Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lara Hathout | -- | 2123 | 2023-07-19 15:31:02 | | | |
| 2 | Fanny Huang | -25 word(s) | 2098 | 2023-07-21 07:53:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sherwani, Z.; Parikh, S.; Yegya-Raman, N.; Mckenna, K.; Deek, M.; Jabbour, S.; Hathout, L. Stereotactic Body Radiation Therapy in Gynecologic Oligometastases. Encyclopedia. Available online: https://encyclopedia.pub/entry/46988 (accessed on 08 February 2026).
Sherwani Z, Parikh S, Yegya-Raman N, Mckenna K, Deek M, Jabbour S, et al. Stereotactic Body Radiation Therapy in Gynecologic Oligometastases. Encyclopedia. Available at: https://encyclopedia.pub/entry/46988. Accessed February 08, 2026.
Sherwani, Zohaib, Shreel Parikh, Nikhil Yegya-Raman, Kelly Mckenna, Matthew Deek, Salma Jabbour, Lara Hathout. "Stereotactic Body Radiation Therapy in Gynecologic Oligometastases" Encyclopedia, https://encyclopedia.pub/entry/46988 (accessed February 08, 2026).
Sherwani, Z., Parikh, S., Yegya-Raman, N., Mckenna, K., Deek, M., Jabbour, S., & Hathout, L. (2023, July 19). Stereotactic Body Radiation Therapy in Gynecologic Oligometastases. In Encyclopedia. https://encyclopedia.pub/entry/46988
Sherwani, Zohaib, et al. "Stereotactic Body Radiation Therapy in Gynecologic Oligometastases." Encyclopedia. Web. 19 July, 2023.
Copy Citation
Stereotactic body radiation therapy (SBRT) allows for the high-precision delivery of a large dose of radiation to a small area. SBRT has shown overall survival benefits in a variety of oligometastatic cancers. No prospective randomized study specifically evaluated the impact of SBRT in recurrent or oligometastatic gynecologic cancer (ROMGC). SBRT has been shown to provide excellent local control with low toxicity in gynecologic oligometastases, even in chemorefractory disease.
stereotactic body radiation therapy
oligometastases
ovarian cancer
1. Introduction
Stereotactic body radiation therapy (SBRT) is a promising technique in the local treatment of oligometastatic disease with the delivery of high biologically equivalent doses (BED) to small volumes of gross disease over a hypofractionated or abbreviated schedule. While there is no universally accepted definition for the oligometastatic state, it commonly refers to an intermediate state between locally advanced and widely metastatic disease, defined as the presence of 1–5 metastases in which radical local treatment might improve systemic control [1][2][3][4]. The maximum number of metastases that can be treated is not agreed upon. In a consensus guideline recently published by ESTRO-ASTRO [5], “the feasibility of safely delivering curative intent metastasis-directed radiation therapy determines the maximum number of lesions and sites”. As a result, SBRT-directed local therapy to oligometastatic sites offers a potential for systemic control; and the number of lesions treated are largely dependent on the safe administration of large doses of radiation.
The SABR-COMET phase II clinical trial randomized 99 patients with oligometastatic cancer and multiple tumor types (lung, breast, prostate, etc.) in a 1:2 ratio to palliative standard of care treatment versus Stereotactic Ablative Radiotherapy (SABR) to all metastases plus standard of care. Patients must have had a controlled primary tumor and 1 to 5 metastases amenable to SABR. SABR improved median overall survival from 28 months to 41 months and exhibited an overall favorable toxicity profile [1]. However, only 2 out of the 99 total patients were reported to have a gynecologic primary. Several studies were published over the last decade evaluating the role of SBRT in recurrent or oligometastatic gynecologic malignancies.
2. SBRT in Ovarian Primary Oligometastases
Radiation was historically used to treat metastatic ovarian cancer in a palliative setting, with a well-established efficacy in the treatment of pain and bleeding [6][7][8]. Local recurrence within the abdomen or pelvis remained a significant factor contributing to poor prognosis despite multiple courses of chemotherapy. Definitive intent treatment using involved field radiation therapy (IFRT) resulted in modest benefits in overall survival and in-field disease control [9][10]. Intensity-modulated radiation therapy (IMRT) was more promising, with 2-year local control (LC) and overall survival (OS) rates of 82% and 63%, respectively, in refractory disease despite a median of three regimens of chemotherapy for recurrence within the abdomen or pelvis [11]. More recently, the use of SBRT has been evaluated in oligometastatic ovarian cancer.
