Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | kunqian li | -- | 1873 | 2023-07-20 10:43:36 | | | |
| 2 | Conner Chen | + 67 word(s) | 1940 | 2023-07-24 05:49:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, K.; Wu, F.; Chen, M.; Xiao, Z.; Xu, Y.; Xu, M.; Liu, J.; Xu, D. Identification of Secondary Metabolites in Medicinal Orchids. Encyclopedia. Available online: https://encyclopedia.pub/entry/47025 (accessed on 03 February 2026).
Li K, Wu F, Chen M, Xiao Z, Xu Y, Xu M, et al. Identification of Secondary Metabolites in Medicinal Orchids. Encyclopedia. Available at: https://encyclopedia.pub/entry/47025. Accessed February 03, 2026.
Li, Kunqian, Fengju Wu, Mengzhu Chen, Zhihao Xiao, Ya Xu, Mengwei Xu, Jingyi Liu, Delin Xu. "Identification of Secondary Metabolites in Medicinal Orchids" Encyclopedia, https://encyclopedia.pub/entry/47025 (accessed February 03, 2026).
Li, K., Wu, F., Chen, M., Xiao, Z., Xu, Y., Xu, M., Liu, J., & Xu, D. (2023, July 20). Identification of Secondary Metabolites in Medicinal Orchids. In Encyclopedia. https://encyclopedia.pub/entry/47025
Li, Kunqian, et al. "Identification of Secondary Metabolites in Medicinal Orchids." Encyclopedia. Web. 20 July, 2023.
Copy Citation
The secondary metabolites present in medicinal orchids are diverse and possess a vast array of biological activities. They represent valuable raw materials for modern pharmaceuticals and clinical medicine and have tremendous potential for future development. A systematic collation of secondary metabolites’ composition and a summary of the biological activities of medicinal orchids represent a crucial step in unlocking the potential of these valuable resources in drug development.
medicinal orchid
metabolite biosynthesis
chemical identification
1. Introduction
Orchids have a long history of medicinal use in China due to their cultivation for over 2000 years. The orchid family is diverse, and so far, over 28,000 species have been identified in 736 genera [1]. In the 2020 edition of the Chinese Pharmacopoeia, several species were recorded as Chinese herbal medicines (CHM). These include Gastrodia elata, Bletilla striata, Dendrobium nobile, D. huoshanense, D. chrysotoxum, D. fimbriatum, D. officinale, Cremastra appendiculata, Dendrobium spp., Pleione bulbocodioides and P. yunnanensis. In China, wild orchids have a long history as medicinal and ornamental plants. Due to their ability to treat diseases and enhance beauty and health, orchids have now become a new economic crop in agriculture. The development of orchids has resulted in several social benefits, particularly in promoting “green and natural” dietary options and daily essentials, including decoction pieces, oral liquids, beauty products, and skincare solutions. Moreover, the active elements identified in orchid plants can be leveraged to formulate new drugs. These genera include Bletilla, Anoectochilus, Liparis and several others. Research findings have shown that the types and quantities of the secondary metabolites present in CHM are known to play a significant role in their pharmacological effects and medicinal quality [2]. To effectively utilize medicinal orchid resources, it is particularly important to excavate their secondary metabolites.
In recent years, secondary metabolites have gained public attention due to their diverse biological activities and pharmacological effects, making them promising candidates for developing new drugs for stubborn diseases. The secondary metabolites found in medicinal orchids are small-molecule substances with various characteristics, including diversity, high specificity and rich biological functions. This makes them a potential source of highly specific active substances to treat stubborn diseases. However, research on medicinal orchids primarily focuses on species identification, classification, conservation and cultivation, as well as compound identification. More attention needs to be paid to the analysis and use of active substances derived from secondary metabolites, which are still in early stages. Despite their potential, medicinal orchids face natural limitations, such as their growth cycle, season, climate and natural accumulation of compounds, resulting in a situation similar to paclitaxel’s in which there is low market supply and high market demand. To address this, it is crucial to elucidate the active secondary metabolites and biosynthesis of medicinal orchid secondary metabolites leading towards resource development and utilization.
2. Identification of Secondary Metabolites
These compounds were first proposed by the German chemist Kossel in 1891, and they are small molecules synthesized by organisms in response to environmental stress and resistance. They serve as plant protectants that aid in disease resistance and defense against natural enemies [3][4]. The biological effects of secondary metabolites are the result of species evolution and adaptation to the environment. The secondary metabolites of medicinal orchids can be classified into three groups: terpenoids, phenols and nitrogen compounds, each of which contain tens of thousands of compounds. Furthermore, the production and distribution of these secondary metabolites are species specific and only occur in particular organs, tissues and developmental stages of the orchids [4]. For instance, D. nobile has the largest number of dendrobine types, whereas D. chrysotoxum has the highest content of moscatilin, with stem tissue being the primary medicinal component. The active substances in traditional Chinese medicine are the material basis for their pharmacological effects. Therefore, the identification and analysis of secondary metabolites are necessary prerequisites for the development and application of medicinal orchids’ active substances.
