Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yucang Liang | -- | 3071 | 2023-07-18 13:42:22 | | | |
| 2 | Lindsay Dong | Meta information modification | 3071 | 2023-07-20 02:58:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ren, Y.; Ma, Z.; Gao, T.; Liang, Y. Luminescent Sensing of Nitroaromatics by Crystalline Lanthanide–Organic Complexes. Encyclopedia. Available online: https://encyclopedia.pub/entry/46926 (accessed on 08 February 2026).
Ren Y, Ma Z, Gao T, Liang Y. Luminescent Sensing of Nitroaromatics by Crystalline Lanthanide–Organic Complexes. Encyclopedia. Available at: https://encyclopedia.pub/entry/46926. Accessed February 08, 2026.
Ren, Yixia, Zhihu Ma, Ting Gao, Yucang Liang. "Luminescent Sensing of Nitroaromatics by Crystalline Lanthanide–Organic Complexes" Encyclopedia, https://encyclopedia.pub/entry/46926 (accessed February 08, 2026).
Ren, Y., Ma, Z., Gao, T., & Liang, Y. (2023, July 18). Luminescent Sensing of Nitroaromatics by Crystalline Lanthanide–Organic Complexes. In Encyclopedia. https://encyclopedia.pub/entry/46926
Ren, Yixia, et al. "Luminescent Sensing of Nitroaromatics by Crystalline Lanthanide–Organic Complexes." Encyclopedia. Web. 18 July, 2023.
Copy Citation
Water environment pollution is becoming an increasingly serious issue due to industrial pollutants with the rapid development of modern industry. Among many pollutants, the toxic and explosive nitroaromatics are used extensively in the chemical industry, resulting in environmental pollution of soil and groundwater. Therefore, the detection of nitroaromatics is of great significance to environmental monitoring, citizen life and homeland security. Lanthanide–organic complexes with controllable structural features and excellent optical performance have been rationally designed and successfully prepared and used as lanthanide-based sensors for the detection of nitroaromatics.
lanthanide–organic complex
luminescent material
luminescence sensing
1. Introduction
At present, the analytical methods for detecting harmful substances in the environment mainly include atomic absorption spectroscopy [1], mass spectrometry [2], optical gas chromatography-mass spectrometry [3], gas chromatography-electron capture detection [4], surface-enhanced Raman spectroscopy [5][6], X-ray imaging [6], thermal neutron analysis [6], electrochemical procedures [5] and ion mobility spectroscopy (IMS) [5][6] and molecularly imprinted polymers (MIPs) [7]. Note that fluorescence detection is a simple, convenient, highly sensitive method for monitoring pollutants in wastewater [8][9][10] and vapor phases [11][12][13], and even in commercial honey samples [14]; no special instructions are required compared with other methods, while rapid and real-time detection is provided, and fluorescence detection has demonstrated good sensitivity and selectivity.
Lanthanide ions have high coordination numbers and poor stereochemical preferences, sharp characteristic emission in the visible and near-infrared (NIR) ranges, large antenna-induced offsets and long lifetimes. Lanthanide–organic complexes have been widely investigated and applied in catalysis, adsorption, detection, luminescence, magnetism, separation, and so on [15][16][17]. In particular, lanthanide–organic complexes have the advantages of good luminescence performance, long emission lifetime, large Stokes shift and high quantum yield, and have broad application prospects in the field of fluorescence sensing [18][19].
As shown in Figure 1, the specific process of energy absorption and conversion of lanthanide–organic complexes is embodied in three steps: First, the ground-state organic-ligand chrominance group in the lanthanide coordination polymer absorbs the excitation light and is excited from the ground state (S0) to the first excited singlet state (S1), and the excited electrons return to the ground state by radiation or non-radiation means, or the first singlet state (S1) can shift the system to the triplet excited state (T1). Finally, the T1 state of the ligand is transferred to the metal ion, resulting in the highly sensitive luminescence of the lanthanide ion. Subsequently, the energy of the lanthanide ion decreases from the excited state to the ground state, and the characteristic fluorescence of the lanthanide ion is emitted. However, the energy transfer from ligands to lanthanide ions is incomplete, and the intramolecular transfer of ligands exists in the form of radiation (π-π*, n-π*). Most lanthanide coordination polymers exhibit the characteristic emission colors of lanthanide metal ions, such as Tb3+, Tm3+, Eu3+ and Sm3+, which are green, blue, red and orange, respectively, while Nd3+, Yb3+ and Er3+ emit in the near infrared region [20].