A recent systematic review on SBRT in gynecologic malignancies [12] analyzed sixteen studies including one prospective phase II trial, two prospective phase I trials and thirteen retrospective reviews dating back to 2009. A total of 667 patients with 1071 oligometastatic lesions from varying ROMGC histological subtypes were evaluated. More than half (57.6%) of patients had primary ovarian oligometastases and 65.4% had a single lesion treated. Most lesions that were treated were at nodal sites (64%) within the abdomen (44.2%) or pelvis (18.8%) to a median BED10 of 50.7 Gy with response rates and local control exceeding 75% and 80%, respectively. Despite the high local control rates, disease progression occurred outside of the SBRT field. In addition, over half (56%) of the studies found no grade 3 or higher toxicities, suggesting that SBRT is well tolerated overall.
The MITO RT1 study is the largest retrospective review of ovarian primary oligometastases treated with SBRT, including 261 patients with 449 oligometastatic lesions with a median follow up of 22 months [13]. Most patients had a single lesion (55.9%) in abdominal lymph nodes (65%) with a median size of 15.7 cc. Inclusion criteria included any site of disease and up to five synchronous lesions. The most common histology was high-grade serous in 71.3% of patients. All patients underwent chemotherapy prior to radiation and 88.2% were treated with SBRT while 11.8% were treated with single-fraction radiotherapy (SRS). The median SBRT dose was 27 Gy (range 18–75) with a median BED10 of 48 Gy (range, 28–262.5). The most frequent SBRT prescriptions were 8 Gy × 3 fractions, 5 Gy × 5 fractions and 9 Gy × 3 fractions. Complete response was achieved in 65.2% of patients, with a 2-year actuarial local control of 81.9%, 2-year progression-free survival of 15.4%, and 2-year overall survival of 73.6%. On multivariate analysis, the following factors were identified as independent predictors for a higher likelihood of complete response and increased local control: patients younger than 60 years old, planning target volume (PTV) < 18 cc, lymph node disease and BED10 > 70. SBRT was well tolerated with 95.1% late toxicity-free survival.
Two other studies have reported comparable complete response rate with SBRT in oligometastatic ovarian cancer. An Italian retrospective study reported their experience with SBRT in 26 patients with 44 metastatic lesions [14]. Most patients had lymph node metastases (63.6%) treated to a median total dose of 45 Gy (range, 36–60 Gy) in six fractions (range, 4–8). After a median follow-up of 28.5 months, complete response was 59.1%, the 2-year local control was 92.9% and the median progression-free survival was 19 months. No grade 3 or 4 toxicities were reported [14].
Similarly, a single-institution retrospective study by Lazzari et al. evaluated the efficacy of SBRT in patients with oligometastatic ovarian cancer ineligible for surgery or systemic therapy [15]. A total of 82 patients with 156 lesions were included and received a median dose of 24 Gy in three fractions. Complete response was reported in 60% of patients after a median follow-up of 17.4 months. The median systemic treatment-free interval after SBRT was 7.4 months while the 2-year local progression-free survival was 68%. Most failures (90%) were outside of the radiation field [15].
Overall, SBRT is an effective and well-tolerated treatment option for ovarian primary oligometastases, with high rates of complete response and local control, as well as favorable overall survival and low rates of late toxicity. Prospective studies are ongoing, including the MITO RT3/RAD study (NCT04593381) [16], which is a multicenter prospective phase II study evaluating SBRT for the treatment of oligometastatic, persistent or recurrent ovarian cancer not amenable to surgery, local therapy and systemic therapy. SBRT is delivered in one, three or five daily fractions to a total dose of 30–50 Gy to all sites of active metastatic disease reported on imaging. The primary endpoint is a clinical complete response on a per lesion basis which aims to complete enrollment and analysis in 2023.
While awaiting the results of prospective data, select patients with oligometastatic, recurrent or persistent ovarian cancer should be considered for treatment with SBRT especially in patients who are not candidates for surgery and systemic therapy.
