Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Subhanullah Khan | -- | 4655 | 2023-07-19 14:35:14 | | | |
| 2 | Rita Xu | Meta information modification | 4655 | 2023-07-20 03:15:27 | | | | |
| 3 | Rita Xu | Meta information modification | 4655 | 2023-07-20 03:18:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khan, S.; Lang, M. Metals in Insects. Encyclopedia. Available online: https://encyclopedia.pub/entry/46983 (accessed on 02 March 2026).
Khan S, Lang M. Metals in Insects. Encyclopedia. Available at: https://encyclopedia.pub/entry/46983. Accessed March 02, 2026.
Khan, Subhanullah, Minglin Lang. "Metals in Insects" Encyclopedia, https://encyclopedia.pub/entry/46983 (accessed March 02, 2026).
Khan, S., & Lang, M. (2023, July 19). Metals in Insects. In Encyclopedia. https://encyclopedia.pub/entry/46983
Khan, Subhanullah and Minglin Lang. "Metals in Insects." Encyclopedia. Web. 19 July, 2023.
Copy Citation
Insects and microbial pathogens are ubiquitous and play significant roles in various biological processes, while microbial pathogens are microscopic organisms that can cause diseases in multiple hosts. Insects and microbial pathogens engage in diverse interactions, leveraging each other’s presence. Metals are crucial in shaping these interactions between insects and microbial pathogens.
insects
microbial pathogens
metals
1. Introduction
Insects and microbial pathogens carry out many essential biological functions because they are present in varying amounts in nature [1]. Insects are a versatile group of organisms occupying various ecological niches and are requisite for many reasons, including pollination, decomposition, and pest control. However, some insects are pests that pose significant economic and health risks to humans, animals, and plants.
Microbial pathogens, however, are microscopic organisms that can cause disease in various hosts, including insects, humans, plants, and animals [2]. Insects and microbial pathogens interact in a variety of ways. Some insect-specific microbial pathogens have evolved to exploit their insect hosts’ unique physiological and behavioral properties [3]. Other pathogens have a broader host range and can infect multiple species of insects. Insects, in turn, have evolved various mechanisms such as physical barriers, immune responses, and behavioral adaptation to defend themselves against microbial pathogens [4]. The interactions between insects and microbial pathogens involve various physiological and biochemical processes [5]. Metals are a critical factor that plays a crucial role in these interactions. Moreover, the study investigated that metals are pertinent to many physiological processes in insects, plants, and microbial pathogens, including digestion, respiration, and immunity [6]. However, metals can also limit the growth and virulence of microbial pathogens and act as a defense mechanism for insects against infection [7]. Understanding the role of metals in the interactions between insects and microbial pathogens can provide insights into the mechanisms of disease transmission and the development of new strategies to control insect pests and microbial pathogens.
Extensive documentation highlights that metals are essential for various biological processes and important for facilitating interactions among plants, animals, and their environment [8]. Metals involve physiological and biochemical processes, including energy production, enzyme activation, and cellular signaling [9][10]. They also play an indispensable role as a cofactor in many proteins and enzymes, such as hemoglobin, myoglobin, and cytochrome c, involved in respiration and other metabolic pathways [11]. However, various studies have identified that metals play a crucial role in immunological reactions and act as cofactors for enzymes accountable for producing reactive oxygen species (ROS) and other antimicrobial compounds [12]. These metals have toxic effects on living organisms when present in higher amounts. Metals toxicity can disrupt the structure and functions of proteins and enzymes by binding to their functional groups nonspecifically [13]. Toxic metals in higher concentrations produce higher ROS, which has been observed to produce harmful effects on cells and tissues [14].
2. Role of Metals in Insects
2.1. Iron (Fe)
Iron (Fe) plays several vital roles in insects. It is an essential part of hemoglobin and acts as a cofactor in several enzymes involved in various metabolic pathways, such as respiration and energy production [15].
Fe is also crucial for insects’ proper development and growth, as it synthesizes proteins and DNA [16]. The mechanism of Fe absorption in insects encompasses acidification of the midgut, interaction with Fe-binding proteins like transferrin, direct uptake of heme, and storage of excess Fe as ferritin [17]. Acidification of the midgut creates an acidic environment that enhances Fe solubility, allowing for its absorption by gut epithelial cells. Insects also utilize transferrin receptors on gut cells to facilitate the endocytosis of Fe-transferrin complexes [18]. Blood-feeding insects can directly absorb heme from host blood, which is transported across the gut epithelium for various physiological processes. Excess Fe is stored in ferritin, acting as a Fe reservoir that can be utilized when Fe availability is limited. These mechanisms ensure efficient Fe acquisition and utilization in insects for essential physiological functions. It can also improve the insect immune system by producing ROS to inhibit the growth of microbial pathogens [19].
Moreover, the study showed that a protein known as ferroportin helps transport and regulate Fe in various types of cells, whether absorbed from the diet via intestinal enterocytes, recycled by macrophages, or stored in hepatocytes. These proteins, such as transferrin, cross the cell membrane to reach the plasma Fe carrier protein [20]. The precise topology and mechanism of Fe transport through ferroportin are not well-understood, and these are considered to be significant unresolved questions in Fe biology. Ferroportin is abundant in specific cells known for Fe export, such as duodenal enterocytes, splenic and hepatic macrophages, and to a lesser extent, hepatocytes [21]. It is also found in the lung, renal tubules, and erythrocyte precursors in the bone marrow [22], although its function in these locations is unclear. Ferroportin transports Fe into the bloodstream on the basal side of enterocytes, and divalent metal transporters expressed on the luminal side of enterocytes regulate Fe absorption in the gut. In the transferrin cycle, the divalent metal transporter actively transfers Fe into enterocytes and is also expressed in macrophages and endosomes [23]. The release of free Fe from the transferrin-receptor complex causes a pH-mediated conformational shift in the endosomes, where it is then transported to the cytoplasm via a divalent metal transporter [24]. Serum transferrin, a member of the transferrin superfamily of proteins, including ovotransferrin and lactoferrin, transports Fe throughout the body. This export protein enables Fe efflux from macrophages and Fe acquisition by enterocytes [25]. Heptaglobin binds hemoglobin to the Heme that is secured by hemopexin. Circulating Hp can effectively manage moderate hemolysis, saturating at 1.5 g/L free Hb [26]. Hepatocytes and macrophages have receptors that uniquely recognize the Hp/Hb complex, as shown in Figure 1.
