
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mastalir Ágnes | -- | 2088 | 2023-07-19 08:59:29 | | | |
| 2 | Fanny Huang | -34 word(s) | 2054 | 2023-07-19 10:46:55 | | | | |
| 3 | Fanny Huang | Meta information modification | 2054 | 2023-08-02 08:43:19 | | |
Video Upload Options
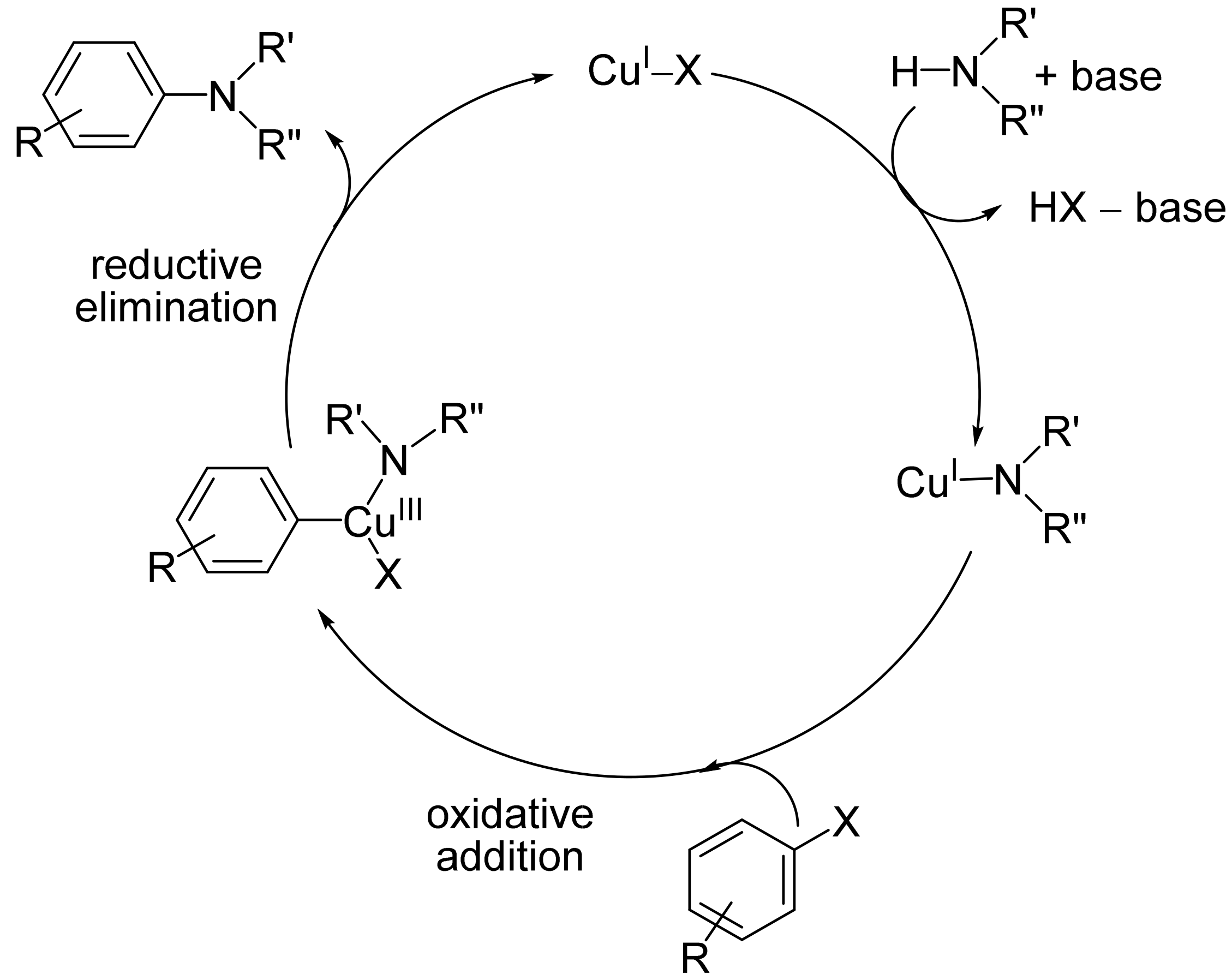
Ullmann-type C–N heterocoupling reactions have been applied for the syntheses of N-arylated amines. Transition metal-catalyzed N-arylations have been recognized as particularly efficient procedures for the preparation of nitrogen-containing aromatic systems. These reactions typically carried out under optimized conditions, have also been found to be suitable for the synthesis of complex molecules with other functional groups, including natural products, drugs, or pharmaceuticals. Most importantly, copper-catalyzed N-arylations have been studied and employed in the total synthesis of biologically active compounds. The construction of fused N-heterocyclic compounds also remained the subject of extensive research because of their potential applications in drug discovery and the development of functional materials.