2.1. Alkaloids
Alkaloids are the most commonly found nitrogen-containing compounds in the secondary metabolites of medicinal orchids and an essential source of bioactive compounds. More than 50 species of orchids have yielded the discovery of over 140 alkaloids [5], which can be classified into various groups based on their structural characteristics, such as sesquiterpenes, indolizines, amides and indoles. In 2019, 52 alkaloid components were detected in 19 orchid species [6]. However, the alkaloid content in medicinal orchids is typically low, with only five out of thirty-five Dendrobium plants having an alkaloid content of over 0.1% [7]. This paper summarizes 27 newly identified alkaloids, whose chemistry structure are shown in Figure 1. It is worth noting that three new alkaloids were identified from D. nobile, including a new pair of amide tautomers [8]. Four new indolizine alkaloids were identified from D. crepidatum [9][10]. Furthermore, a pair of enantiomers and three new indolizine alkaloids have been discovered from D. crepidatum [11]. These twenty-seven alkaloids are listed in Table 1 and include eight sesquiterpene alkaloids (1–8), two amides (9–10), eleven indolizines (11–21), four indoles (22–25), a spiral-shaped alkaloid (26) and a Lycodine-type Lycopodium alkaloid (27).
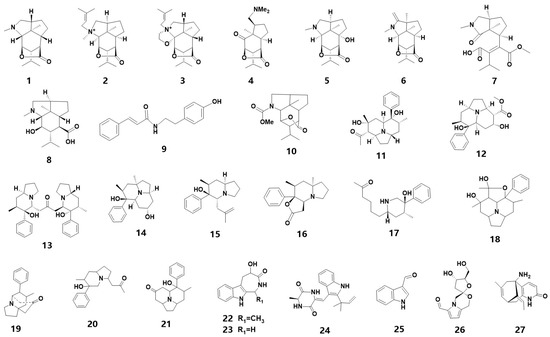
Figure 1. The chemical structure of alkaloids found in orchids.
2.2. Phenanthrenes
Phenanthrenes are a class of substances with three benzene rings as the parent ring and are commonly found in medicinal orchids. Based on their structural properties, they can be classified into simple phenanthrene, dihydrophenanthrene (DHP), phenanthraquinone (ketone), phenanthrene furan and phenanthrene dimer. Table 1 summarizes the 39 phenanthrenes that were identified from medicinal orchids between 2018 and 2023 (for their chemical structures, refer to Figure 2). Notably, a new DHP trimer was identified from B. striata [12], and silk grass was found to contain six new DHP [13]. Furthermore, monolithic orchids were discovered to have four optically rotating phenanthraquinones [14], and two new phenanthraquinones were found in Dendrobium flowers [15]. Additionally, three secondary metabolites were identified for the first time from plants in the genus Bletilla [12]. These thirty-nine phenanthrenes are listed in Table 1 and include one DHP dimer (28), twenty DHP (29–48), seven simple phenanthrenes (49–55) and nine phenanthraquinones (56–64), as well as furans (65) and biphenanthrene (66).
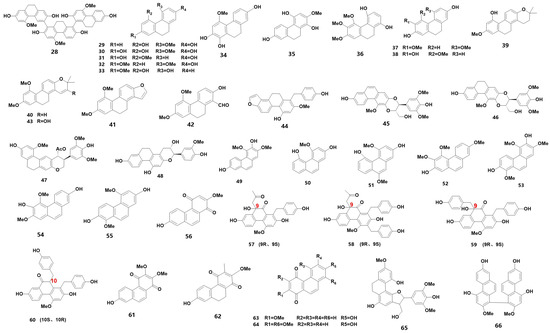
Figure 2. The chemical structure of phenanthrene found in orchids.
2.3. Bibenzylates
Bibenzyl is an essential precursor for synthesizing phenanthrene substances, with 1,2-diphenylethane as the parent ring. Among medicinal orchids, the bibenzyls of Dendrobium have been studied in depth. For instance, eight bibenzyl compounds were isolated from D. officinale leaves [16], and its stems contained 15 bibenzyl compounds, including dendrocandin X and 3,4′-dihydroxy-4,5-dimethoxybibenzyl, which were isolated from D. officinale for the first time [17]. Additionally, dendrocandin Y, a new bibenzyl derivative, was discovered from the stem extract [18]. Using UPLC-Q-TOF-MS technology, five bibenzyloids, including densiflorol A, aloifol I and isomoniliformin A, were identified in D. pendulum for the first time [19]. The discovery of new benzyl compounds in medicinal orchids serves as a research resource for developing their medicinal value. Table 1 (67–101) summarizes the information of 35 bibenzyls (for their chemical structures, refer to Figure 3). In Table 1, composition (67) was identified as new from Bletilla, whereas compositions (75, 83, 89) were determined to be new from Dendrobium. Three new substances (90, 98, 101) were found in D. plicatile [20], D. hercoglossum [21] and D. hancockii [22], respectively, within the genus.