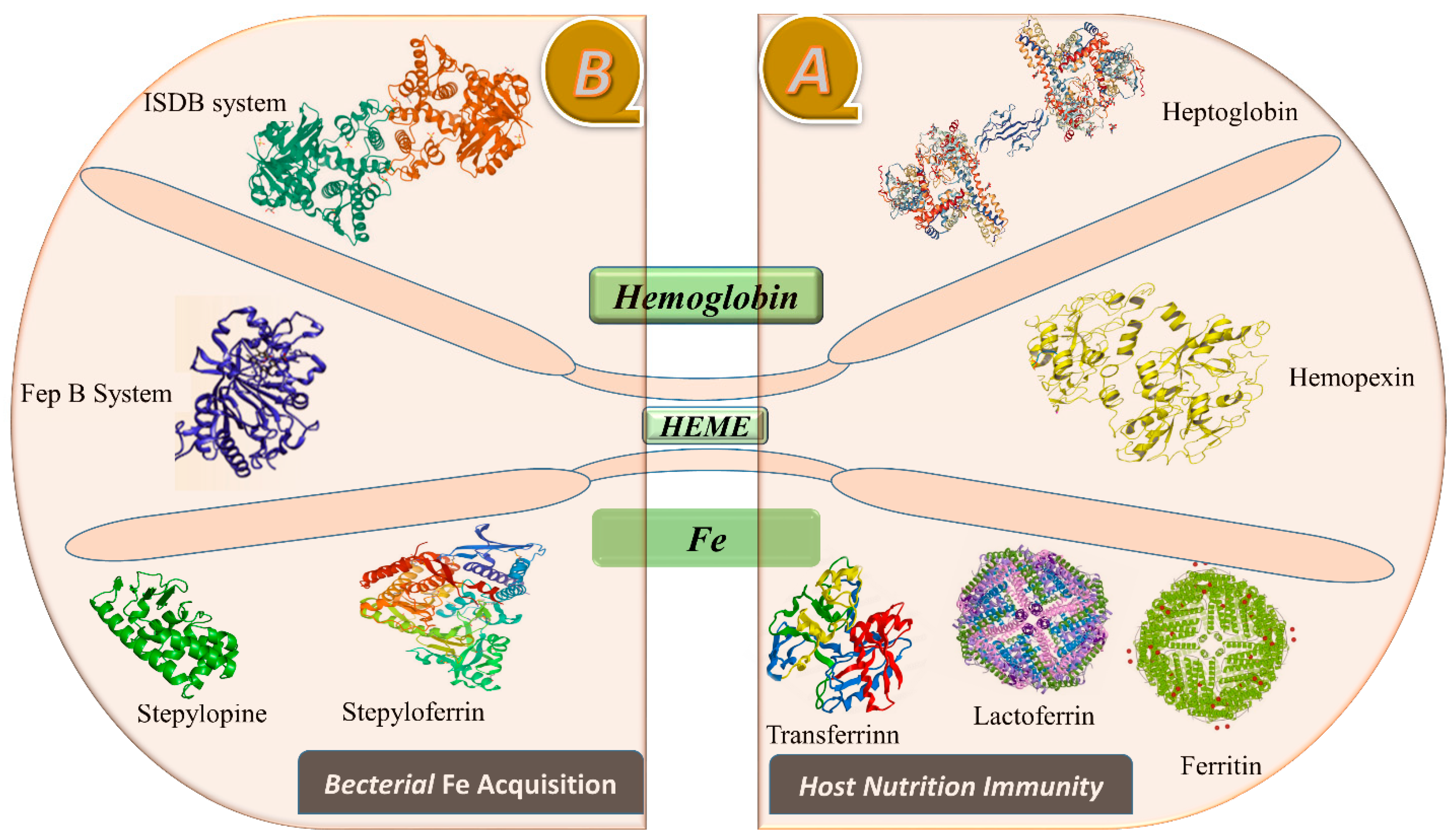
Figure 1. This figure illustrates the proteins recruited in the sequestration of Fe by host nutritional immunity, as shown in (A). In contrast, (B) depicts the bacterial effectors in Fe retrieval.
Fe is an essential nutrient for the survival and growth of microbial pathogens, as it is required for several critical physiological processes [27]. However, Fe is not readily available in the host environment, as it is tightly bound to host proteins such as transferrin and lactoferrin to prevent microbial growth [28]. Therefore, microbial pathogens have evolved multiple strategies to harvest Fe from the host. One such strategy is the production of siderophores, small molecules that chelate Fe and facilitate its acquisition from cells [29]. Many bacterial pathogens produce siderophores; some can even intercept siderophores from other microbes. Another strategy is the expression of high-affinity Fe transporters, which actively enable the pathogen to take up Fe from host proteins. However, the Gram-positive pathogen Staphylococcus aureus expresses the ScaABC transporter, specific for transferrin-bound Fe [30]. Siderophores are increasingly recognized for their contribution to virulence beyond simple Fe chelation. They also act as signals that elicit a strong host defense, promoting mitophagy, hypoxic responses, and cytokine production [31][32]. Therefore, microbial pathogens must overcome these obstacles to acquire sufficient Fe for survival and virulence. Scrutinizing the mechanisms of Fe acquisition in pathogens is crucial for developing novel antimicrobial strategies [33]. However, targeting siderophores or Fe transporters could limit the growth and virulence of pathogens. For pathogenic bacteria, obtaining sufficient Fe during and after infection inside the host is one of the main barriers. At the host–pathogen interface, an analytical structure describes the flow of signaling and the struggle for shared resources between the host and pathogen, and host–pathogen competition for this valuable transition metal take place, as shown in Figure 2. In addition, Fe-based therapies, such as Fe chelation therapy, have also been studied as potential treatments for infectious diseases [34]. However, the efficacy of such therapies has yet to be determined, as Fe is integral to host physiology and immune function.
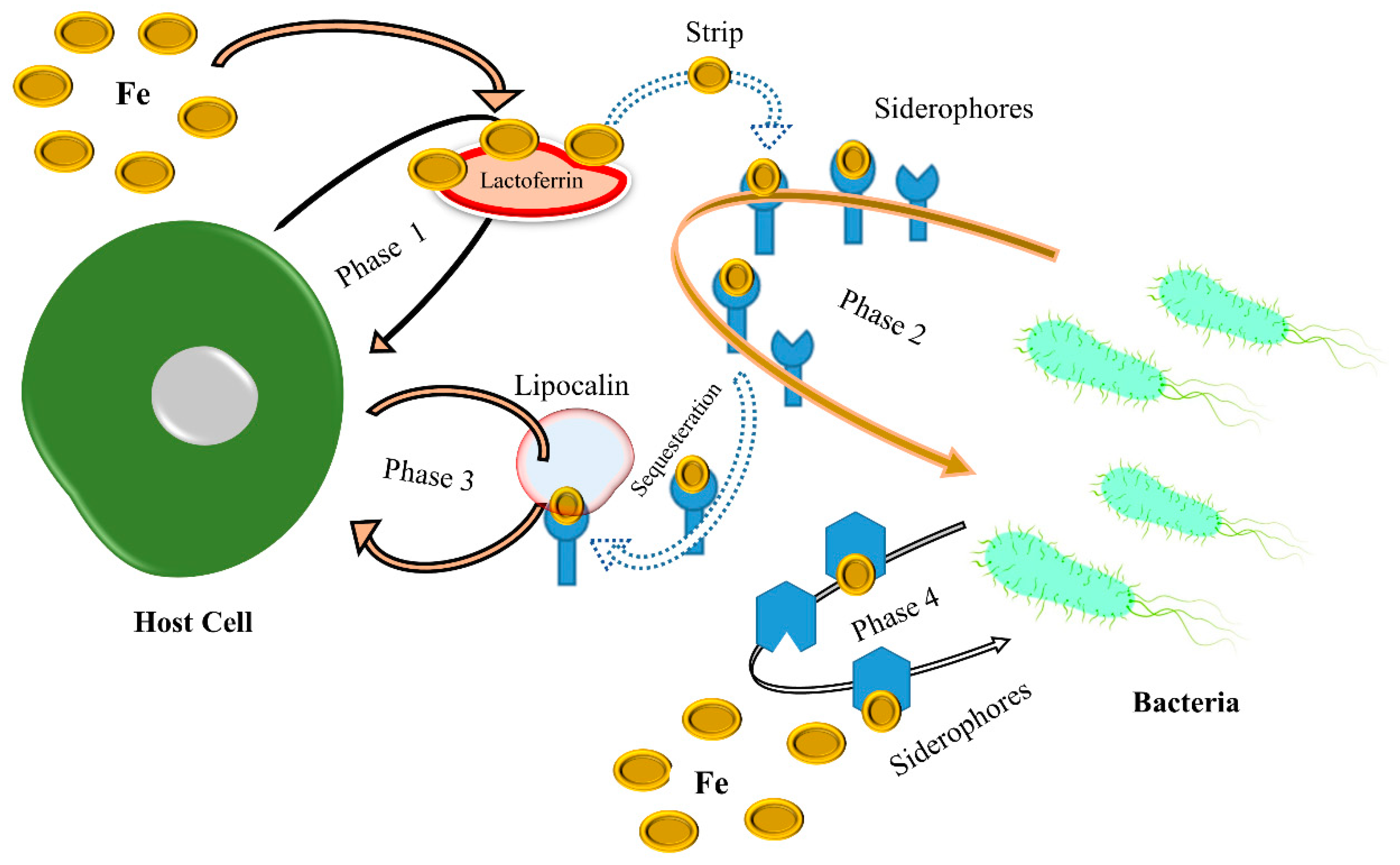
Figure 2. In phase 1, this figure shows that when in a tug-of-war with Fe, host cells produce Fe-binding proteins such as lactoferrin to prevent pathogens from taking up Fe. However, while the pathogens in phase 2 use high-affinity siderophores as a defense mechanism to extract Fe from host proteins, the host cells produce siderophore-binding proteins such as lipocalin to neutralize the siderophores and stop pathogen acquisition, as shown in phase 3. Pathogens can generate siderophores to which lipocalin cannot bind to ensure pathogen survival, as shown in phase 4.