3. SBRT in Non-Ovarian Gynecologic Primary Oligometastases
Most retrospective studies evaluating the role of SBRT in gynecologic malignancies included multiple primary histologies; although ovarian cancer was studied most, cervical, and uterine cancer were included as well. Cuccia et al. reported the outcomes of 40 patients with 60 extracranial oligometastases from gynecological cancers. Metastases were from ovarian cancer (43%), endometrial cancer (41%), cervical cancer (13%) and vaginal cancer (3%). Lymph node metastases were the most common site of radiation (55.5%) and most patients had a single lesion (65%). SBRT was delivered to a total median dose of 42 Gy (range, 24–70) and a median BED10 of 72 Gy10 (range, 48–180 Gy10). After a median follow-up 27 months, the median local control was 19 months, the 2-year local control rate was 100% and the 2-year progression-free survival was 23%.
In a retrospective study by Onal et al. [17], 29 patients with 35 oligometastatic cervical cancer (72%) and ovarian cancer (28%) were treated with SBRT for either de novo oligometastatic disease (24%) or oligoprogressive disease (76%). The 2-year local control was 85% and disease progression occurred at a median time of 7.7 months after SBRT. Complete response after SBRT was correlated with improved overall and progression-free survival.
SBRT has been evaluated in primary oligometastatic cervical cancer in the recently published MITO RT2/RAD study [18] which represents the largest retrospective cohort of patients with oligometastases from primary cervical cancer treated with SBRT. A total of 83 patients with 125 oligometastatic cervical cancer lesions were treated with SBRT. Most patients had single (69.9%) pelvic (36.8%) lymph node (55.2%) disease treated to a total dose of 35 Gy in five fractions with a median BED10 of 59.5 Gy. After a median follow-up of 14.5 months, complete response was noted in 58.4% of lesions and in 55.4% of patients. Interestingly, the authors noted significant improvements in local control, progression-free survival and overall survival in patients who had complete response (CR), as opposed to any other response (partial response (PR), stable disease (SD) or progression of disease (PD)). Two-year local control was 89% for CR and 22.1% for PR/SD/PD, respectively. Two-year progression-free survival was 42.5% for CR and 7.8% for PR/SD/PD, respectively. Two-year overall survival was 68.9% for CR and 44.4% for PR/SD/PD, respectively. However, no factors independently predicted for complete response.
While the MITO RT1 study demonstrated the effectiveness and tolerability of SBRT for ovarian primary oligometastases, the MITO RT2/RAD study showed less favorable outcomes in cervical primary oligometastases treated with SBRT, but still suggested a promising avenue for further research in patients with comparatively unfavorable prognostic factors. In fact, early evidence in patients with cervical cancer with supraclavicular lymph node involvement without distant metastases showed that treatment with standard fractionation led to long term disease control which helped provide early rationale for selective targeting of oligometastatic disease with curative intent [19][20][21].
As for uterine cancer, most of the current data regarding uterine primary oligometastases are from retrospective studies with mixed cohorts [22][23][24][25][26][27][28]. The only recent study to researchers knowledge that exclusively included uterine primary oligometastases treated with SBRT is a single-institution retrospective analysis including 27 patients with 61 biopsy-proven lesions [29]. Most patients (74%) were treated for oligorecurrence and half (51.9%) had adenocarcinoma. The authors reported favorable response in 80.3% of cases and a median 1-year local progression-free survival rate of 75.9%, which was maintained at 3 years. The 1-year and 3-year overall survival rates were 65.4% and 28.7%, respectively. Liver lesions were found to be associated with a less favorable response on multivariate analysis, with only 37.5% of liver lesions with a favorable response. Tumor size < 3.8 cm was associated with a favorable response in the univariate analysis, which trended towards significance in the multivariate analysis.
The delivery of a high BED over a small volume with rapid dose fall off is an advantage of SBRT, as seen in Figure 1. However, a limitation of SBRT is its inability to target the adjacent, potentially clinically relevant, at-risk nodal echelon. It has been commonly reported in the literature that despite local ablative radiation to oligometastatic sites, disease progression varies widely, with rates ranging between 23.1% and 75%. Disease progression outside of the radiated field remains the main site of progression and occurs in 78.9–100% of cases [12]. Superior overall survival and progression-free survival have been noted in early detection of oligometastatic lesions and complete response following SBRT [12][17][30], which may suggest micrometastatic spread in the neighboring lymphatics that is not clinically apparent at the time of evaluation, resulting in out-of-field progression despite local ablative radiation.