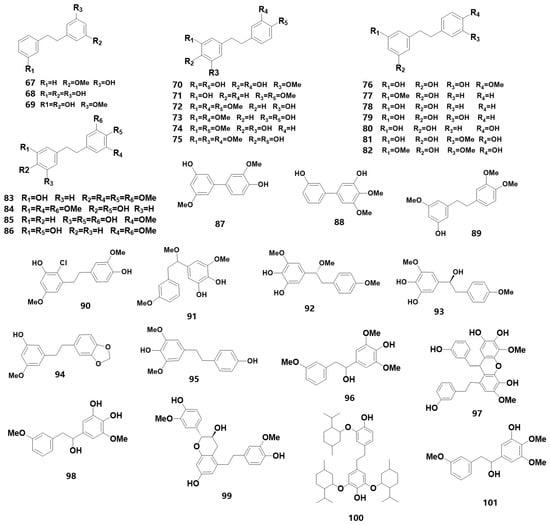
Figure 3. The chemical structure of bibenzyl substances found in orchids.
2.4. Other Secondary Metabolites
Medicinal orchids encompass a wide range of species, each characterized by a diverse array of secondary metabolites. These metabolites can be further categorized into various material types. For instance, flavonoids can be further sub-classified into flavanones, flavonols and other categories. These flavonoids are widely distributed in medicinal orchid plants and are a primary source of plant pigments. In fact, 66 flavonoids have been detected in 24 orchid plants [23], while 34 different species of Dendrobium have been found to contain flavonoids [24], including D. devonianum [25], D. officinale [26][27][28], D. fimbriatum [29] and D. huoshanense [30]. In the family of Orchidaceae, many types of secondary metabolites still exist, and 54 species are summarized in Table 1 (102–155) (for their chemical structures, refer to Figure 4).
Table 1. A total of 155 components were first identified from medicinal orchids in 2018–2023.
| NO. | Compound | Plant Source | Reference |
|---|---|---|---|
| Alkaloids | |||
| 1 | Dendrobine | C, P | [31][32] |
| 2 | N-isopentenyl-dendrobinium | C, P | [31][32] |
| 3 | N-isopentenyl-dendroxinium | C, P | [31][32] |
| 4 | 诺比洛宁 | C, P | [31,[32] |
| 5 | 树蜀葵碱 | P | [32] |
| 6 | 穆比罗宁A | P | [32] |
| 7 | 芬德莱因· | P | [32] |
| 8 * | 石斛酸 | N | [8] |
| 9 | N-对肉桂酰酪胺 | P | [32] |
| 10 * | N-甲氧基羰基石斛 | N | [8] |
| 11 | 克雷皮定 | P | [32] |
| 12 | 石斛比利比胺B | P | [32] |
| 13 | 石斛红素 | P | [32] |
| 14 | 石斛红素C | P | [32] |
| 15 | 克雷达明 | P | [32] |
| 16 | 石斛酸偶啶D | P | [32] |
| 17 | 高顺替啶B | P | [32] |
| 18 * | 克雷替图明 A | C | [10] |
| 19 * | 克雷替坦 B | C | [10] |
| 20 * | Crepidatumines C | C | [9] |
| 21 * | 克雷匹妥胺D | C | [9] |
| 22 | 人参皂苷 | 布,R | [33,[33] |
| 23 | 安诺西托钦 | 埠 | [34] |
| 24 | 新奇努林A | L | [35] |
| 25 | 吲哚-3-醛代 | 圣 | [36] |
| 26# | 可口水素A | J | [37] |
| 27 | 石杉碱甲 | 埠 | [34] |
| 菲 | |||
| 28 * | 2,2′,2“,7,7′,7”-六羟基-4,4′,4“-三甲氧基-[9,9′,9”,10,10′,10“]-六氢-1,8,1′,6”-三菲 | 圣 | [12] |
| 29 | 2,5-二羟基-4-甲氧基-9,10-二氢菲 | PL, M | [20,[38] |
| 30 | 2,5,7-三羟基-4-甲氧基-9,10-二氢菲 | B | [39] |
| 31 | 腔肠素 | H | [40] |
| 32 | 4,7-二甲氧基-9,10-二氢菲-2-醇 | 圣 | [36] |
| 33 | 2-甲氧基-9,10-二氢菲-4,5-二醇 | 圣 | [36] |
| 34 | 4-甲氧基-9,10-二氢菲-1,2,7-三醇 | 圣 | [36] |
| 35 | 1,4,7-三羟基-2-甲氧基-9,10-二氢菲 | .PL | [20] |
| 36 | 卡兰氢醌C | .PL | [20] |
| 37# | 2,7-二羟基-3,5-二甲氧基-9,10-二氢菲 | 圣 | [12] |
| 38# | 2,3,7-三羟基-4-甲氧基-9,10-二氢菲 | 圣 | [12] |
| 39 * | 螺蒽菲 A | 四 | [13] |
| 40 * | 螺嘟 B | 四 | [13] |
| 41 * | 螺妖菲 C | 四 | [13] |
| 42 * | 螺蝶咽D | 四 | [13] |
| 43 * | 螺嘟嘟 E | 四 | [13] |
| 44 * | 螺孢菲 F | 四 | [13] |
| 45 | 石斛红素P1 | O | [41] |
| 46 | 石斛红素P2 | O | [41] |
| 47 * | 布莱蒂洛尔 A | 圣 | [36] |
| 48 | 金氧托酚A | L | [35] |
| 49 | 3,7-二羟基-2,4-二甲氧基菲 | .PL | [20] |
| 50 | 2,5-二羟基-4-甲氧基菲 | 哈, 米 | [22,[38] |
| 51 | 2,5-二羟基-4,9-二甲氧基菲 | 医 管 局 | [22] |
| 52 * | 2-hydroxy-3,4,7-trimethoxyphenanthrene | O | [42] |
| 53 | 2,4,8-trimethoxy phenanthrene-3,7-diol | N | [43] |
| 54 | 5,7-dimethoxyphenanthrene-2,6-diol | ST | [36] |
| 55 | 1,5-dimethoxyphenanthrene-2,7-diol | ST | [36] |
| 56 | 7-hydroxy-2-methoxy-1,4-phenanthrenequinone | HA | [22] |