1. Introduction
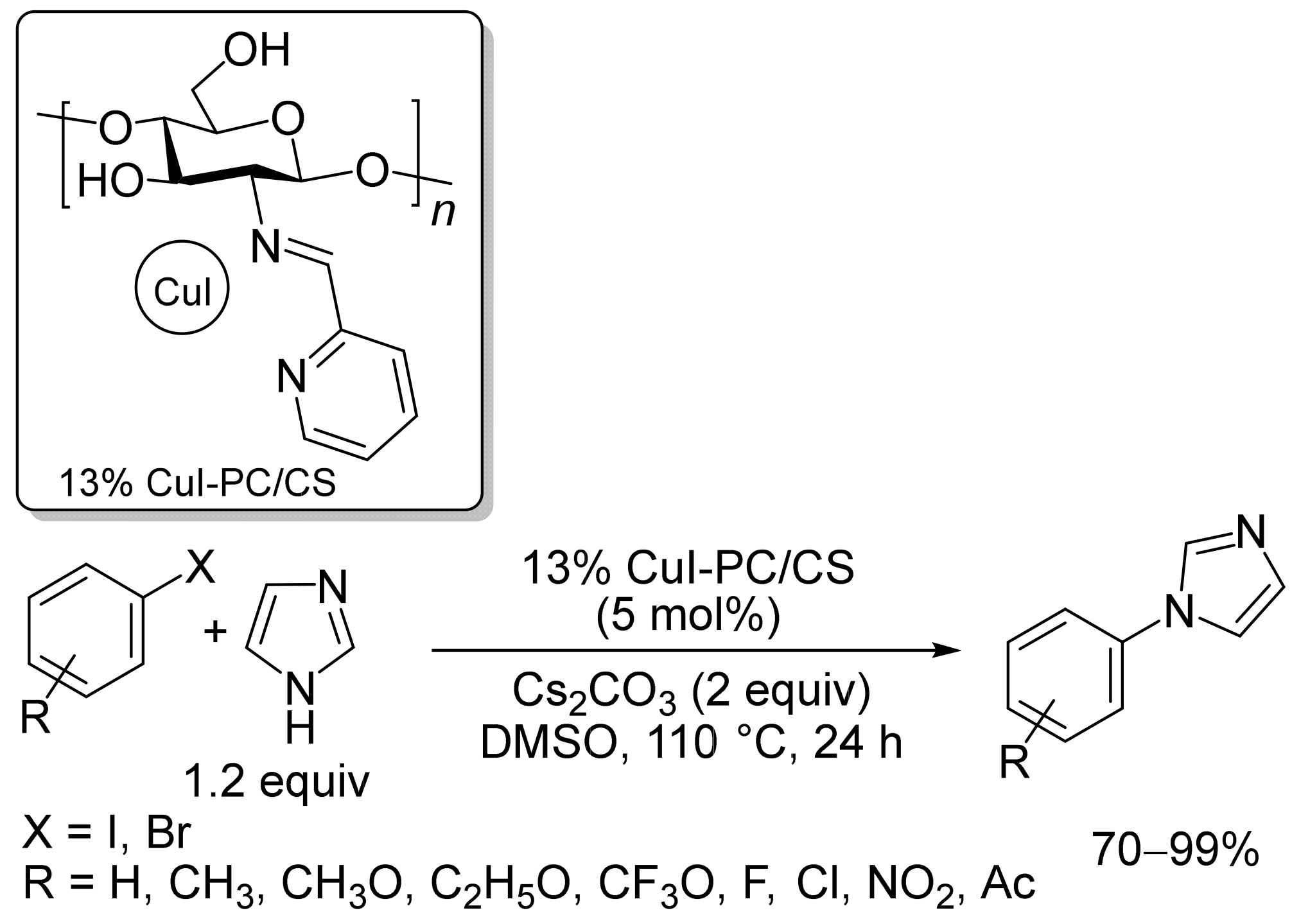
2. Magnetic Catalysts