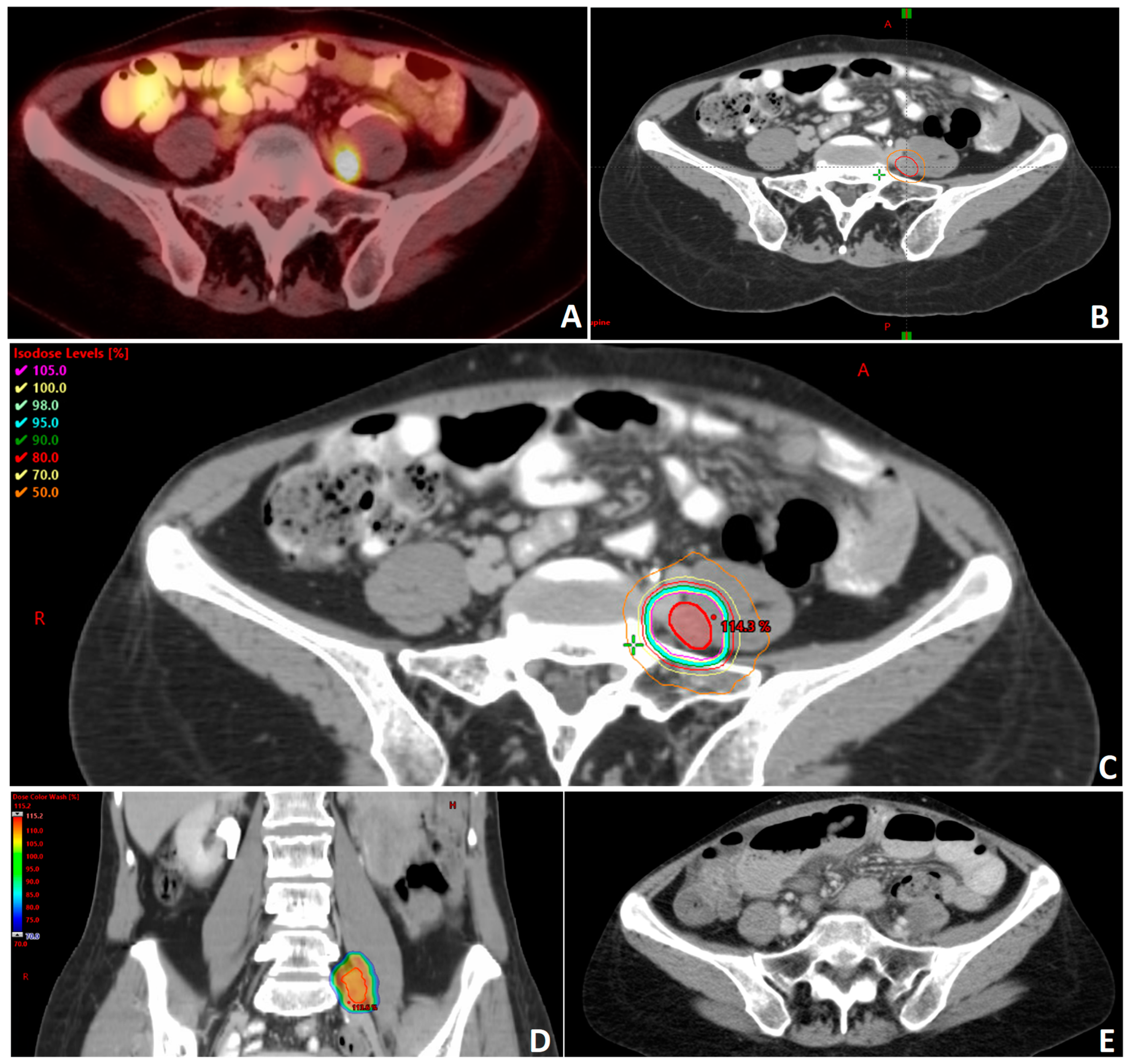
Figure 1. Clinical example of a patient with recurrent and oligometastatic high grade serous carcinoma of the ovary following surgery and multiple lines of systemic therapy. (A) Patient was found to have a hypermetabolic nodule medial to the left psoas muscle on positron emission tomography (PET) compatible with metastatic disease. (B) Gross tumor volume (GTV) delineation of oligometastatic nodule (red) with 0.5 mm planning tumor volume (PTV) radial expansion (orange). The treatment plan for this patient was generated to deliver a dose of 3000 cGy in 5 fractions prescribed to the PTV. The technique used in this plan is stereotactic body radiotherapy (SBRT) utilizing volumetric modulated arc therapy (VMAT) with a 10 MV beam energy. The plan is prescribed to the PTV. Axial (C) and coronal (D) images showing dose distribution. There is a max dose of 115.2% of the prescription dose with the PTV. (E) Representative axial image of follow-up computed tomography (CT) showing resolution of the treated nodule.