| 57 * | Bulbocodioidins A (9R,9S) | BUL | [14] |
| 58 * | Bulbocodioidins B (9R,9S) | BUL | [14] |
| 59 * | Bulbocodioidins C (9R,9S) | BUL | [14] |
| 60 * | Bulbocodioidins D (10S,10R) | BUL | [14] |
| 61 * | 2,3-dimethoxyl-7-hydroxyl-1,4-phenanthrenedione | F | [15] |
| 62 * | 2-methoxyl-3-methyl-7-hydroxyl-9,10-dihydro-1,4-phenanthrenedione | F | [15] |
| 63 | densiflorol B | N | [43] |
| 64 | cypripedin | N | [43] |
| 65 | 3-hydroxymethyl-9-methoxy-2-(4′-hydroxy-3′, 5′-dimethoxyphenyl)-2,3,6,7-tetrahydrophenanthro [4, 3-b] furan-5, 11-diol | M | [38] |
| 66 # | 4,7,4′,7′-tetrahydroxy-2,2′-dimethoxy-1,1′-biphenanthrene | ST | [12] |
| Bibenzylates | |||
| 67 # | 3-Hydroxy-5-methoxybibenzyl | ST | [12] |
| 68 | 3,3′,5-trihydroxybibenzyl | L | [35] |
| 69 | batatasin III | L, M, B, H | [35][38][39][40] |
| 70 | 3,4′-dihydroxy-5-methoxybibenzyl | H | [40] |
| 71 | 3-hydroxy-4′,5-dimethoxybibenzyl | H | [40] |
| 72 | 3-O-methylgigantol | H | [40] |
| 73 | gigantol | H | [40] |
| 74 | 3,4-dihydroxy-4′,5-dimethoxybibenzyl | H | [40] |
| 75 # | Moscatilin | PL, H | [20][40] |
| 76 | 3,5,5′-trihydroxy-4′-methoxybibenzyl | ST | [36] |
| 77 | 3-O-methyldihydropinosylvin | ST, M | [36][38] |
| 78 | Dihydropinosylvin | ST | [36] |
| 79 | 3,5,5′-trihydroxybibenzyl | ST | [36] |
| 80 | 3,5,4′-trihydroxybibenzyl | ST | [36] |
| 81 | 4,3′,5′-trihydroxy-3-methoxybibenzyl | HA | [22] |
| 82 | 4,3′-dihydroxy-3,5′-dimethoxybibenzyl | HA | [22] |
| 83# | 3′-hydroxy-3,4,4′,5-tetramethoxybibenzyl | PL | [20] |
| 84 | 4,4′-dihydroxy-3,3′,5-trimethoxybibenzyl | M, HA | [22][38] |
| 85 | dendrosinen B | B | [39] |
| 86 | 4,3′-dihydroxy-3,5-dimethoxybibenzyl | HE | [21] |
| 87 | 4′, 5-dihydroxy-3, 3′-dimethoxybiphezyl | B | [39] |
| 88 | 3, 3′-dihydroxy-4, 5-dimethoxybiphezyl | B | [39] |
| 89 # | 3-methylgigantol | PL | [20] |
| 90 * | 2-chloro-3,4′-dihydroxy-3′,5-dimethoxybibenzyl | PL | [20] |
| 91 | 4,5-dihydroxy-3,3′,α-trimethoxybibenzyl | HE | [21] |
| 92 | dendrocandin A | H | [40] |
| 93 | (S)-3,4,α-trihydroxy-4′,5-dimethoxybibenzyl | H | [40] |
| 94 | densiflorol A | H | [40] |
| 95 | 4,4′-dihydroxyl-3,5-dimethoxylbibenzyl | L | [35] |
| 96 | 4,α-dihydroxy-3,5,3′-trimethoxybibenzyl | HE | [21] |
| 97 | dendrosinen D | B | [39] |
| 98 * | 3,4,α-trihydroxy-5,3′-dimethoxybibenzyl | HE | [21] |
| 99 | trigonopol B | L | [35] |
| 100 | 4,4′-dihydroxy-3,5,3′-trimethoxybibenzyl | HE | [21] |
| 101 * | 3, α-dihydroxy-4,5,3′-trimethoxybibenzyl | HA | [22] |
| Other secondary metabolites | |||
| 102 # | p-hydroxybenzyl methyl ether | ST | [12] |
| 103 | p-Hydroxybenzyl ether | ST | [12] |
| 104 | p-hydroxybenzyl alcohol | ST | [12] |
| 105 | 4-methoxy-phenylethanol | S | [44] |
| 106 | dihydroconiferyl alcohol | HU | [45] |
| 107 | anoectosterol | BU, R | [33][34] |
| 108 # | (E) -4- (2-methoxyvinyl) benzene-1,2-diol | N | [43] |
| 109 | p-hydroxybenzyl alcohol | ST | [36] |
| 110 | 3-hydroxybenzaldehyde | S | [44] |
| 111 | 3,4-dihydroxy-5-methoxy benzaldehyde | HU | [45] |
| 112 # | 3,5-dihydroxy-4-hydroxy benzaldehyde | HU | [45] |
| 113 | 4-hydroxy-3-methoxy benzaldehyde | HU | [45] |
| 114 | 5-hydroxymethyl furfural | HU | [45] |
