription factor of durum wheat, TdSHN1, confers cadmium, copper, and zinc tolerance to yeast and transgenic tobacco plants. Protoplasma 2022, 259, 19–31.
- Rono, J.K.; Le Wang, L.; Wu, X.C.; Cao, H.W.; Zhao, Y.N.; Khan, I.U.; Yang, Z.M. Identification of a new function of metallothionein-like gene OsMT1e for cadmium detoxification and potential phytoremediation. Chemosphere 2021, 265, 129136.
- Sterckeman, T.; Thomine, S. Mechanisms of cadmium accumulation in plants. Crit. Rev. Plant Sci. 2020, 39, 322–359.
- Zulfiqar, U.; Jiang, W.; Xiukang, W.; Hussain, S.; Ahmad, M.; Maqsood, M.F.; Ali, N.; Ishfaq, M.; Kaleem, M.; Haider, F.U. Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils; a comprehensive review. Front. Plant Sci. 2022, 13, 773815.
- Ying, Z.; Yushen, M.; Yamei, W.; Zehang, L.; Yuejun, X.; Hucheng, X.; Yucheng, J. Genotype differences in cadmium accumulation ability of ramie on cadmium-polluted Field. J. Agricul. Sci. Tech. 2021, 23, 54.
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576.
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311.
- Rehman, S.; Chattha, M.U.; Khan, I.; Mahmood, A.; Hassan, M.U.; Al-Huqail, A.A.; Salem, M.Z.; Ali, H.M.; Hano, C.; El-Esawi, M.A. Exogenously applied trehalose augments cadmium stress tolerance and yield of mung bean (Vigna radiata L.) grown in soil and hydroponic systems through reducing cd uptake and enhancing photosynthetic efficiency and antioxidant defense systems. Plants 2022, 11, 822.
- Yeboah, A.; Lu, J.; Gu, S.; Liu, H.; Shi, Y.; Amoanimaa-Dede, H.; Agyenim-Boateng, K.G.; Payne, J.; Yin, X. Evaluation of two wild castor (Ricinus communis L.) accessions for cadmium tolerance in relation to antioxidant systems and lipid peroxidation. Environ. Sci. Pollut. Res. 2021, 28, 55634–55642.
- Liu, T.; Zhu, S.; Tang, Q.; Tang, S. Genome-wide transcriptomic profiling of ramie (Boehmeria nivea L. Gaud) in response to cadmium stress. Gene 2015, 558, 131–137.
- Yeboah, A.; Lu, J.; Ting, Y.; Karikari, B.; Gu, S.; Xie, Y.; Liu, H.; Yin, X. Genome-wide association study identifies loci, beneficial alleles, and candidate genes for cadmium tolerance in castor (Ricinus communis L.). Indust. Crops Prod. 2021, 171, 113842.
- Hou, F.; Zhou, X.; Liu, P.; Yuan, G.; Zou, C.; Lübberstedt, T.; Pan, G.; Ma, L.; Shen, Y. Genetic dissection of maize seedling traits in an IBM Syn10 DH population under the combined stress of lead and cadmium. Mol. Genet. Gen. 2021, 296, 1057–1070.
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Reg. 2019, 38, 739–752.
- Kapoor, D.; Bhardwaj, S.; Gautam, S.; Rattan, A.; Bhardwaj, R.; Sharma, A. Brassinosteroids in plant nutrition and heavy metal tolerance. In Brassinosteroids in Plant Developmental Biology and Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2022; pp. 217–235.
- Rehman, R.S.; Ali, M.; Zafar, S.A.; Hussain, M.; Pasha, A.; Naveed, M.S.; Ahmad, M.; Waseem, M. Abscisic Acid Mediated Abiotic Stress Tolerance in Plants. Asian J. Res. Crop. Sci. 2022, 7, 1–17.
- Gray, W.M. Hormonal regulation of plant growth and development. PLoS Biol. 2004, 2, e311.
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386.
- Xu, Y.-X.; Mao, J.; Chen, W.; Qian, T.-T.; Liu, S.-C.; Hao, W.-J.; Li, C.-F.; Chen, L. Identification and expression profiling of the auxin response factors (ARFs) in the tea plant (Camellia sinensis (L.) O. Kuntze) under various abiotic stresses. Plant Physiol. Biochem. 2016, 98, 46–56.
- Yaashikaa, P.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035.
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop. J. 2016, 4, 162–176.
- Shaukat, K.; Zahra, N.; Hafeez, M.B.; Naseer, R.; Batool, A.; Batool, H.; Raza, A.; Wahid, A. Role of salicylic acid–induced abiotic stress tolerance and underlying mechanisms in plants. In Emerging Plant Growth Regulators in Agriculture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 73–98.