Given the high rate of disease progression outside of the SBRT field, elective nodal radiotherapy as metastasis-directed treatment was evaluated in a recently published landmark analysis [24], with a median follow up of 11.7 years. Definitive standard fractionation 2D/3D conformal radiation therapy (CRT) or IMRT including the entire at-risk nodal echelon was delivered to 48 patients with gynecologic oligometastases, resulting in disease-free survival and overall survival of 73 months and 200 months, respectively. Nodal sites were treated to a median dose of 62 Gy to the gross tumor volume (GTV) in 2.0–2.2 Gy per fraction with the clinical target volume (CTV) covering the adjacent nodal echelon to a dose of 45–50 Gy in 1.8–2.0 Gy fractions. Fifty percent of patients in this cohort had recurrence after a median of 28 months, predominantly with an out-of-field component, despite the extension of coverage to neighboring lymphatics. Interestingly, there was a significant improvement in disease-free survival in patients receiving any chemotherapy with radiation as opposed to radiation alone (93 months vs. 34 months, respectively). This highlights the importance of systemic therapies in the treatment of oligometastatic disease.
References
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy versus Standard of Care Palliative Treatment in Patients with Oligometastatic Cancers (SABR-COMET): A Randomised, Phase 2, Open-Label Trial. Lancet 2019, 393, 2051–2058.
- Gomez, D.R.; Blumenschein, G.R.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local Consolidative Therapy versus Maintenance Therapy or Observation for Patients with Oligometastatic Non-Small-Cell Lung Cancer without Progression after First-Line Systemic Therapy: A Multicentre, Randomised, Controlled, Phase 2 Study. Lancet Oncol. 2016, 17, 1672–1682.
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e173501.
- Ruers, T.; Punt, C.; Van Coevorden, F.; Pierie, J.P.E.N.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.A.; Mauer, M.; et al. Radiofrequency Ablation Combined with Systemic Treatment versus Systemic Treatment Alone in Patients with Non-Resectable Colorectal Liver Metastases: A Randomized EORTC Intergroup Phase II Study (EORTC 40004). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2619–2626.
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Méndez Romero, A.; Nevens, D.; Palma, D.; Park, C.; et al. Defining Oligometastatic Disease from a Radiation Oncology Perspective: An ESTRO-ASTRO Consensus Document. Radiother. Oncol. 2020, 148, 157–166.
- Adelson, M.D.; Taylor Wharton, J.; Delclos, L.; Copeland, L.; Gershenson, D. Palliative Radiotherapy for Ovarian Cancer. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 17–21.
- Choan, E.; Quon, M.; Gallant, V.; Samant, R. Effective Palliative Radiotherapy for Symptomatic Recurrent or Residual Ovarian Cancer. Gynecol. Oncol. 2006, 102, 204–209.
- Jiang, G.; Balboni, T.; Taylor, A.; Liu, J.; Lee, L.J. Palliative Radiation Therapy for Recurrent Ovarian Cancer: Efficacy and Predictors of Clinical Response. Int. J. Gynecol. Cancer 2018, 28, 43–50.
- Albuquerque, K.; Patel, M.; Liotta, M.; Harkenrider, M.; Guo, R.; Small, W.; Ronald, P. Long-Term Benefit of Tumor Volume-Directed Involved Field Radiation Therapy in the Management of Recurrent Ovarian Cancer. Int. J. Gynecol. Cancer 2016, 26, 655–660.