It has been widely recognized that microbial pathogens depend on acquiring and utilizing Fe from their host for survival. However, the host immune system’s mechanisms for retaining Fe limit this process [35]. Several Fe acquisition mechanisms have evolved in microbial pathogens to overcome this limitation, including the production of siderophores, Fe transporters, heme acquisition systems, and Fe-regulated surface proteins. Siderophores are small Fe-chelating molecules secreted by pathogens that bind and transport Fe into the cell [36]. Fe transporters and heme acquisition systems help pathogens acquire Fe from host proteins, while Fe-regulated surface proteins capture Fe from host transferrin and lactoferrin. Fe-responsive regulators such as Fur tightly regulate Fe acquisition in pathogens, which regulate the expression of genes involved in Fe acquisition and metabolism [37][38]. Fe acquisition mechanisms such as siderophores and Fe transporters in bacterial infections are crucial virulence factors that allow bacteria to overcome host Fe retention mechanisms and establish conditions [39]. Fe acquisition mechanisms such as high-affinity Fe transporters and Fe-responsive regulators are pertinent to fungal virulence and pathogenicity in fungal infections. In parasitic infections, Fe is involved in several essential processes, such as energy production, DNA synthesis, and oxidative metabolism, and is prominent for the parasite’s survival and growth in the host [40]. The role of Fe in interactions between insects and microbial pathogens is complex and diverse, with both essentiality and toxicity playing integral parts.
2.2. Interaction of Plants with Insects and Microbes via Fe
There is substantial proof that Fe is an essential nutrient for plants and many microbes and necessary for their growth and development. The availability of Fe can influence plant–insect and plant–microbe interactions [41], and Fe-rich soils can increase the abundance and activity of certain beneficial microbial species, such as mycorrhizal fungi. These rhizobacteria can help plants become more herbivore-tolerant and improve their defenses against herbivores [42][43]. Moreover, these beneficial microbial species may also prevent and reduce the populations of certain insect pests [44].
The plant Fe deficiency response is regulated at the transcriptional and post-translational levels. Hormones like auxin, ethylene, nitric oxide, cytokinin, and gibberellic acid play vital roles in this process [45]. In Arabidopsis, ethylene and gibberellic acid enhance Fe uptake by increasing FRO2 and IRT1 expression. Ethylene and auxin promote nitric oxide accumulation, stabilizing FIT and improving Fe uptake. Auxin also stimulates lateral root formation for increased Fe absorption [46].
Conversely, cytokinin inhibits root growth and suppresses Fe deficiency response genes: salicylic acid and jasmonic acid, two major defense hormones, influence plant Fe acquisition [47]. Salicylic acid positively affects Fe uptake gene expression in Arabidopsis through auxin and ethylene signaling [48]. On the other hand, jasmonic acid negatively regulates Fe acquisition by downregulating Fe uptake genes independently of FIT [49]. Ethylene and auxin hormones are crucial in the plant immune signaling network. This connection between Fe availability and immunity highlights their potential role in Fe uptake responses in plant roots.
2.3. Zinc (Zn) and Copper (Cu)
Like humans, insects rely on dietary intake of trace metals like Zn and Cu for proper physiological functioning. These specified metals are responsible for multiform insect processes, including DNA synthesis, oxidation reactions, cuticle biosynthesis, and acting as essential cofactors for numerous enzymes [50]. Their presence is indispensable for insects’ everyday functioning and overall well-being at the molecular and biochemical levels. In insect studies, two families of Zn transporters: ten dZip and seven dZnT proteins, analogous to human Zip (SLC39) and ZnT (SLC30) families [51]. These transporters play roles in Zn influx and efflux, with specific expression in the midgut (dZip1) and Malpighian tubules (dZnT35C), contributing to Zn absorption and excretion. Zn is distributed throughout the gastrointestinal tract, with higher accumulation in the posterior midgut, crop, and Malpighian tubules [52]. dZip1 and dZip2 import Zn from the lumen into the enterocyte, while dZnt1 and ZnT77C release imported Zn into circulation from the basolateral membrane. The silencing of dZnt1, specifically in the gut, increases lethality under Zn-deficient conditions, highlighting its crucial role in Zn absorption [53]. Zn repletion leads to the suppression of dZip1 and dZip2 mRNA expression and the protein expression of dZnt1 [54].
The expression of dZnt1 and dZnT35C, a potential ZnT2 homolog, is regulated by dMTF1. FOI, an ortholog of dZip6 and dZip10, is essential for cell migration and gonad morphogenesis by controlling DE-cadherin expression at the posttranscriptional level [53]. A Catsup mutant with a defective dZip7 exhibits high levels of catecholamines and shows signs of semi-dominant lethality. The mutant also displays defects in membrane protein trafficking and increased ER stress [55]. On the other hand, Zn is embarked on regulating gene expression in insects, where it acts as a cofactor for several transcription factors that control gene expression [56]. Zn is also involved in regulating insect development, which plays a crucial role in insect cuticle formation and the regulation of molting.
The most prominent finding to emerge is that Cu is a crucial part of the innate immune system of insects, where it acts as a cofactor for the enzyme phenoloxidase. Phenoloxidase plays a significant role in the insect immune response by catalyzing the oxidation of phenolic compounds to quinones, which are toxic to microorganisms [57][58]. The quinones also contribute to the formation of melanin, which is pivotal for encapsulating pathogens via insect defense cells.
Phenol + O2 + Cu2+ → Quinone + H2O + Cu+
Quinone + Quinone + Cu+ → Melanin + Cu2+
In this reaction, the copper becomes an ion (Cu2+), acting as a cofactor for the phenoloxidase enzyme and facilitating the transfer of electrons during the oxidation of phenols. The reaction cannot proceed without the Cu ion, and melanization cannot occur, making the insect vulnerable to foreign invaders.
Furthermore, Cu also regulates the insect’s antioxidant defense system, which protects the insect’s cells from oxidative damage caused by the ROS produced during the immune response [59]. However, it has been reported that dMTF-1 is a crucial regulator of essential metal homeostasis, controlling gene expression in metal pathways [60]. DmATP7 is vital for Cu uptake and efflux in insects, particularly during larval development. DmATP7 term depends on functional dMTF-1, while its background expression is maintained in dMTF-1 knockout flies [61]. dMTF-1 also regulates the Cu importer protein Ctr1B in response to Cu-specific stress, facilitating increased Cu uptake. Knockout flies lacking dMTF-1 exhibit decreased survival and prolonged development due to impaired metal regulation [62]. dMTF-1 is also crucial for transcription factors; an insect tightly regulates these metals. It controls the expression of ZnT and Zn exporter proteins involved in the uptake and efflux of Zn while also regulating Cu-related genes. dMTF-1’s concentration gradient between the cytosol and nucleus governs the regulation of Zn exporters [63]. Zn toxicity induces dMTF-1 upregulation and translocation into the nucleus, where it binds to the MRE upstream of ZnT, promoting ZnT transcription and Zn exporter production [64]. Although the regulation of Zn importers via dMTF-1 in insects is not yet established, it is possible that some Zn importers may also be influenced by dMTF-1. This regulatory mechanism involving dMTF-1 ensures the maintenance of Zn and Cu homeostasis, essential for RNA and DNA metabolism. On the other hand, Zn is required for the proper functioning of several immune-related enzymes, including alkaline phosphatase and carbonic anhydrase. These enzymes play an integral role in the insect’s immune response by regulating the insect’s tissue pH and modulating the immune cells’ activity [65]. Zn is also immersed in regulating the expression of several immune-related genes in insects, including genes encoding antimicrobial peptides, which are essential in the insect’s defense against microbial pathogens [66].