- Wang, H.; Zhong, G.; Shi, G.; Pan, F. Toxicity of Cu, Pb, and Zn on seed germination and young seedlings of wheat (Triticum aestivum L.). In Proceedings of the International Conference on Computer and Computing Technologies in Agriculture, Nanchang, China, 22–25 October 2010; pp. 231–240.
- Bulak, P.; Walkiewicz, A.; Brzezińska, M. Plant growth regulators-assisted phytoextraction. Biol. Plant. 2014, 58, 1–8.
- Chen, K.; Chen, P.; Qiu, X.; Chen, J.; Gao, G.; Wang, X.; Zhu, A.; Yu, C. Regulating role of abscisic acid on cadmium enrichment in ramie (Boehmeria nivea L.). Sci. Rep. 2021, 11, 1–10.
- He, Y.; He, S.; Luo, J.; Zeng, Y.; Zhang, X.; Huo, Y.; Jie, Y.; Xing, H. Exogenous Plant growth Regulator and Foliar Fertilizers for Phytoextraction of Cadmium with Boehmeria nivea Gaudich from Contaminated Field Soil. 2022. Available online: https://www.researchsquare.com/article/rs-1197456/latest.pdf (accessed on 20 May 2023).
- Mroue, S.; Simeunovic, A.; Robert, H.S. Auxin production as an integrator of environmental cues for developmental growth regulation. J. Exp. Bot. 2018, 69, 201–212.
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735.
- Cao, X.; Yang, H.; Shang, C.; Ma, S.; Liu, L.; Cheng, J. The roles of auxin biosynthesis YUCCA gene family in plants. Int. J. Mol. Sci. 2019, 20, 6343.
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18.
- Chaca, P.; Vigliocco, A.; Reinoso, H.; Molina, A.; Abdala, G.; Zirulnik, F.; Pedranzani, H. Effects of cadmium stress on growth, anatomy and hormone contents in Glycine max (L.) Merr. Acta Physiol. Plant. 2014, 36, 2815–2826.
- Ahmad, P.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Ahanger, M.A.; Alamri, S.A. Jasmonic acid alleviates negative impacts of cadmium stress by modifying osmolytes and antioxidants in faba bean (Vicia faba L.). Arch. Agron. Soil Sci. 2017, 63, 1889–1899.
- Shahid, M.; Javed, M.T.; Mushtaq, A.; Akram, M.S.; Mahmood, F.; Ahmed, T.; Noman, M.; Azeem, M. Microbe-mediated mitigation of cadmium toxicity in plants. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 427–449.
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-induced cadmium stress tolerance in rice seedlings: Coordinated action of antioxidant defense, glyoxalase system and nutrient homeostasis. Comptes Rendus Biol. 2016, 339, 462–474.
- Erguuml, N. Effects of some heavy metals and heavy metal hormone interactions on wheat (Triticum aestivum L. cv. Gun 91) seedlings. Afri. J. Agricul. Res. 2012, 7, 1518–1523.
- Villiers, F.; Jourdain, A.; Bastien, O.; Leonhardt, N.; Fujioka, S.; Tichtincky, G.; Parcy, F.; Bourguignon, J.; Hugouvieux, V. Evidence for functional interaction between brassinosteroids and cadmium response in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 1185–1200.
- Munzuroglu, O.; Zengin, F.K. Effect of cadmium on germination, coleoptile and root growth of barley seeds in the presence of gibberellic acid and kinetin. J. Environ. Biol. 2006, 27, 671–677.
- Jie, H.; Zhao, L.; Ma, Y.; Rasheed, A.; Jie, Y. Integrated transcriptome and metabolome analysis reveal that exogenous gibberellin application regulates lignin synthesis in ramie. Agronomy 2023, 13, 1450.
- Jie, H.; Ma, Y.; Xie, D.-Y.; Jie, Y. Transcriptional and metabolic characterization of feeding ramie growth enhanced by a combined application of gibberellin and ethrel. Int. J. Mol. Sci. 2022, 23, 12025.
- Karcz, W.; Kurtyka, R. Effect of cadmium on growth, proton extrusion and membrane potential in maize coleoptile segments. Biol. Plant. 2007, 51, 713–719.
- Krantev, A.; Yordanova, R.; Popova, L. Salicylic acid decreases Cd toxicity in maize plants. Gen. App. Plant Physiol. 2006, 3, 45–52.
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931.