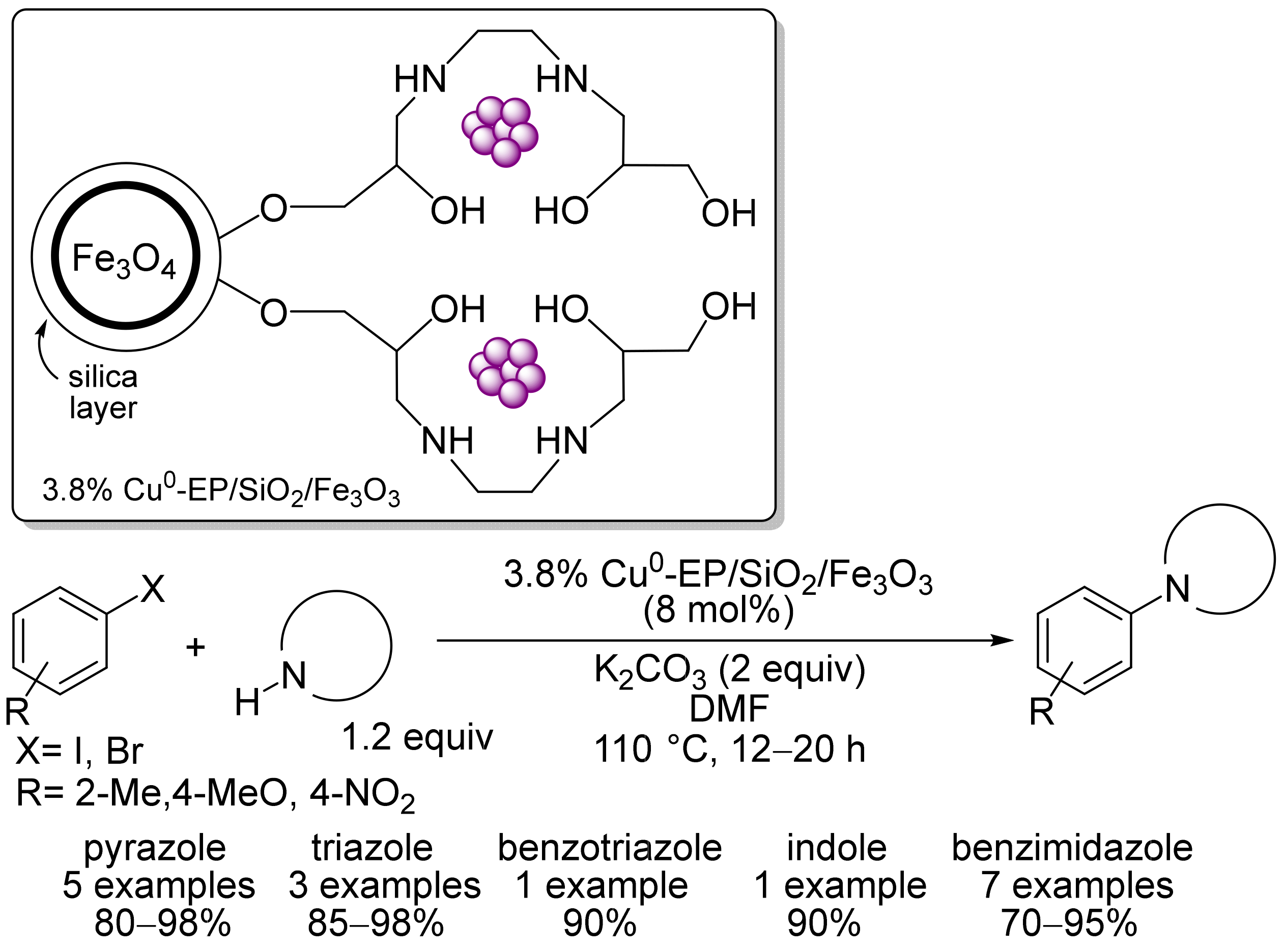
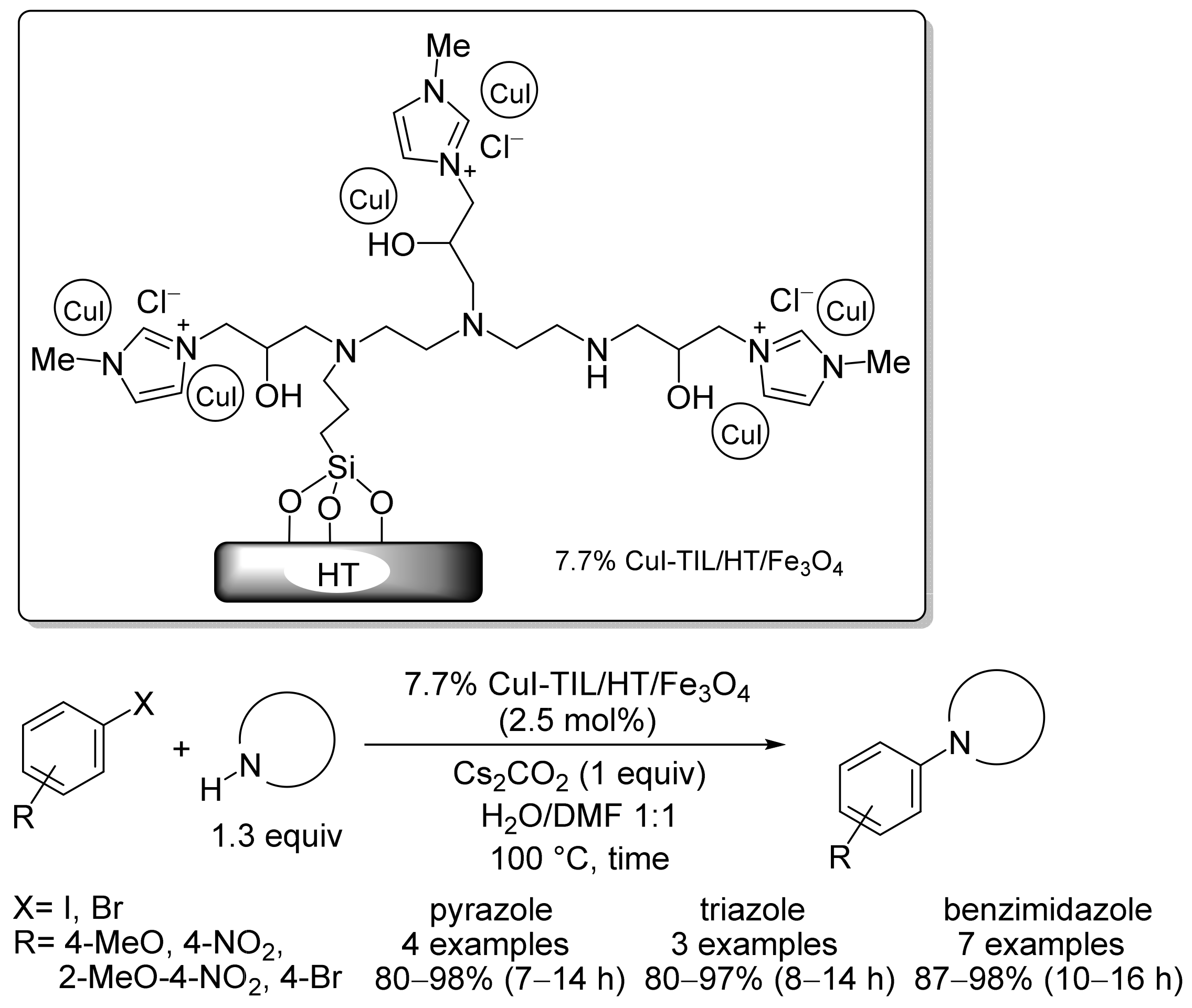
References
- Dai, L.X. C-S coupling with nitro group as leaving group via inorganic salt catalysis. Prog. Chem. 2018, 30, 1257–1297.