| 115 | coumarin | N | [43] |
| 116 # | moellendorffiline | N | [43] |
| 117 # | isopimpinellin | N | [43] |
| 118 | syringaresinol | L, HE | [21][35] |
| 119 | neoolivil | B | [39] |
| 120 | Matairesinol | S | [44] |
| 121 # | methyl melilotate | M | [38] |
| 122 # | ethyl melilotate | M | [38] |
| 123 * | methyl 2-(acetyloxy) benzenepropanoate | M | [38] |
| 124 | Dihydroconiferyl dihydrop-hydroxycinnamate | B | [39] |
| 125 | eis-p-hydroxyl ethyl cinnamate | S | [44] |
| 126 | p-hydroxyphenylpropionic ethyl ester | S | [44] |
| 127 | methyl 3-(4-hydroxyphenyl) propionate | HU | [45] |
| 128 | (9Z,12Z)-methyl octadeca-9,12-dienoate | S | [44] |
| 129 | hexadecanoic acid 2,3-dihydroxypropyl ester | HE | [21] |
| 130 | p-hydroxyphenyl-propionic acid | B | [39] |
| 131 | p-hydroxybenzoic acid | HU | [45] |
| 132 | p-hydroxycinnamic ac | B | [39] |
| 133 | ferulic acid | B | [39] |
| 134 | caffeic acid | B | [39] |
| 135 | 4-Hydroxy-2-methoxy-3,6-dimethylbenzoic acid | H | [40] |
| 136 # | 2-Hydroxy-4-methoxy-3,6-dimethylbenzoic acid | B | [39] |
| 137 | dihydroferulic acid | HU | [45] |
| 138 # | 4-O-β-D-glucopyranosyl coniferyl aldehyde | HU | [45] |
| 139 | 4-allyl-2,6-dimethoxyphenyl glucopyranoside | HU | [45] |
| 140 # | 3,4,5-trihydroxyallylbenzene-3-O-β-D-glucopyranosyl-4-O-β-D-glucopyranoside | HU | [45] |
| 141 | (7S,8R)-syringylglycerol-8-O-4′-sinapyl ether 4-O-β-D-glucopyranoside | HU | [45] |
| 142 # | 3,4,5-trimethoxyphenol-1-O-β-D-glucopyranoside | HU | [45] |
| 143 | gastrodin | HU | [45] |
| 144 | (+)-syringaresinol-4-O-β-D-glucopyranoside | HU | [45] |
| 145 | Liriodendrin | HU | [45] |
| 146 | quercetin | BU | [34] |
| 147 | 8-p-hydroxybenzyl quercetin | BU | [34] |
| 148 | 5,4′-dihydroxy-6,7,3′-trimethoxyflavone | BU | [34] |
| 149 | naringenin | L, N, HU | [35][43][45] |
| 150 | 5,4′-dihydroxy-7, 3′, 5′-trimethoxyflavanone | L | [35] |
| 151 | 5,7, 4′-trihydroxy-3′, 5′-dimethoxyflavanone | L | [35] |
| 152 # | carthamidin | H | [40] |
| 153 # | periloyrine | HU | [45] |
| 154 | N-trans-cinnamoyltyramine | HE | [21] |
| 155 # | (9Z,11E) -13-hydroxy-9,11-octadecadienoic acid | M | [38] |
Notes: Compounds 1 to 27 are alkaloids, 28 to 66 are phenanthophylls, 67 to 101 are bibenzylls and 102 to 155 are classified as other compounds. * denotes newly identified compounds; # indicates the first isolation from this genus; the rest indicate the first isolation from this plant species. C refers to D. crepidatum, P refers to D. pendulum, N refers to D. nobile, L refers to D. loddigesii, PL refers to D. plicatile, M refers to D. moschatum, H refers to D. heterocarpum, B refers to D. bellatulum, O refers to D. officinale, HA refers to D. hancockii, F refers to Flickingeria fimbriata, HE refers to D. hercoglossum, HU refers to D. huoshanense, S refers to D. sinense, CH refers to D. chrysotoxum, W refers to D. wardianum, BU refers to Anoectochilus burmannicus, R refers to A. roxburghii, ST refers to B. striata, J refers to Liparis japonica, NE refers to L. nervosa, SI refers to Spiranthes sinensis, BUL refers to P. bulbocobioides, Y refers to Pholidota yunnanensis, SU refers to Pecteilis susannae and MI refers to Phaius mishmensis.