A number of studies have disclosed that Zn and Cu are crucial cofactors for various enzymes involved in multiple biochemical reactions in insects. Zn is required to function in enzymes integrated into DNA synthesis, RNA transcription, and protein synthesis, as well as repair enzymes properly [67]. Zn ions (Zn2+) are coordinated to the amino acid residues of DNA polymerases, such as DNA polymerase III. This coordination stabilizes the enzyme’s binding to the DNA template and allows for the accurate replication of genetic information [68][69]. Zn ions interact with the negatively charged phosphate backbone of the DNA molecule and form coordination complexes that stabilize the enzyme-DNA complex. This coordination also helps to properly position the deoxynucleoside triphosphate (dNTP) substrates for incorporation into the growing DNA chain, resulting in the actual complexity of the genetic information [70]. In addition to its role in DNA polymerase activity, Zn is also involved in the movement of other enzymes involved in DNA synthesis and repair, such as DNA ligases and topoisomerases. Zn ions cooperate in binding these enzymes to DNA substrates, allowing for the efficient repair of DNA strand breaks and the accurate replication of genetic information [71].
Moreover, the host immune system tightly regulates the availability of these metals in the host environment, which can limit their accessibility to invading pathogens. In microbial pathogens, Zn can also control the expression of virulence factors and the formation of biofilms, which can enhance their ability to colonize and infect the host [72]. However, the host immune system can absorb Zn through various mechanisms, such as the production of metal-binding proteins, to limit its availability to invading pathogens [73]. Cu is an essential cofactor for many enzymes involved in cellular processes, including respiration, response to oxidative stress, and Fe acquisition, as well as control the virulence factor and biofilm formation in pathogens. However, excess Cu can be toxic to cells by generating ROS, which can damage DNA, proteins, and lipids [74], as shown in Figure 3. Hence, microbial pathogens have evolved various mechanisms to deal with excess Cu, such as producing Cu-binding proteins and activating detoxification systems. Overall, the roles of Zn and Cu in microbial pathogens are complex and tightly regulated by the host immune system [73].
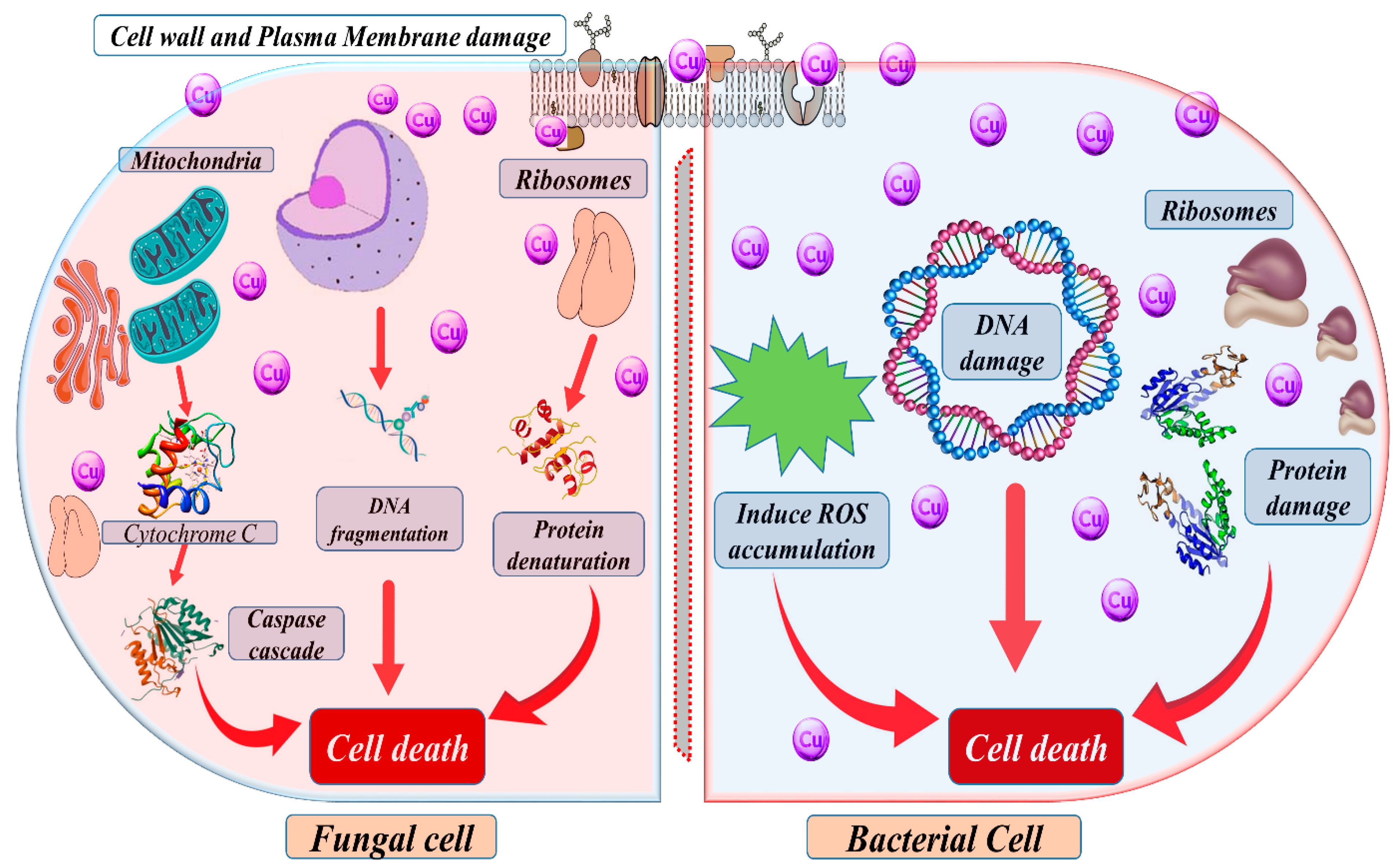
Figure 3. A schematic diagram depicting Cu’s potential mechanism of action on microorganisms, which can be proposed as follows: Cu impacts the cell wall of microbes, disrupting its constituents and damaging the membrane. This membrane damage subsequently reduces the electrochemical potential, compromising the integrity of the membrane. Furthermore, Cu specifically targets microorganisms’ DNA, interfering with the synthesis of proteins and inducing detrimental effects that eventually lead to the demise of the microbial cell.
2.4. Interaction of Plants with Insects and Microbes through Zn and Cu Metals
Plants interact with insects and microbes by competing with them to gain metals such as Zn and Cu. Within the realm of enzyme activity, Zn is essential for the proper function of DNA/RNA polymerase enzymes, ribosomes, and superoxide dismutase (SOD) [75]. It exhibits specific significance in plants, present in carbonic anhydrase and stromal processing peptides, thereby contributing to photosynthesis [76]. Furthermore, Zn contributes to protein structure, with approximately 4% of Arabidopsis proteins containing Zn finger domains, emphasizing its functional importance in plant physiology [77]. Cu is imperative for active functioning critical enzymes such as cytochrome oxidases, ascorbate oxidase, superoxide dismutase (SOD), and polyphenol oxidase [78][79]. In plants, Cu is also required for the receptor signaling of the hormone ethylene, which plays a crucial role in plant development and disease resistance [80]. These metals are needed to form chlorophyll, which is pivotal for photosynthesis, and for auxin production. This hormone stimulates cell division, elongation, tissue differentiation, and tropism (responses to environmental stimuli) [81]. In addition, these metals can be used by insects and microbes as energy sources and for the biotransformation of compounds, such as nitrogen and sulfur. In addition, Zn and Cu can be used by plants to ward off herbivores, as they are toxic to certain insects [82]. However, Zn toxicity occurs in agricultural soils treated with sewage sludge, in urban and suburban soils enhanced via anthropogenic inputs of Zn, especially in soils with low pH, and in soils affected by mining and smelting activities [83]. Mechanisms for creating either low or high Zn scenarios in plant and animal systems are essential for Zn-based disease and pest control.
Eukaryotic cells have an impressive ability to regulate the levels of Zn within their interiors. Despite Zn being commonly present in lower concentrations at the interiors of the cell, it is present in higher ranges outside the eukaryotic cells [84]. A diverse range of proteins, such as ZIP (ZRT- and IRT-like proteins), ZNT (Zn transporter), and metallothioneins (MTs) that sequester Zn, are involved in regulating Zn equilibrium in plants. ZNT proteins transport Zn within cells, averting cytotoxicity by sequestering it within vacuoles, while ZIP proteins facilitate the absorption of Zn from the soil into plant root cells [85]. MTs act as Zn chelators, storing excess Zn in a harmless form, protecting against Zn deficiency. This cooperative system ensures Zn’s controlled distribution, storage, and acquisition. ZIP transporters respond to low Zn levels by upregulation, whereas ZNT transporters and MTs become active when Zn levels are high, guaranteeing proper physiological development and plant function [14][86].