- Gondor, O.K.; Pál, M.; Darkó, É.; Janda, T.; Szalai, G. Salicylic acid and sodium salicylate alleviate cadmium toxicity to different extents in maize (Zea mays L.). PLoS ONE 2016, 11, e0160157.
- Kalai, T.; Bouthour, D.; Manai, J.; Kaab, L.B.B.; Gouia, H. Salicylic acid alleviates the toxicity of cadmium on seedling growth, amylases and phosphatases activity in germinating barley seeds. Arc. Agro. Soil Sci. 2016, 62, 892–904.
- Tang, C.; Zhang, R.; Hu, X.; Song, J.; Li, B.; Ou, D.; Hu, X.; Zhao, Y. Exogenous spermidine elevating cadmium tolerance in Salix matsudana involves cadmium detoxification and antioxidant defense. Int. J. Phytoremed. 2019, 21, 305–315.
- Nahar, K.; Rahman, M.; Hasanuzzaman, M.; Alam, M.; Rahman, A.; Suzuki, T.; Fujita, M. Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ. Sci. Pollut. Res. 2016, 23, 21206–21218.
- Yang, H.; Shi, G.; Li, W.; Wu, W. Exogenous spermidine enhances Hydrocharis dubia cadmium tolerance. Russian J. Plant Physiol. 2013, 60, 770–775.
- Rady, M.M.; Hemida, K.A. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185.
- Gong, X.; Liu, Y.; Huang, D.; Zeng, G.; Liu, S.; Tang, H.; Zhou, L.; Hu, X.; Zhou, Y.; Tan, X. Effects of exogenous calcium and spermidine on cadmium stress moderation and metal accumulation in Boehmeria nivea (L.) Gaudich. Environ. Sci. Pollut. Res. 2016, 23, 8699–8708.
- She, W.; Cui, G.-X.; Jie, Y.-C.; Bai, Y.-C.; Cao, Y.; Xiao, C.-X. Comparative effects of chelants on plant growth, cadmium uptake and accumulation in nine cultivars of Ramie (Boehmeria nivea). Acta Agricul. Scand. Sect. B-Soil Plant Sci. 2014, 64, 71–78.
- Yin, Y.; Wang, Y.; Liu, Y.; Zeng, G.; Hu, X.; Hu, X.; Zhou, L.; Guo, Y.; Li, J. Cadmium accumulation and apoplastic and symplastic transport in Boehmeria nivea (L.) Gaudich on cadmium-contaminated soil with the addition of EDTA or NTA. RSC Adv. 2015, 5, 47584–47591.
- Wu, S.; Xue, S.; Iqbal, Y.; Xing, H.; Jie, Y. Seasonal nutrient cycling and enrichment of nutrient-related soil microbes aid in the adaptation of ramie (Boehmeria nivea L.) to nutrient-deficient conditions. Front. Plant Sci. 2021, 12, 644904.
- Zheng, J.; Liu, T.; Zheng, Q.; Li, J.; Qian, Y.; Li, J.; Zhan, Q. Identification of cold tolerance and analysis of genetic diversity for major wheat varieties in Jianghuai region of China. Pak. J. Bot. 2019, 52, 839–849.
- Das, S.; Pandit, E.; Guru, M.; Nayak, D.; Tasleem, S.; Barik, S.; Mohanty, D.; Mohanty, S.; Patra, B.; Pradhan, S. Genetic diversity, population structure, marker validation and kinship analysis for seedling stage cold tolerance in indica rice. ORYZA-An Int. J. Rice 2018, 55, 396–405.
- Swarup, S.; Cargill, E.J.; Crosby, K.; Flagel, L.; Kniskern, J.; Glenn, K.C. Genetic diversity is indispensable for plant breeding to improve crops. Crop. Sci. 2021, 61, 839–852.
- Negisho, K.; Shibru, S.; Pillen, K.; Ordon, F.; Wehner, G. Genetic diversity of Ethiopian durum wheat landraces. PLoS ONE 2021, 16, e0247016.
- Govindaraj, M.; Vetriventhan, M.; Srinivasan, M. Importance of genetic diversity assessment in crop plants and its recent advances: An overview of its analytical perspectives. Genet. Res. Int. 2015, 2015, 1–14.
- Esquinas-Alcázar, J. Protecting crop genetic diversity for food security: Political, ethical and technical challenges. Nat. Rev. Gen. 2005, 6, 946–953.
- Choudhury, B.I.; Khan, M.L.; Dayanandan, S. Patterns of nucleotide diversity and phenotypes of two domestication related genes (OsC1 and Wx) in indigenous rice varieties in Northeast India. BMC Genet. 2014, 15, 71.