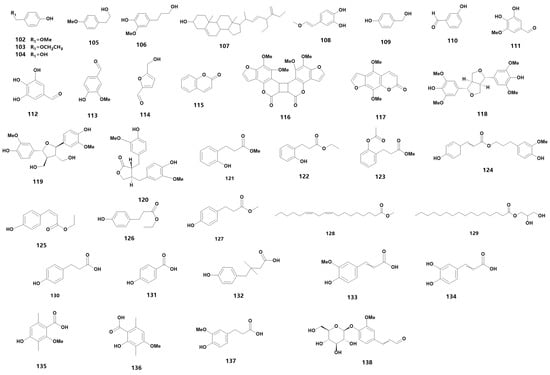
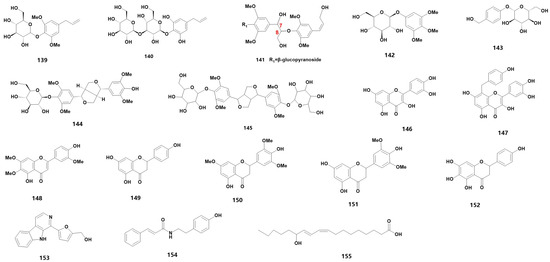
Figure 4. The chemical structure of other substances found in orchids.
References
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Van den Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174.
- Su, W.H.; Zhang, G.F.; Li, X.H.; Ou, X.K. Relationship between accumulation of secondary metabolism in medicinal plant and environmental condition. Chin. Tradit. Herb. Drugs 2005, 36, 139–142.
- Liu, S.J. Distribution and functions of plants’ secondary metabolites. J. Taishan Univ. 2003, 25, 91–94.
- Dong, Y.L.; Pan, X.W. Introduction to plant secondary metabolites. Biotechnol. Bull. 2002, 37, 17–19.
- Liu, H.D.; Pan, L.L.; Zhou, X.; Wan, N.; Wu, Y.F.; Li, B. Research progress on chemical constituents and pharmacological activities of alkaloids in Orchidaceae plants. Chin. Tradit. Herb. Drugs 2019, 50, 731–744.
- Li, Z.J.; Wang, Y.C.; Han, B.; Yang, Y.B.; Wang, Z.; Sun, Z.Y. Research progress on constituents of alkaloids in plants from Dendrobium Sw. Chin. Tradit. Herb. Drugs 2019, 50, 3246–3254.
- Wang, Z.H.; Li, J.; Zhang, J.H.; Zhu, G.F.; Liang, C.L.; Ye, Q.S. Comparison of Polysaccharide and Alkaloid Contents in Dendrobium. Chin. Agric. Sci. Bull. 2015, 31, 242–246.
- Zhang, M.S.; Linghu, L.; Wang, G.H.; He, Y.Q.; Sun, C.X.; Xiao, S.J. Dendrobine-type alkaloids from Dendrobium nobile. Nat. Prod. Res. 2022, 36, 5393–5399.
- Xu, X.; Li, Z.; Yang, R.; Zhou, H.; Bai, Y.; Yu, M.; Ding, G.; Li, B. Crepidatumines C and D, Two New Indolizidine Alkaloids from Dendrobium crepidatum Lindl. ex Paxt. Molecules 2019, 24, 3071.
- Xu, X.; Chen, X.; Yang, R.; Li, Z.; Zhou, H.; Bai, Y.; Yu, M.; Li, B.; Ding, G. Crepidtumines A and B, Two Novel Indolizidine Alkaloids from Dendrobium crepidatum. Sci. Rep. 2020, 10, 9564.
- Hu, Y.; Yang, H.; Ding, X.; Liu, J.; Wang, X.; Hu, L.; Liu, M.; Zhang, C. Anti-inflammatory octahydroindolizine alkaloid enantiomers from Dendrobium crepidatum. Bioorg. Chem. 2020, 100, 103809.
- Pan, Y.C. Establishment of Bletilla Striata Liquid Suspension Culture System and Determination of Secondary Metabolites. Master’s Degree, Zunyi Medical University, Zunyi, China, 2019.