In contrast, ZNT proteins decrease intracellular Zn levels by promoting Zn release from the cell or its uptake into intracellular vesicles. The sequestration of Zn is primarily controlled by Zn-dependent mechanisms that regulate the transcription, translation, and intracellular trafficking of these transporters [87]. Indeed, many studies show that the expression levels of Zn transporters in plant tumors have been found to correlate with the severity of malignancy, indicating that disruptions in intracellular Zn homeostasis can contribute to cancer progression. In various types of cancer, specific Zn importers are upregulated, potentially enabling tumor cells to evade programmed cell death (apoptosis) and activate survival mechanisms through autophagy [88][89]. ZIPs and ZNTs are among the critical Zn transporters involved in these processes, as shown in Figure 4. In addition, microbes can use these metals to make antibiotics that plants can use to protect against infection [90].

Figure 4. The localization and transport of Zn within crucial cells. This figure illustrates the processes involved in ZIP and ZNT Zn transporter families. The arrows indicate the movement of Zn. When Zn levels are low, ZIP1, ZIP2, and ZIP4 transporters become active, while Zn administration stimulates the expression of ZNT-1 and ZNT-2 transporters. Typically, a higher Zn efflux increases susceptibility to apoptosis, whereas elevated Zn levels offer protection and promote autophagy.
Zn and Cu can be found in soil and taken up by plants, and are also present in the bodies of insects and microbes [91]. Zn also helps protect plants from diseases caused by fungi, bacteria, and viruses by producing phytohormones, such as salicylic acid, and activating the plant’s defense mechanisms [92]. Zn can also induce the expression of defense-related genes, such as those involved in synthesizing phytoalexins, which are antimicrobial compounds that may help plants ward off disease [93]. In addition, Zn can stimulate the production of secondary metabolites, such as flavonoids, with antimicrobial properties [94][95]. Zn also helps maintain the structural integrity of plant tissues, which can prevent pathogen invasion.
Cu is significant for enzymes to function properly and to protect plants from environmental factors such as cold, heat, and drought [96]. One of the main functions of Cu in plants is as a cofactor for enzymes implicated in various metabolic pathways, including photosynthesis, respiration, and lignin synthesis. It serves as a component of the primary electron donor in the photosystem 1 of plants [97]. Due to its ability to readily gain and lose electrons, Cu acts as a cofactor for oxidase, mono, and di-oxygenase enzymes such as amine oxidases, ammonia monooxidase, ceruloplasmin, and lysyl oxidase. Additionally, Cu is involved in the function of enzymes responsible for eliminating superoxide radicals, including superoxide dismutase and ascorbate oxidase [98]. It can also improve plants’ resistance to these stresses by increasing antioxidant activity and reducing oxidative damage. Additionally, Cu is pertinent in maintaining membrane integrity and stability, which can help prevent water loss during drought and cold stress [99].
Insects and microbes are vital to plants’ health, as they help to provide plants with essential nutrients and water [100]. Microbes also help break down soil nutrients, making them available to plants. Insects can also provide prominent nutrients to the plant, such as nitrogen, phosphorus, and potassium, and other beneficial compounds such as Ca, Mg, and S, as well as the vitamins and hormones necessary for the proper functioning of enzymes and other metabolic processes in plants [101][102]. In addition to providing essential nutrients, insects can transfer other beneficial compounds to plants. However, some insects can transmit plant-growth-promoting hormones such as gibberellins and auxins to stimulate plant growth and development [103]. Insects can also transfer vitamins and antioxidants such as vitamins C and E, which may help protect plants from oxidative damage [104].
2.5. Metals Other Than Fe, Cu, and Zn
Metals other than Fe, Cu, and Zn, like manganese (Mn), nickel (Ni), cobalt (Co), and molybdenum (Mo), are also essential trace elements that play prominent roles in the growth and development of plants, insects, and microbes [105]. Metal ions, mainly through the Haber–Weiss reaction, are pivotal for oxidative modifications of free amino acids and proteins [106]. Commonly oxidized amino acid residues include histidine, arginine, lysine, proline, methionine, and cysteine. These site-specific modifications occur at metal binding sites within proteins [107]. One significant consequence of oxygen-free radical-induced protein damage is their susceptibility to protease degradation [108].
Additionally, protein oxidation can release its binding metals, such as Fe2+ from [4Fe-4S] clusters found in certain dehydratases like aconitases [109]. On the other hand, metal (e.g. Mn, Ni, Co, etc.) binding to the cell nucleus leads to genotoxic damage, including DNA base modifications, DNA–protein cross-linkages, DNA strand breaks, rearrangements, and depurination [110]. Reactive oxygen species generated via metal-mediated production induce pro-mutagenic adducts, such as 8-oxoG (8-oxo guanine), which can cause C to T transversion mutations without DNA repair [111]. Metal-induced carcinogenicity and acute toxicity involve oxidative damage, DNA methylation aberration, and chromatin condensation [112].
Manganese (Mn) is involved in synthesizing chitin, a component of the insect’s exoskeleton. In addition, it also plays a significant role in the development of reproductive organs and is necessary for the appropriate maturation of eggs in certain insect species [113]. Ni is implicated in the metabolism of carbohydrates, amino acids, and lipids and is necessary for synthesizing enzymes that are pivotal for insect growth and development [114]. Co is essential for the metabolism of carbohydrates, amino acids, and lipids and is involved in the synthesis of hemoglobin, which is vital for insect oxygen transport [115]. However, Mo is an essential nutrient utilized as a prosthetic group in oxidoreductases. Its molybdoenzymes, identified in Drosophila, play crucial roles in metabolism, including the breakdown of acetaldehyde and purines [116].
Furthermore, these metals also play a pivotal role in the physiology and pathogenesis of the microbial pathogens, in which delicate mechanisms have evolved to acquire and regulate their levels from the host environment [117]. Mn is vital for the growth and survival of bacterial pathogens and contributes to biological processes like oxidative stress management and DNA protection [118]. Mn is also enlisted in expressing virulence factors such as adhesins and capsules in bacterial pathogens [119]. Moreover, it can affect the stability and folding of proteins involved in the virulence factor synthesis, such as capsule polysaccharides [120]. Subsequently, Ni is considered the most critical cofactor for several enzymes involved in energy metabolism and nitrogen fixation and is required for the growth and survival of many bacterial pathogens. Bacterial pathogens have undergone evolutionary adaptations in their acquisition mechanisms, enabling them to effectively regulate nickel (Ni) levels from the host environment [121]. Ni is also a critical component of some virulence factors such as urease in bacterial pathogens [122]. Co is a component of vitamin B12, which is pivotal for the growth and survival of many bacterial pathogens [123][124], while it can also regulate virulence factors, such as siderophores, in the bacterial pathogens [73]. Moreover, the bacterial virulence factors such as adhesins and capsules also require an optimum concentration of Mo for their expression [125], and Mo is also an essential cofactor for the function of various enzymes involved in redox reactions, including nitrate reductase, formate dehydrogenase, and aldehyde oxidase [126].
2 Mo(VI) + 3 NADH + 9 H+ + 2 NO3− → 2 Mo(IV) + 3 NAD+ + 6 H2O + 2 NO2−
Mo(VI) + NADH + H+ + HCOOH → Mo(IV) + NAD+ + H2O + CO2
Mo(VI) + H2O + RCHO → Mo(IV) + 2 H+ + RCOOH
In redox reactions, Mo acts as a catalytic virtuoso, facilitating the transfer of electrons and protons between the substrates, esteemed cofactors (such as NADH), and the ultimate products. The presence of Mo is crucial for the proper functioning of these enzymes and their involvement in redox processes.