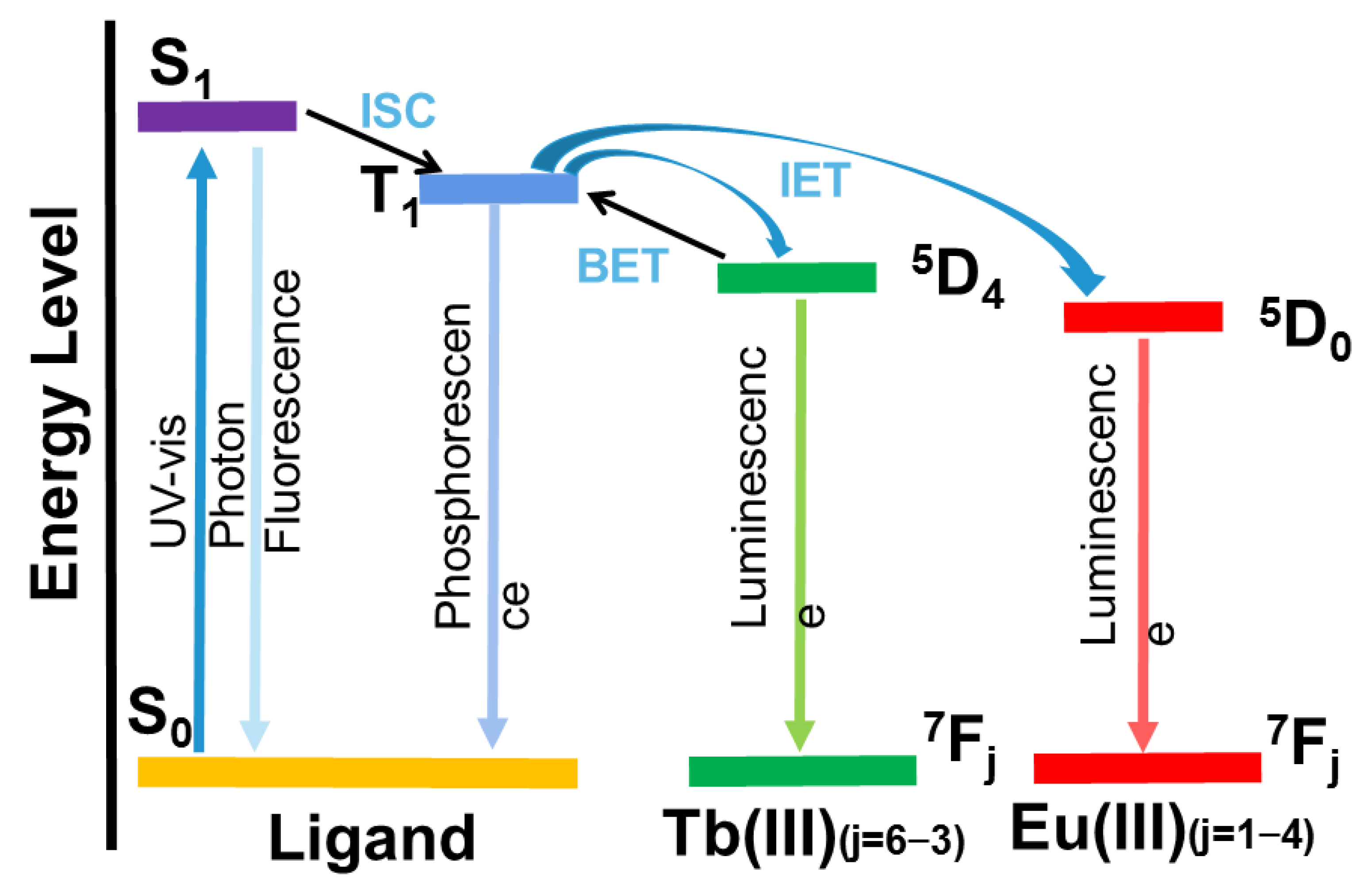
Figure 1. Process of energy absorption and conversion of Tb(III)/Eu(III) complexes. (ISC: intersystem crossing; IET: intramolecular energy transfer; BET: back energy transfer).