- Fischer, C.; Koenig, B. Palladium and copper-mediated N-aryl bond formation reactions for the synthesis of biological active compounds. Beilstein J. Org. Chem. 2011, 7, 59–74.
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550.
- Yashwantrao, G.; Saha, S. Sustainable strategies of C-N bond formation via Ullmann coupling employing earth abundant copper catalyst. Tetrahedron 2021, 97, 132406.
- Majumder, A.; Gupta, R.; Mandal, M.; Babu, M.; Chakraborty, D. Air-stable palladium(0) phosphine sulfide catalysts for Ullmann-type C–N and C–O coupling reactions. J. Organomet. Chem. 2015, 781, 23–34.
- Kuil, M.; Bekedam, E.K.; Visser, G.M.; van den Hoogenband, A.; Terpstra, J.W.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; van Strijdonck, G.P.F. Mild copper-catalyzed N-arylation of azaheterocycles with aryl halides. Tetrahedron Lett. 2005, 46, 2405–2409.
- Verma, A.K.; Singh, J.; Sankar, V.K.; Chaudhary, R.; Chandra, R. Benzotriazole: An excellent ligand for Cu-catalyzed N-arylation of imidazoles with aryl and heteroaryl halides. Tetrahedron Lett. 2007, 48, 4207–4210.
- Phipps, R.J.; Grimster, N.P.; Gaunt, M.J. Cu(II)-catalyzed direct and site-selective arylation of indoles under miled conditions. J. Am. Chem. Soc. 2008, 130, 8172–8174.
- Liu, L.; Frohn, M.; Xi, N.; Dominguez, C.; Hungate, R.; Reider, P.J. A soluble base for the copper-catalyzed imidazole N-arylations with aryl halides. J. Org. Chem. 2005, 70, 10135–10138.
- Beletskaya, I.P.; Cheprakov, A.V. Copper in cross-coupling reactions: The post-Ullmann chemistry. Coord. Chem. Rev. 2004, 248, 2337–2364.
- Ley, S.V.; Thomas, A.W. Modern synthetic methods for copper-mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S bond formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449.
- Hisana, K.N.; Abdulla, C.M.A.; Anilkumar, G. Copper-catalyzed N-arylation of indoles. Curr. Org. Chem. 2022, 26, 857–886.
- Oeser, P.; Koudelka, J.; Petrenko, A.; Tobrman, T. Recent progress concerning the N-arylation of indoles. Molecules 2021, 26, 5079.
- Panda, N.; Jena, A.K.; Mohapatra, S.; Rout, S.R. Copper ferrite nanoparticle-mediated N-arylation of heterocycles: A ligand-free reaction. Tetrahedron Lett. 2011, 52, 1924–1927.
- Mastalir, Á.; Molnár, Á. A novel insight into the Ullmann homocoupling reactions performed in heterogeneous catalytic systems. Molecules 2023, 28, 1769.
- Shafir, A.; Lichtor, P.A.; Buchwald, S.L. N-versus O-arylation of aminoalcohols: Orthogonal selectivity in copper-based catalysts. J. Am. Chem. Soc. 2007, 129, 3490–3491.
- Khatri, P.K.; Jain, S.L. Glycerol ingrained copper: An efficient recyclable catalyst for the N-arylation of amines with aryl halides. Tetrahedron Lett. 2013, 54, 2740–2743.
- Danqing, L.; Ming, J.; Li, L.; Mohammadnia, M. Preparation and characterization of Cu supported on 2-(1H-benzolimidazole-2-yl)aniline-functionalized Fe3O4 nanoparticles as a novel magnetic catalyst for Ullmann and Suzuki cross-coupling reactions. Appl. Organomet. Chem. 2020, 34, e5820.
- Kundu, D.; Bhadra, S.; Mukherjee, N.; Sreedhar, B.; Ranu, B.C. Heterogeneous CuII-catalyzed solvent-controlled selective N-arylation of cyclic amides and amines with bromo-iodoarenes. Chem. Eur. J. 2013, 19, 15759–15768.