References
- Zhang, L.; Su, Q.F.; Wang, L.S.; Lv, M.W.; Hou, Y.X.; Li, S.S. Linalool: A ubiquitous floral volatile mediating the communication between plants and insects. J. Syst. Evol. 2022, 61, 538–549.
- Leitão, J.H. Microbial Virulence Factors. IJMS 2020, 21, 5320.
- Biere, A.; Bennett, A.E. Three-way interactions between plants, microbes and insects. Functional 2013, 27, 567–573.
- Zhang, Q.; Chen, X.; Xu, C.; Zhao, H.; Zhang, X.; Zeng, G.; Qian, Y.; Liu, R.; Guo, N.; Mi, W. Horizontal gene transfer allowed the emergence of broad host range entomopathogens. Proc. Natl. Acad. Sci. USA 2019, 116, 7982–7989.
- Guo, Z.; Guo, L.; Bai, Y.; Kang, S.; Sun, D.; Qin, J.; Ye, F.; Wang, S.; Wu, Q.; Xie, W. Retrotransposon-mediated evolutionary rewiring of a pathogen response orchestrates a resistance phenotype in an insect host. Proc. Natl. Acad. Sci. USA 2023, 120, e2300439120.
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E. Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability. Microb. Ecol. 2023, 1–32.
- Perveen, N.; Muhammad, K.; Muzaffar, S.B.; Zaheer, T.; Munawar, N.; Gajic, B.; Sparagano, O.A.; Kishore, U.; Willingham, A.L. Host-pathogen interaction in arthropod vectors: Lessons from viral infections. Front. Immunol. 2023, 14, 1061899.
- Gebre, S.H. Bio-inspired Synthesis of Metal and Metal Oxide Nanoparticles: The Key Role of Phytochemicals. J. Clust. Sci. 2023, 34, 665–704.
- Page, M.G.P. The Role of Iron and Siderophores in Infection, and the Development of Siderophore Antibiotics. Clin. Infect. Dis. 2019, 69 (Suppl. 7), S529–S537.
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40.
- Zhang, Y.-Y.; Li, X.-S.; Ren, K.-D.; Peng, J.; Luo, X.-J. Restoration of metal homeostasis: A potential strategy against neurodegenerative diseases. Ageing Res. Rev. 2023, 87, 101931.
- Dharmaraja, A.T. Role of Reactive Oxygen Species (ROS) in Therapeutics and Drug Resistance in Cancer and Bacteria. J. Med. Chem. 2017, 60, 3221–3240.
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 544–568.
- Lin, Y.F.; Aarts, M.G. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. CMLS 2012, 69, 3187–3206.
- Thallaj, N. Review of a Few Selected Examples of Intermolecular Dioxygenases Involving Molecular Oxygen and Non-Heme Iron Proteins. Int. J. Adv. Parmacutical Sci. Res. (IJAPSR) 2023, 3, 1–18.
- Rout, G.R.; Sahoo, S. ROLE OF Iron in Plant Growth and Metabolism. Rev. Agric. Sci. 2015, 3, 1–24.
- Stijlemans, B.; Beschin, A.; Magez, S.; Van Ginderachter, J.A.; De Baetselier, P. Iron homeostasis and Trypanosoma brucei associated immunopathogenicity development: A battle/quest for iron. BioMed Res. Int. 2015, 2015, 1–15.
- Aziz, D.A.A.; Penyelidikan, P.P. The Development and Optimization of Processes for The expression of Sialylated Recombinant Human Therapeutic Glycoprotein in Insect Cell-Baculovirus System; University Teknologi Malaysia: Johor, Malaysia, 2001.
- Macaluso, G.; Grippi, F.; Di Bella, S.; Blanda, V.; Gucciardi, F.; Torina, A.; Guercio, A.; Cannella, V. A Review on the Immunological Response against Trypanosoma cruzi. Pathogens 2023, 12, 282.
- Ganz, T. Hepcidin and iron regulation, 10 years later. Blood J. Am. Soc. Hematol. 2011, 117, 4425–4433.
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 1434–1443.
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out ferroportin. Cell Metab. 2015, 22, 777–787.
- Winter, W.E.; Bazydlo, L.A.; Harris, N.S. The molecular biology of human iron metabolism. Lab. Med. 2014, 45, 92–102.
- Kalinowski, D.S.; Richardson, D.R. Cellular and molecular biology of iron-binding proteins. In Cellular and Molecular Biology of Metals; CRC Press: Boca Raton, FL, USA, 2010; pp. 177–190.
- Tandara, L.; Salamunić, I. Iron metabolism: Current facts and future directions. Biochem. Medica 2012, 22, 311–328.
- Smith, A.; McCulloh, R.J. Hemopexin and haptoglobin: Allies against heme toxicity from hemoglobin not contenders. Front. Physiol. 2015, 6, 187.
- Payne, S.M. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1993, 1, 66–69.
- Chu, B.C.; Garcia-Herrero, A.; Johanson, T.H.; Krewulak, K.D.; Lau, C.K.; Peacock, R.S.; Slavinskaya, Z.; Vogel, H.J. Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals 2010, 23, 601–611.
- Marchetti, M.; De Bei, O.; Bettati, S.; Campanini, B.; Kovachka, S.; Gianquinto, E.; Spyrakis, F.; Ronda, L. Iron metabolism at the interface between host and pathogen: From nutritional immunity to antibacterial development. Int. J. Mol. Sci. 2020, 21, 2145.
- Barton, L.L. Transmembrane Movement: Mechanisms and Examples. Struct. Funct. Relatsh. Prokaryotes 2005, 21, 468–526.
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in iron metabolism: From mechanism to therapy potential. Trends Mol. Med. 2016, 22, 1077–1090.
- Wang, W.; Lu, Y.; Wang, Y.; Zhang, Y.; Xia, B.; Cao, J. Siderophores induce mitophagy-dependent apoptosis in platelets. Ann. Transl. Med. 2020, 8, 14.
- Dolezal, T.; Krejcova, G.; Bajgar, A.; Nedbalova, P.; Strasser, P. Molecular regulations of metabolism during immune response in insects. Insect Biochem. Mol. Biol. 2019, 109, 31–42.
- Scott, Z.W.; Choi, S.-r.; Britigan, B.E.; Narayanasamy, P. Development of Gallium (III) as an Antimicrobial Drug Targeting Pathophysiologic Iron Metabolism of Human Pathogens. ACS Infect. Dis. 2023, 9, 716–738.
- Laranjeira-Silva, M.F.; Hamza, I.; Pérez-Victoria, J.M. Iron and heme metabolism at the leishmania–host interface. Trends Parasitol. 2020, 36, 279–289.
- Dolan, S.K. Illuminating Siderophore Transporter Functionality with Thiopeptide Antibiotics. mBio 2023, 14, e0332622.
- Sánchez-Jiménez, A.; Marcos-Torres, F.J.; Llamas, M.A. Mechanisms of iron homeostasis in Pseudomonas aeruginosa and emerging therapeutics directed to disrupt this vital process. Microb. Biotechnol. 2023, 16, 1475–1491.
- Banerjee, S.; Farhana, A.; Ehtesham, N.Z.; Hasnain, S.E. Iron acquisition, assimilation and regulation in mycobacteria. Infect. Genet. Evol. 2011, 11, 825–838.
- Rementeria, A.; López-Molina, N.; Ludwig, A.; Vivanco, A.B.; Bikandi, J.; Pontón, J.; Garaizar, J. Genes and molecules involved in Aspergillus fumigatus virulence. Rev. Iberoam Micol. 2005, 22, 1–23.
- Kita, K.; Nihei, C.; Tomitsuka, E. Parasite Mitochondria as Drug Target: Diversity and Dynamic Changes During the Life Cycle. Curr. Med. Chem. 2003, 10, 2535–2548.
- Huber, D.; Römheld, V.; Weinmann, M. Relationship between nutrition, plant diseases and pests. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 283–298.
- Haschka, D.; Hoffmann, A.; Weiss, G. Iron in immune cell function and host defense. In Seminars in Cell & Developmental Biology, 2021; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–36.
- Khan, S.; Subhan, F.; Haleem, K.S.; Khattak, M.N.K.; Khan, I.; Sultan, T.; Tauseef, I. Impact of plant growth-promoting rhizobacteria on yield and disease control of Nicotiana tabacum. Arch. Biol. Sci. 2018, 70, 717–725.