- Liu, L.; Yin, Q.M.; Yan, X.; Hu, C.; Wang, W.; Wang, R.K.; Luo, X.; Zhang, X.W. Bioactivity-Guided Isolation of Cytotoxic Phenanthrenes from Spiranthes sinensis. J. Agric. Food Chem. 2019, 67, 7274–7280.
- Shao, S.Y.; Wang, C.; Han, S.W.; Sun, M.H.; Li, S. Phenanthrenequinone enantiomers with cytotoxic activities from the tubers of Pleione bulbocodioides. Org. Biomol. Chem. 2019, 17, 567–572.
- Chen, D.N.; Wu, Y.P.; Chen, Y.J.; Liu, W.J.; Wang, J.X.; He, F.; Jiang, L. Two new stilbenoids from aerial parts of Flickingeria fimbriata. J. Asian Nat. Prod. Res. 2019, 21, 117–122.
- Ren, G.; Chen, Y.T.; Ye, J.B.; Zhong, G.Y.; Xiao, C.Y.; Deng, W.Z.; Chen, Y.L. Phytochemical investigation of leaves of Dendrobium officinale. Chin. Tradit. Herb. Drugs 2020, 51, 3637–3644.
- Zhou, Y.J.; Wang, J.H.; Xu, H.; Chou, G.X.; Wang, Z.T. Bibenzyls from Dendrobium officinale. China J. Chin. Mater. Medica 2021, 46, 3853–3858.
- Meng, W.T.; Meng, X.; Niu, L.T.; Zhang, S.S.; Ouyang, C.J.; Ding, C.H.; Zhu, L.J.; Zhang, X. A new bibenzyl derivative from stems of Dendrobium officinale. China J. Chin. Mater. Medica 2023, 48, 700–706.
- Wang, Y.C.; Han, B.; Li, Z.J.; Nie, X.T.; Yang, M.Z.; Wang, W.; Sun, Z.Y. Determination of the Compounds of Dendrobium pendulum Roxb.by UPLC-Q-TOF-MS. Chin. Pharm. J. 2021, 56, 708–714.
- Chen, D.N.; Wang, Y.Y.; Liu, W.J.; Chen, Y.J.; Wu, Y.P.; Wang, J.X.; He, F.; Jiang, L. Stilbenoids from aerial parts of Dendrobium plicatile. Nat. Prod. Res. 2020, 34, 323–328.
- Cheng, L.; Chen, Z.Y.; Shang, Z.M.; Zhang, M.S.; Li, X.F.; Zhang, J.Y.; Xiao, S.J. Chemical constituents of Dendrobium hercoglossum. Chin. Tradit. Herb. Drugs 2020, 51, 3126–3130.
- Shang, Z.M.; Xia, D.; Cheng, L.; Liu, G.Y.; Zhang, M.S.; Zhang, J.Y.; Li, X.F.; Xiao, S.J. Chemical constituents from Dendrobium hancockii. Chin. Tradit. Herb. Drugs 2019, 50, 3760–3763.
- Liu, H.; Wei, X.Y.; Ma, H.; Qu, H.; Sun, Y. Analysis on pigment composition of petals of several native Cymbidium Sw.species. Jiangsu J. Agric. Sci. 2022, 38, 1657–1677.
- Zeng, Y.Y.; Nie, X.T.; Li, Z.J.; Zhang, M.; Yang, Y.B.; Wang, W.; Sun, Z.Y. Research Progress on Active Ingredients of Flavonoids in Traditional Chinese Medicine Dendrobium. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 197–206.
- Zhou, C.; Luo, Y.; Lei, Z.; Wei, G. UHPLC-ESI-MS Analysis of Purified Flavonoids Fraction from Stem of Dendrobium denneaum Paxt. and Its Preliminary Study in Inducing Apoptosis of HepG2 Cells. Evid. Based Complement. Altern. Med. 2018, 2018, 8936307.
- Lin, J.; Wang, W.Y.; Zou, H.; Dai, Y.M. Transcriptome Analysis on Pathway of and Genes Related to Flavonoid Synthesis in Dendrobium officinale. Fujian J. Agric. Sci. 2019, 34, 1019–1025.
- Lyu, C.G.; Yang, J.; Kang, C.Z.; Li, Z.H.; Ma, Z.H.; Guo, L.P.; Wang, Y.P. Determination of 10 Flavonoids by UPLC-MS/MS and Analysis of Polysaccharide Contents and Compositions in Dendrobii Officinalis Caulis from Different Habitats. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 47–52.
- Zhang, X.F.; Zhou, C.H.; Zhang, L.K.; Jiang, M.; Xie, Z.S.; Yuan, Y.; Huang, Y.C.; Luo, Y.Y.; Wei, G. Isolation and Identification of Main Flavonoid Glycosides of Dendrobium officinale from Danxia Species and Yunnan Guangnan Species. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 29–34.
- Li, Z.B.; Meng, Y.J.; Hu, L.; Zhang, L.; Huang, Y.H.; Yang, L.E.; Liang, Z.Y.; Huang, Y.C.; Wei, G. Analysis of the Chemical Composition of Flavonoids in Dendrobium fimbriatum Hook. Based on HPLCESI-MSn. Tradit. Chin. Drug Res. Clin. Pharmacol. 2022, 33, 1254–1260.