- Haun, W.J.; Hyten, D.L.; Xu, W.W.; Gerhardt, D.J.; Albert, T.J.; Richmond, T.; Jeddeloh, J.A.; Jia, G.; Springer, N.M.; Vance, C.P. The composition and origins of genomic variation among individuals of the soybean reference cultivar Williams 82. Plant Physiol. 2011, 155, 645–655.
- Van de Wouw, M.; van Hintum, T.; Kik, C.; van Treuren, R.; Visser, B. Genetic diversity trends in twentieth century crop cultivars: A meta analysis. Theoret. App. Genet. 2010, 120, 1241–1252.
- Fukuoka, S.; Suu, T.D.; Ebana, K.; Trinh, L.N.; Nagamine, T.; Okuno, K. Diversity in phenotypic profiles in landrace populations of Vietnamese rice: A case study of agronomic characters for conserving crop genetic diversity on farm. Genet. Res. Crop Evolut. 2006, 53, 753–761.
- Wendel, J.F.; Greilhuber, J.; Dolezel, J.; Leitch, I.J. Plant Genome Diversity Volume 1: Plant Genomes, Their Residents, and Their Evolutionary Dynamics; Springer: Berlin/Heidelberg, Germany, 2012.
- Murat, F.; Peer, Y.V.D.; Salse, J. Decoding plant and animal genome plasticity from differential paleo-evolutionary patterns and processes. Gen. Biol. Evol. 2012, 4, 917–928.
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34.
- Wu, X.; Cai, K.; Zhang, G.; Zeng, F. Metabolite profiling of barley grains subjected to water stress: To explain the genotypic difference in drought-induced impacts on malting quality. Fronti. Plant Sci. 2017, 8, 1547.
- Belete, Y.; Shimelis, H.; Laing, M.; Mathew, I. Genetic diversity and population structure of bread wheat genotypes determined via phenotypic and SSR marker analyses under drought-stress conditions. J. Crop Imp. 2021, 35, 303–325.
- Pandey, J.; Scheuring, D.C.; Koym, J.W.; Coombs, J.; Novy, R.G.; Thompson, A.L.; Holm, D.G.; Douches, D.S.; Miller, J.C.; Vales, M.I. Genetic diversity and population structure of advanced clones selected over forty years by a potato breeding program in the USA. Sci. Rep. 2021, 11, 1–18.
- Vaughan, D.; Balazs, E.; Heslop-Harrison, J. From crop domestication to super-domestication. Ann. Bot. 2007, 100, 893–901.
- Andersen, J.R.; Lübberstedt, T. Functional markers in plants. Trend. Plant Sci. 2003, 8, 554–560.
- Ren, Y.; Wei, Q.; Lin, L.; Shi, L.; Cui, Z.; Li, Y.; Huang, C.; Wei, C. Physicochemical properties of a new starch from ramie (Boehmeria nivea) root. Int. J. Biol. Macromol. 2021, 174, 392–401.
- Glaszmann, J.-C.; Kilian, B.; Upadhyaya, H.D.; Varshney, R.K. Accessing genetic diversity for crop improvement. Curr. Opin. Plant Biol. 2010, 13, 167–173.
- Zhu, G.; Huang, D.; Zhu, Q.; Liu, S.; Liu, G.; Jia, L. Tolerance and phytoremediation potential of ramie for cadmium contaminated soil. Res. Agricul. Modern. 2009, 30, 752–755.
- She, W.; Jie, Y.; Xing, H.; Lu, Y.; Huang, M.; Kang, W.; Wang, D. Comparison and screening indicators for ramie (Boehmeria nivea) genotypes tolerant to cadmium. Acta Agro. Sin. 2011, 37, 348–354.
- She, W.; Jie, Y.; Xing, H.; Huang, M.; Kang, W.; Lu, Y.; Wang, D. Uptake and accumulation of heavy metal by ramie growing on antimony mining area in Lengshuijiang City of Hunan Province. J. Agro-Environ. Sci. 2010, 29, 91–96.
- Wu, Z.; Tang, Q.; Wang, Y.; Qiu, C.; Long, S.; Zhao, X.; Hu, Z.; Guo, Y. Ramie (Boehmeria nivea) as Phytoremediation Crop for heavy metal-contaminated paddy soil in Southern China: Variety comparison, cd accumulation, and assessment of fiber recycling. J. Nat. Fib. 2021, 19, 11078–11091.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
827
Revisions:
2 times
(View History)
Update Date:
21 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