- Brown, A.P.; Jhingran, A.; Klopp, A.H.; Schmeler, K.M.; Ramirez, P.T.; Eifel, P.J. Involved-Field Radiation Therapy for Locoregionally Recurrent Ovarian Cancer. Gynecol. Oncol. 2013, 130, 300–305.
- Chundury, A.; Apicelli, A.; Dewees, T.; Powell, M.; Mutch, D.; Thaker, P.; Robinson, C.; Grigsby, P.W.; Schwarz, J.K. Intensity Modulated Radiation Therapy for Recurrent Ovarian Cancer Refractory to Chemotherapy. Gynecol. Oncol. 2016, 141, 134–139.
- Yegya-Raman, N.; Cao, C.D.; Hathout, L.; Girda, E.; Richard, S.D.; Rosenblum, N.G.; Taunk, N.K.; Jabbour, S.K. Stereotactic Body Radiation Therapy for Oligometastatic Gynecologic Malignancies: A Systematic Review. Gynecol. Oncol. 2020, 159, 573–580.
- Macchia, G.; Lazzari, R.; Colombo, N.; Laliscia, C.; Capelli, G.; D’Agostino, G.R.; Deodato, F.; Maranzano, E.; Ippolito, E.; Ronchi, S.; et al. A Large, Multicenter, Retrospective Study on Efficacy and Safety of Stereotactic Body Radiotherapy (SBRT) in Oligometastatic Ovarian Cancer (MITO RT1 Study): A Collaboration of MITO, AIRO GYN, and MaNGO Groups. Oncologist 2020, 25, e311–e320.
- Iftode, C.; D’Agostino, G.R.; Tozzi, A.; Comito, T.; Franzese, C.; De Rose, F.; Franceschini, D.; Di Brina, L.; Tomatis, S.; Scorsetti, M. Stereotactic Body Radiation Therapy in Oligometastatic Ovarian Cancer: A Promising Therapeutic Approach. Int. J. Gynecol. Cancer 2018, 28, 1507–1513.
- Lazzari, R.; Ronchi, S.; Gandini, S.; Surgo, A.; Volpe, S.; Piperno, G.; Comi, S.; Pansini, F.; Fodor, C.; Orecchia, R.; et al. Stereotactic Body Radiation Therapy for Oligometastatic Ovarian Cancer: A Step Toward a Drug Holiday. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 650–660.
- MacChia, G.; Jereczek-Fossa, B.A.; Lazzari, R.; Cerrotta, A.; Deodato, F.; Ippolito, E.; Aristei, C.; Gambacorta, M.A.; Scambia, G.; Valentini, V.; et al. Efficacy and Safety of Stereotactic Body Radiotherapy (SBRT) in Oligometastatic/Persistent/Recurrent Ovarian Cancer: A Prospective, Multicenter Phase II Study (MITO-RT3/RAD). Int. J. Gynecol. Cancer 2022, 32, 939–943.
- Onal, C.; Gultekin, M.; Oymak, E.; Guler, O.C.; Yilmaz, M.T.; Yuce Sari, S.; Akkus Yildirim, B.; Yildiz, F. Stereotactic Radiotherapy in Patients with Oligometastatic or Oligoprogressive Gynecological Malignancies: A Multi-Institutional Analysis. Int. J. Gynecol. Cancer 2020, 30, 865–872.
- Macchia, G.; Nardangeli, A.; Laliscia, C.; Fodor, A.; Draghini, L.; Gentile, P.C.; D’Agostino, G.R.; Balcet, V.; Bonome, P.; Ferioli, M.; et al. Stereotactic Body Radiotherapy in Oligometastatic Cervical Cancer (MITO-RT2/RAD Study): A Collaboration of MITO, AIRO GYN, and MaNGO Groups. Int. J. Gynecol. Cancer 2022, 32, 732–739.
- Lee, S.H.; Lee, S.H.; Lee, K.C.; Lee, K.B.; Shin, J.W.; Park, C.Y.; Sym, S.J.; Lee, J.-H. Radiation Therapy with Chemotherapy for Patients with Cervical Cancer and Supraclavicular Lymph Node Involvement. J. Gynecol. Oncol. 2012, 23, 159–167.