- Liang, Z.Y.; Zhang, J.Y.; Huang, Y.C.; Zhou, C.J.; Wang, Y.W.; Zhou, C.H.; Xing, S.P.; Shun, Q.S.; Xu, Y.X.; Wei, G. Identification of flavonoids in Dendrobium huoshanense and comparison with those in allied species of Dendrobium by TLC, HPLC and HPLC coupled with electrospray ionization multi-stage tandem MS analyses. J. Sep. Sci. 2019, 42, 1088–1104.
- Li, Z.J.; Zhou, W.Y.; Han, B.; Wang, Y.C.; Zeng, Y.Y.; Lu, S.C.; Sun, Z.Y. Study on alkaloids from stems of Dendrobium crepidatum based on UPLC-Q-TOF-MS. Nat. Prod. Res. Dev. 2020, 32, 482–488+426.
- Wang, Y.C.; Zhang, M.; Han, B.; Zhai, F.F.; Liu, L.; Sun, Z.Y.; Li, Z.J. Study on alkaloids in the stems of Dendrobium pendulum based on UPLC-Q-TOF-MS. Nat. Prod. Res. Dev. 2021, 33, 2019–2028.
- Qian, L.P.; Que, H.Q.; Peng, H.Y.; Li, W.; Chen, A.H.; Guo, S.M.; Li, Y.C.; Lin, S. A Study on the Chemical Constituents of Anoectochilus burmannicus Rolfe. Chin. J. Ethnomed. Ethnopharm. 2020, 29, 43–48.
- Qian, L.P.; Li, W.; Peng, H.Y.; Que, H.Q.; Shun-min, G.; Sui, L. Research on chemical constituents of Anoectochilus roxburghii. China Med. Pharm. 2021, 11, 73–76.
- Li, X.W.; Chen, H.P.; He, W.B.; Yang, W.L.; Ni, F.Y.; Huang, Z.W.; Hu, H.Y.; Wang, J. Polyphenols from Dendrobium loddigesii and their biological activities. Acta Sci. Nat. Univ. Sunyatseni 2019, 58, 96–102.
- Gang, Y.Q. Studies on Chemical Constituents from Bletilla Striata and Their Biological Activities. Master’s Thesis, South-Central University for Nationalities, Wuhan, China, 2020.
- Song, S.P.; Jiang, F.; Li, C.H.; Wei, T.; Yu, Y.F.; Tian, X.H. Chemical Constituents from Liparis japonica. Chin. Pharm. J. 2018, 53, 104–108.
- Yang, Z.Y.; Zhang, Y.; Yang, J.; Luo, W.L.; Zhang, M.S.; Wang, G.; Sun, C.X.; Dong, M.J.; Xiao, S.J. Chemical constituents from Dendrobium moschatum. Chin. Tradit. Pat. Med. 2022, 44, 3517–3521.
- Shang, Z.M.; Cheng, L.; Liu, G.Y.; Zhang, M.S.; Li, X.F.; Xiao, S.J. Chemical constituents of Dendrobium bellatulum. Chin. Tradit. Herb. Drugs 2019, 50, 2036–2040.
- Yang, X.B.; Yan, S.; Hu, J.M.; Zhou, J. Chemical constituents from Dendrobium heterocarpum Lindl. Nat. Prod. Res. Dev. 2019, 31, 1745–1752.
- Zhao, G.Y.; Deng, B.W.; Zhang, C.Y.; Cui, Y.D.; Bi, J.Y.; Zhang, G.G. New phenanthrene and 9,10-dihydrophenanthrene derivatives from the stems of Dendrobium officinale with their cytotoxic activities. J. Nat. Med. 2018, 72, 246–251.
- Sarakulwattana, C.; Mekboonsonglarp, W.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. New bisbibenzyl and phenanthrene derivatives from Dendrobium scabrilingue and their α-glucosidase inhibitory activity. Nat. Prod. Res. 2020, 34, 1694–1701.
- Zhou, W.; Zeng, Q.F.; Xia, J.; Wang, L.; Tao, L.; Shen, X.C. Antitumor Phenanthrene Constituents of Dendrobium nobile. Chin. Pharm. J. 2018, 53, 1722–1725.
- Cai, C.H.; Tan, C.Y.; Chen, H.Q.; Wang, H.; Mei, W.L.; Song, X.Q.; Dai, H.F. Chemical constituents from Dendrobium sinense (Ⅱ). Guihaia 2020, 40, 1368–1374.
- Zhao, H.S.; Xu, F.G.; Chen, X.X.; Hu, J.M.; Zeng, F.J.; Peng, D.Y.; Wu, D.L. Chemical constituents of Dendrobium huoshanense C.Z.Tang et S.J.Cheng. Nat. Prod. Res. Dev. 2021, 33, 1491–1498.
More
Information
Subjects:
Plant Sciences; Endocrinology & Metabolism
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
24 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