- Iwama, R.E.; Moran, Y. Origins and diversification of animal innate immune responses against viral infections. Nat. Ecol. Evol. 2023, 7, 1–12.
- Vidhyasekaran, P. Plant Hormone Signaling Systems in Plant Innate Immunity; Springer: Berlin, Germany, 2015; Volume 2.
- García, M.J.; Lucena, C.; Romera, F.J. Ethylene and Nitric Oxide Involvement in the Regulation of Fe and P Deficiency Responses in Dicotyledonous Plants. Int. J. Mol. Sci. 2021, 22, 4904.
- Maurer, F.; Müller, S.; Bauer, P. Suppression of Fe deficiency gene expression by jasmonate. Plant Physiol. Biochem. 2011, 49, 530–536.
- Brumbarova, T.; Bauer, P.; Ivanov, R. Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci. 2015, 20, 124–133.
- Cui, Y.; Chen, C.-L.; Cui, M.; Zhou, W.-J.; Wu, H.-L.; Ling, H.-Q. Four IVa bHLH Transcription Factors Are Novel Interactors of FIT and Mediate JA Inhibition of Iron Uptake in Arabidopsis. Mol. Plant 2018, 11, 1166–1183.
- Nawaz, A.; Rehman, H.U.; Usman, M.; Wakeel, A.; Shahid, M.S.; Alam, S.; Sanaullah, M.; Atiq, M.; Farooq, M. Nanobiotechnology in crop stress management: An overview of novel applications. Discov. Nano 2023, 18, 74.
- Mwangi, M.N.; Oonincx, D.G.; Stouten, T.; Veenenbos, M.; Melse-Boonstra, A.; Dicke, M.; Van Loon, J.J. Insects as sources of iron and zinc in human nutrition. Nutr. Res. Rev. 2018, 31, 248–255.
- Jones, M.W.M.; de Jonge, M.D.; James, S.A.; Burke, R. Elemental mapping of the entire intact Drosophila gastrointestinal tract. JBIC J. Biol. Inorg. Chem. 2015, 20, 979–987.
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784.
- Navarro, J.A.; Schneuwly, S. Copper and Zinc Homeostasis: Lessons from Drosophila melanogaster. Front. Genet. 2017, 8, 223.
- Groth, C.; Sasamura, T.; Khanna, M.R.; Whitley, M.; Fortini, M.E. Protein trafficking abnormalities in Drosophila tissues with impaired activity of the ZIP7 zinc transporter Catsup. Development 2013, 140, 3018–3027.
- Guo, Z.; Qin, J.; Zhou, X.; Zhang, Y. Insect Transcription Factors: A Landscape of Their Structures and Biological Functions in Drosophila and beyond. Int. J. Mol. Sci. 2018, 19, 3691.
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of the insect immune system. Entomol. Exp. Appl. 2011, 142, 1–16.
- Kanost, M.; Gorman, M. Phenoloxidases in insect immunity. Insect Immunol. 2008, 1, 69–96.
- Li, Y.; Wang, Y.; Jiang, H.; Deng, J. Crystal structure of Manduca sexta prophenoloxidase provides insights into the mechanism of type 3 copper enzymes. Proc. Natl. Acad. Sci. USA 2009, 106, 17002–17006.
- Xiao, G. Molecular physiology of zinc in Drosophila melanogaster. Curr. Opin. Insect Sci. 2022, 51, 100899.
- Balamurugan, K.; Egli, D.; Hua, H.; Rajaram, R.; Seisenbacher, G.; Georgiev, O.; Schaffner, W. Copper homeostasis in Drosophila by complex interplay of import, storage and behavioral avoidance. EMBO J. 2007, 26, 1035–1044.
- Selvaraj, A.; Balamurugan, K.; Yepiskoposyan, H.; Zhou, H.; Egli, D.; Georgiev, O.; Thiele, D.J.; Schaffner, W. Metal-responsive transcription factor (MTF-1) handles both extremes, copper load and copper starvation, by activating different genes. Genes Dev. 2005, 19, 891–896.
- Slobodian, M.R.; Petahtegoose, J.D.; Wallis, A.L.; Levesque, D.C.; Merritt, T.J. The effects of essential and non-essential metal toxicity in the Drosophila melanogaster insect model: A review. Toxics 2021, 9, 269.
- Bahadorani, S.; Mukai, S.; Egli, D.; Hilliker, A.J. Overexpression of metal-responsive transcription factor (MTF-1) in Drosophila melanogaster ameliorates life-span reductions associated with oxidative stress and metal toxicity. Neurobiol. Aging 2010, 31, 1215–1226.
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006, 50, 85–94.
- Stączek, S.; Cytryńska, M.; Zdybicka-Barabas, A. Unraveling the Role of Antimicrobial Peptides in Insects. Int. J. Mol. Sci. 2023, 24, 5753.
- Hrdina, A.; Iatsenko, I. The roles of metals in insect–microbe interactions and immunity. Curr. Opin. Insect Sci. 2022, 49, 71–77.
- McHenry, C.S. DNA replicases from a bacterial perspective. Annu. Rev. Biochem. 2011, 80, 403–436.
- Ishida, T. Antiviral activities of Zn2+ ions for viral prevention, replication, capsid protein in intracellular proliferation of viruses. World Sci. News 2018, 97, 28–50.
- Kuznetsova, A.A.; Fedorova, O.S.; Kuznetsov, N.A. Structural and molecular kinetic features of activities of DNA polymerases. Int. J. Mol. Sci. 2022, 23, 6373.
- Lee, J.Y.; Chang, C.; Song, H.K.; Moon, J.; Yang, J.K.; Kim, H.-K.; Kwon, S.-T.; Suh, S.W. Crystal structure of NAD+-dependent DNA ligase: Modular architecture and functional implications. EMBO J. 2000, 19, 1119–1129.
- Begg, S.L. The role of metal ions in the virulence and viability of bacterial pathogens. Biochem. Soc. Trans. 2019, 47, 77–87.
- Sharma, K.K.; Singh, D.; Mohite, S.V.; Williamson, P.R.; Kennedy, J.F. Metal manipulators and regulators in human pathogens: A comprehensive review on microbial redox copper metalloenzymes “multicopper oxidases and superoxide dismutases”. Int. J. Biol. Macromol. 2023, 233, 123534.
- Andrés, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841.
- Zaminpira, S.; Niknamian, S. How butterfly effect or deterministic chaos theory in theoretical physics explains the main cause of cancer. EC Cancer 2017, 2, 227–238.
- Stengel, A. Characterisation of components and mechanisms involved in redox-regulation of protein import into chloroplasts. LMU 2009.
- Isernia, C.; Bucci, E.; Leone, M.; Zaccaro, L.; Di Lello, P.; Digilio, G.; Esposito, S.; Saviano, M.; Di Blasio, B.; Pedone, C. NMR structure of the single QALGGH zinc finger domain from the Arabidopsis thaliana SUPERMAN protein. Chembiochem 2003, 4, 171–180.
- Zhu, H.; Chen, C.; Zeng, J.; Yun, Z.; Liu, Y.; Qu, H.; Jiang, Y.; Duan, X.; Xia, R. Micro RNA 528, a hub regulator modulating ROS homeostasis via targeting of a diverse set of genes encoding copper-containing proteins in monocots. New Phytol. 2020, 225, 385–399.
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214.
- Iqbal, N.; Trivellini, A.; Masood, A.; Ferrante, A.; Khan, N.A. Current understanding on ethylene signaling in plants: The influence of nutrient availability. Plant Physiol. Biochem. 2013, 73, 128–138.
- Zekri, M.; Obreza, T.A. Plant Nutrients for Citrus Trees; University of Florida Cooperative Extension Service: Panama, FL, USA, 2003.
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201.
- Gaspéri, J.; Ayrault, S.; Moreau-Guigon, E.; Alliot, F.; Labadie, P.; Budzinski, H.; Blanchard, M.; Muresan, B.; Caupos, E.; Cladière, M.; et al. Contamination of soils by metals and organic micropollutants: Case study of the Parisian conurbation. Environ. Sci. Pollut. Res. 2018, 25, 23559–23573.