- Jeon, W.; Koh, H.K.; Kim, H.J.; Wu, H.-G.; Kim, J.H.; Chung, H.H. Salvage Radiotherapy for Lymph Node Recurrence after Radical Surgery in Cervical Cancer. J. Gynecol. Oncol. 2012, 23, 168–174.
- Kim, J.-Y.; Kim, J.-Y.; Kim, J.H.; Yoon, M.S.; Kim, J.; Kim, Y.S. Curative Chemoradiotherapy in Patients with Stage IVB Cervical Cancer Presenting with Paraortic and Left Supraclavicular Lymph Node Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 741–747.
- Aghdam, N.; Repka, M.C.; McGunigal, M.; Pepin, A.; Paydar, I.; Rudra, S.; Paudel, N.; Pernia Marin, M.; Suy, S.; Collins, S.P.; et al. Stereotactic Body Radiation Therapy: A Versatile, Well-Tolerated, and Effective Treatment Option for Extracranial Metastases From Primary Ovarian and Uterine Cancer. Front. Oncol. 2020, 10, 572564.
- Cuccia, F.; Pastorello, E.; Vitale, C.; Nicosia, L.; Mazzola, R.; Figlia, V.; Giaj-Levra, N.; Ricchetti, F.; Rigo, M.; Attinà, G.; et al. The Use of SBRT in the Management of Oligometastatic Gynecological Cancer: Report of Promising Results in Terms of Tolerability and Clinical Outcomes. J. Cancer Res. Clin. Oncol. 2021, 147, 3613–3618.
- Corrigan, K.L.; Yoder, A.; De, B.; Lin, L.; Jhingran, A.; Joyner, M.M.; Eifel, P.J.; Colbert, L.E.; Lu, K.H.; Klopp, A.H. Long-Term Survival Following Definitive Radiation Therapy for Recurrence or Oligometastases in Gynecological Malignancies: A Landmark Analysis. Gynecol. Oncol. 2022, 164, 550–557.
- Smile, T.; Reddy, C.A.; Qiao-Guan, G.; Amarnath, S.R.; Stephans, K.L.; Woody, N.M.; Balagamwala, E.H.; AlHilli, M.M.; Michener, C.; Mahdi, H.; et al. Stereotactic Body Radiotherapy for the Treatment of Oligometastatic Gynecological Malignancy in the Abdomen and Pelvis: A Single-Institution Experience. Int. J. Radiat. Oncol. 2020, 108, e169.
- Kataria, T.; Naga, P.; Banerjee, S.; Gupta, D.; Narang, K.; Tayal, M.; Bisht, S.S. CyberKnife Stereotactic Ablative Radiotherapy for Recurrent or Oligometastatic Gynecological Cancers. South Asian J. Cancer 2021, 10, 107–111.
- Reshko, L.B.; Baliga, S.; Crandley, E.F.; Harry Lomas, I.V.; Richardson, M.K.; Spencer, K.; Bennion, N.; Mikdachi, H.E.; Irvin, W.; Kersh, C.R. Stereotactic Body Radiation Therapy (SBRT) in Recurrent, Persistent or Oligometastatic Gynecological Cancers. Gynecol. Oncol. 2020, 159, 611–617.
- Ito, M.; Kodaira, T.; Koide, Y.; Okuda, T.; Mizumatsu, S.; Oshima, Y.; Takeuchi, A.; Mori, T.; Abe, S.; Asai, A.; et al. Role of High-Dose Salvage Radiotherapy for Oligometastases of the Localised Abdominal/Pelvic Lymph Nodes: A Retrospective Study. BMC Cancer 2020, 20, 540.
- Reddy, A.V.; Mills, M.N.; Reshko, L.B.; Martin Richardson, K.; Kersh, C.R. Stereotactic Body Radiation Therapy in Oligometastatic Uterine Cancer: Clinical Outcomes and Toxicity. Cancer Investig. 2020, 38, 522–530.
- Laliscia, C.; Fabrini, M.G.; Delishaj, D.; Morganti, R.; Greco, C.; Cantarella, M.; Tana, R.; Paiar, F.; Gadducci, A. Clinical Outcomes of Stereotactic Body Radiotherapy in Oligometastatic Gynecological Cancer. Int. J. Gynecol. Cancer 2017, 27, 396–402.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
598
Revisions:
2 times
(View History)
Update Date:
21 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