- Islam, S.M.; Mondal, S.; Mondal, P.; Roy, A.S.; Tuhina, K.; Salam, N.; Mobarak, M. A reusable polymer supported copper catalyst for the C–N and C–O bond cross-coupling reaction of aryl halides as well as arylboronic acids. J. Organomet. Chem. 2012, 696, 4264–4274.
- Yang, Q.; Zhao, Y.; Ma, D. Cu-mediated Ullmann-type cross-coupling and industrial applications in route design, process development, and scale-up of pharmaceutical and agrochemical processes. Org. Process Res. Dev. 2022, 26, 1690–1750.
- Pirhayati, M.; Veisi, H.; Kakanejadifard, A. Palladium stabilized by 3,4-dihydroxypyridine-functionalized magnetic Fe3O4 nanoparticles as a reusable and efficient heterogeneous catalyst for Suzuki reactions. RSC Adv. 2016, 6, 27252–27259.
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393.
- Wang, D.; Astruc, D. Fast-growing field of magnetically recyclable nanocatalysts. Chem. Rev. 2014, 114, 6949–6985.
- Karimi, B.; Mansouri, F.; Vali, H. A highly water-dispersible /magnetically separable palladium catalyst based on a Fe3O4@SiO2 anchored TEG-imidazolium ionic liquid for the Suzuki-Miyaura coupling reaction in water. Green Chem. 2014, 16, 2587–2596.
- Hemmati, S.; Kamangar, S.A.; Yousefi, M.; Salehi, M.H.; Hekmati, M. Cu(I)-anchored polyvinyl alcohol coated-magnetic nanoparticles as heterogeneous nanocatalyst in Ullmann-type C–N coupling reactions. Appl. Organomet. Chem. 2020, 34, e5611.
- Veisi, H.; Taheri, S.; Hemmati, S. Preparation of polydopamine sdulfamic acid-functionalized magnetic Fe3O4 nanoparticles with a core/shell nanostructure as heterogeneous and recyclable nanocatalysts for the acetylation of alcohols, phenols, amines and thiols under solvent-free conditions. Green Chem. 2016, 18, 6337–6348.
- Rajabzadeh, M.; Eshghi, H.; Khalifeh, R.; Bakavoli, M. Magnetically recoverable copper nanorods and their catalytic activity in Ullmann cross-coupling reactions. Appl. Organomet. Chem. 2017, 31, e3647.
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interf. Sci. 1968, 26, 62–69.
- Rajabzadeh, M.; Eshghi, H.; Khalifeh, R.; Bakavoli, M. Generation of Cu nanoparticles on novel designed Fe3O4@SiO2/EP.EN.EG as reusable nanocatalyst for the reduction of nitro compounds. RSC Adv. 2016, 6, 19331–19340.
- Rajabzadeh, M.; Najdi, N.; Zarei, Z.; Khalifeh, R. CuI immobilized on tricationic ionic liquid anchored on functionalized magnetic hydrotalcite (Fe3O4/HT-TIL-CuI) as a novel, magnetic and efficient nanocatalyst for Ullmann-type C–N coupling reaction. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2696–2711.
- Zarei, Z.; Akhlaghinia, B. ZnII doped and immobilized on functionalized magnetic hydrotalcite (Fe3O4/HT-SMTU-ZnII): A novel, green and magnetically recyclable bifunctional nanocatalyst for the one-pot multi-component synthesis of acridinediones under solvent-free conditions. New J. Chem. 2017, 41, 15485–15500.