- Whitfield, M. Interactions between phytoplankton and trace metals in the ocean. Advances in Marine Biology. 2001, 41, 1–128.
- Aparachita, P. Effects of Water Soluble Zinc on Growth Performance, Immune Response, and Tissue Zinc Transporters in Nursery Pigs; Oklahoma State University: Stillwater, OK, USA, 2020.
- Castro, P.H.; Lilay, G.H.; Assunção, A.G. Regulation of micronutrient homeostasis and deficiency response in plants. In Plant Micronutrient Use Efficiency; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–15.
- Kuliyev, E. Functional and Biochemical Characterization of ZIP4 Extracellular Domain⎯Implications on Acrodermatitis Enteropathica, a Life-Threatening Genetic Disorder. Michigan State University: East Lansing, MI, USA, 2020.
- Murakami, M.; Hirano, T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008, 99, 1515–1522.
- John, E.; Laskow, T.C.; Buchser, W.J.; Pitt, B.R.; Basse, P.H.; Butterfield, L.H.; Kalinski, P.; Lotze, M.T. Zinc in innate and adaptive tumor immunity. J. Transl. Med. 2010, 8, 118.
- Rajkumar, M.; Bruno, L.B.; Banu, J.R. Alleviation of environmental stress in plants: The role of beneficial Pseudomonas spp. Crit. Rev. Environ. Sci. Technol. 2017, 47, 372–407.
- Naz, T.; Iqbal, M.M.; Fahad, S.; Akhtar, J.; Saqib, M.; Alamri, S.; Siddiqui, M.H.; Saud, S.; Khattak, J.Z.K.; Ali, S.; et al. Bio-fortification of Two Wheat Cultivars with Iron and Zinc Through Their Soil and Foliar Application in Salt-Factored Soil: Growth, Ionic, Physiological, and Biochemical Modifications. J. Plant Growth Regul. 2023, 1–19.
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: A review. Curr. Res. Microb. Sci. 2021, 2, 100054.
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’ resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25.
- Kahromi, S.; Khara, J. Chitosan stimulates secondary metabolite production and nutrient uptake in medicinal plantDracocephalum kotschyi. J. Sci. Food Agric. 2021, 101, 3898–3907.
- Khan, T.; Abbasi, B.H.; Khan, M.A. The interplay between light, plant growth regulators and elicitors on growth and secondary metabolism in cell cultures of Fagonia indica. J. Photochem. Photobiol. B Biol. 2018, 185, 153–160.
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019.
- Russo, M.; Petropoulos, V.; Molotokaite, E.; Cerullo, G.; Casazza, A.P.; Maiuri, M.; Santabarbara, S. Ultrafast excited-state dynamics in land plants Photosystem I core and whole supercomplex under oxidised electron donor conditions. Photosynth. Res. 2020, 144, 221–233.
- Winterbourn, C.C. Biological chemistry of superoxide radicals. Chemtexts 2020, 6, 7.
- Turk, H.; Erdal, S. Melatonin alleviates cold-induced oxidative damage in maize seedlings by up-regulating mineral elements and enhancing antioxidant activity. J. Plant Nutr. Soil Sci. 2015, 178, 433–439.
- Song, X.; Zhong, Z.; Gao, L.; Weiss, B.L.; Wang, J. Metabolic interactions between disease-transmitting vectors and their microbiota. Trends Parasitol. 2022, 38, 697–708.
- Soetan, K.; Olaiya, C.; Oyewole, O. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222.
- Bhatla, S.C.A.; Lal, M.; Kathpalia, R.; Bhatla, S.C. Plant mineral nutrition. Plant Physiol. Dev. Metab. 2018, 37–81.
- Jha, C.K.; Saraf, M. Plant growth promoting rhizobacteria (PGPR): A review. J. Agric. Res. Dev. 2015, 5, 108–119.
- Rajak, P.; Roy, S.; Ganguly, A.; Mandi, M.; Dutta, A.; Das, K.; Nanda, S.; Sarkar, S.; Khatun, S.; Ghanty, S. Protective potential of vitamin C and E against organophosphate toxicity: Current status and perspective. J. Ecophysiol. Occup. Health 2022, 456, 141–154.
- Bánfalvi, G. Heavy metals, trace elements and their cellular effects. Cell. Eff. Heavy Met. 2011, 3–28.
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407.
- Zhong, X.; Wright, J.F. Biological Insights into Therapeutic Protein Modifications throughout Trafficking and Their Biopharmaceutical Applications. Int. J. Cell Biol. 2013, 2013, 273086.
- Moskovitz, J.; Yim, M.B.; Chock, P.B. Free radicals and disease. Arch. Biochem. Biophys. 2002, 397, 354–359.
- Calderon, I.L.; Elías, A.O.; Fuentes, E.L.; Pradenas, G.A.; Castro, M.E.; Arenas, F.A.; Perez, J.M.; Vasquez, C.C. Tellurite-mediated disabling of clusters of Escherichia coli dehydratases. Microbiology 2009, 155, 1840–1846.
- Madkour, L.H. Targeted Drug Delivery. J. Target. Drug Deliv. 2019, 3.
- David, S.S.; O’Shea, V.L.; Kundu, S. Base-excision repair of oxidative DNA damage. Nature 2007, 447, 941–950.
- Martinez-Zamudio, R.; Ha, H.C. Environmental epigenetics in metal exposure. Epigenetics 2011, 6, 820–827.
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Annu. Rev. Entomol. 2016, 61, 177–196.
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics: Sensing, Signaling, and Regulation; Urumqi China Wiley: Hoboken, NJ, USA, 2023; pp. 1–18.
- Bretscher, H.; O’connor, M.B. The Role of Muscle in Insect Energy Homeostasis. Front. Physiol. 2020, 11, 580687.
- Mattson, W.J., Jr. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 1980, 11, 119–161.
- Dresen, M.; Valentin-Weigand, P.; Berhanu Weldearegay, Y. Role of Metabolic Adaptation of Streptococcus suis to Host Niches in Bacterial Fitness and Virulence. Pathogens 2023, 12, 541.
- Aggarwal, S.; Kumaraswami, M. Managing Manganese: The Role of Manganese Homeostasis in Streptococcal Pathogenesis. Front. Cell Dev. Biol. 2022, 10, 2022.
- Mitchell, A.; Mitchell, T. Streptococcus pneumoniae: Virulence factors and variation. Clin. Microbiol. Infect. 2010, 16, 411–418.
- Crosby, H.A.; Tiwari, N.; Kwiecinski, J.M.; Xu, Z.; Dykstra, A.; Jenul, C.; Fuentes, E.J.; Horswill, A.R. The Staphylococcus aureus ArlRS two-component system regulates virulence factor expression through MgrA. Mol. Microbiol. 2020, 113, 103–122.
- Mustafa, A.; Zulfiqar, U.; Mumtaz, M.Z.; Radziemska, M.; Haider, F.U.; Holatko, J.; Hammershmiedt, T.; Naveed, M.; Ali, H.; Kintl, A. Nickel (Ni) phytotoxicity and detoxification mechanisms: A review. Chemosphere 2023, 328, 138574.
- Mora, D.; Arioli, S. Microbial urease in health and disease. PLoS Pathog. 2014, 10, e1004472.
- Chen, C.; Xu, C.; Qian, D.; Yu, Q.; Huang, M.; Zhou, L.; Qin, J.G.; Chen, L.; Li, E. Growth and health status of Pacific white shrimp, Litopenaeus vannamei, exposed to chronic water born cobalt. Fish Shellfish. Immunol. 2020, 100, 137–145.
- Gopinath, K.; Venclovas, Č.; Ioerger, T.R.; Sacchettini, J.C.; McKinney, J.D.; Mizrahi, V.; Warner, D.F. A vitamin B12 transporter in Mycobacterium tuberculosis. Open Biol. 2013, 3, 120175.
- Duell, B.L.; Su, Y.C.; Riesbeck, K. Host–pathogen interactions of nontypeable Haemophilus influenzae: From commensal to pathogen. FEBS Lett. 2016, 590, 3840–3853.
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
963
Revisions:
3 times
(View History)
Update Date:
20 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