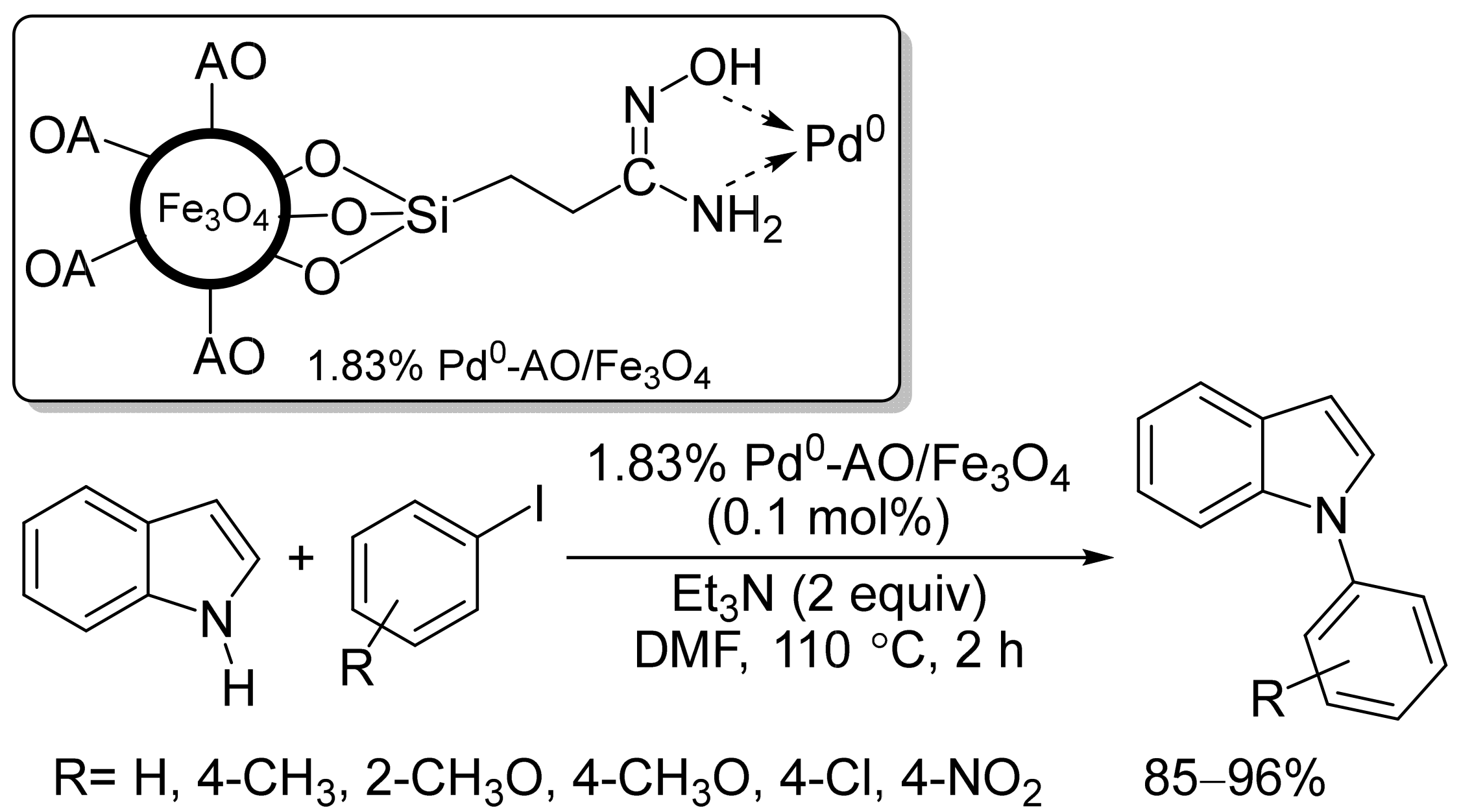
- Ghorbani-Vaghei, R.; Hemmati, S.; Hekmati, M. Pd immobilized on modified magnetic Fe3O4 nanoparticles: Magnetically recoverable and reusable Pd nanocatalyst for Suzuki-Miyaura coupling reactions and Ullmann-type N-arylation of indoles. J. Chem. Sci. 2016, 128, 1157–1162.
- Veisi, H.; Gholami, J.; Ueda, H.; Mohammadi, P.; Noroozi, M. Magnetically palladium catalyst stabilized by diaminoglyoxime-functionalized magnetic Fe3O4 nanoparticles as active and reusable catalyst for Suzuki coupling reactions. J. Mol. Catal. A Chem. 2015, 396, 216–223.
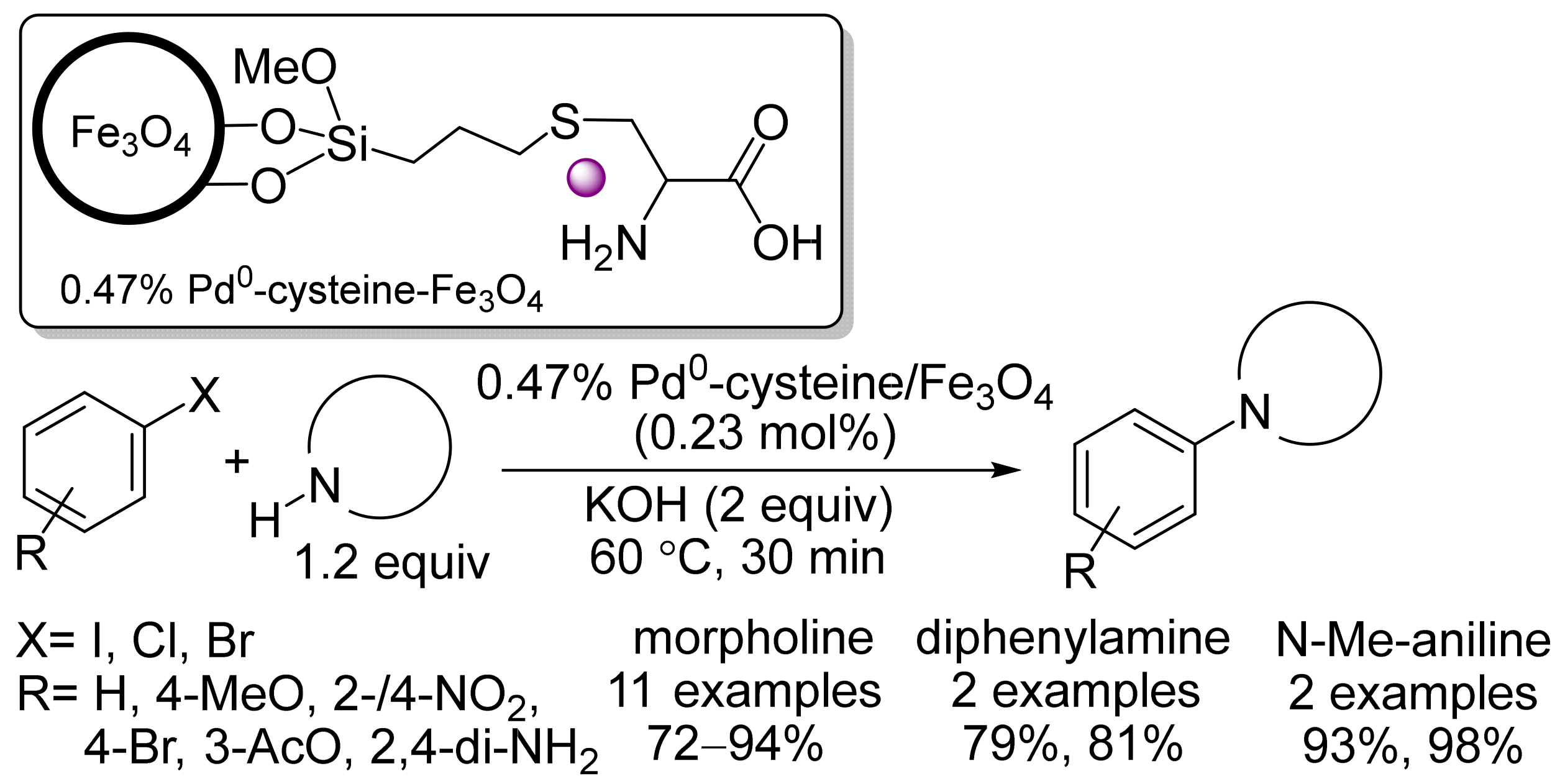
- Hajipour, A.R.; Khorsandi, Z.; Metkazini, S.F.M. Palladium nanoparticles supported on cysteine-functionalized MNPs as robust recyclable catalysts for fast O- and N-arylation reactions in green media. J. Organomet. Chem. 2019, 899, 120793.