2. Fluorescent Sensing of Nitrobenzene Pollutants Based on the Structural Characteristics of Ln–Organic Complexes
2.1. 0D Discrete Structure
The discrete-structured lanthanide complexes usually possess polynuclear metal secondary building units (SBUs) and the weak intramolecular interactions play an important role in the sensing of nitro-based pollutants. The reported discrete lanthanide complexes include Eu2 [24], Cd8Nd4 [25],Yb18 [26], Nd42 [27], and Yb42 [28] metal units, in which the polynuclear metal clusters exhibit characteristic nanoring structures. Ma et al. [25][26] prepared Nd4 and Yb18, and Shi et al. [27][28] synthesized Ln42 (Ln = Nd, La, Yb) nanowheel cluster structures. Due to their unique ring structures, special high-nuclearity lanthanide nanorings may show some advantages during the luminescent sensing, such as a strong capture ability for analytes. A great deal of intramolecular interactions existed in these lanthanide complexes, such as hydrogen bonds or Ar-H–π interactions, which resulted in the formation of high-dimensional supramolecular structures or three-dimensional channel structures, which are conducive to the interaction with analytes [25][26][27][28].
2.2. 1D Ln-Coordination Polymers
Several 1D-fluorescent lanthanide–organic complexes for sensing nitro pollutants were reported, possibly due to their monotonous structural characteristics and indistinct fluorescent sensing performance. One-dimensional Ln–organic coordination polymers [Ln(BDPO)(H2O)4] (Ln = Eu for CUST-623, Tb for CUST-624) can be prepared using the reaction of Eu or Tb ions with the N,N′-bis(3,5-dicarboxyphenyl)-oxalamide ligand (BDPO). [Ln(BDPO)(H2O)4] has a 1D framework structure with two kinds of 1D open channels in the b-axis direction. Such 1D Ln–organic complexes can be used as a fluorescent sensor to detect TNP with the low detection limit of 0.21 μM [29]. Further, 1D [Eu(L)6(DMF)] (L = 2-(2-formylphenoxy) acetic acid) can be packed into a 2D structure through hydrogen bonding, and it has a good selectivity as well as a high sensitivity to TNP with the detection limit of rapid response of 3.39 μM in a CH3CN solution. The good detection ability towards TNP over other nitro explosives may be ascribed to the possible quenching mechanism of competitive absorption, photoinduced electron transfer and hydrogen-bond interaction [30]. In addition, the mixed ligands are used to construct the 1D Ln–organic-coordination polymers, which is regarded as a good strategy. For instance, the reaction of Eu(III) ion with mixed ligands of 2,3,4,5-tetrafluorobenzoic acid and 1,10-phenanthroline afforded a 1D structural tetranuclear [Eu4] complex with two crystallographically independent Eu3+ ions, which exhibited a highly sensitive response toward nitrobenzene at the ppm concentration [31].
2.3. 2D Ln-Coordination Polymers
Chemists explored a few 2D fluorescent lanthanide-based homometallic (f-f group) or heterometallic (d–f group) organic complexes as sensing materials for the detection of nitroaromatics. The studies revealed that the structural characteristics and the presence of weak interactions between 2D sheets (hydrogen bond, X…X bond, π stacking interaction) are beneficial to the interaction of the analyte with material and the fluorescence response of the luminescent species [32][33][34][35][36][37].
Liu et al. reported a new 2D Dy-organic complex [Dy(L)(NO3)(DMF)3]n (H2L = 2,5-di(1H-1,2,4-triazol-1-yl) terephthalic acid, DMF = N, N-dimethylformamide)} with binuclear units as a ratiometric luminescent sensor for nitro compounds (such as 4-NP) in aqueous solutions; the lowest detection limit (0.0676 μM) indicates a high sensitivity [32]. The luminescence response of another 2D complex, [Eu(TFTA)1.5(H2O)2]·H2O (TFTA = tetrafluoroterephthalate), in methanol was influenced by the primary and secondary inner filter effects (IFE) of nitroaromatic compounds (4-NTP, 2,4-DNP and TNP). The modelling and correction of IFE revealed the mechanism of static and dynamic quenching in the complex, and F–F interactions were also involved in the assembly of 2D structures into 3D supramolecular entities; as predicted, these interactions weaken with the lanthanide contraction [33]. The similar Br–Br interactions were found in a series of 2D lanthanide coordination polymers {Eu2(TBrTA)3(H2O)8·2H2O}n (TBrTA = tetrabromoterephthalate), the quenching rate of nitroaromatic compounds was 85%, including 4-nitrophenol, dinitrophenol, and trinitrophenol (picric acid), with the main quenching mechanisms of competitive absorption, photoinduced electron transfer, and the electrostatic process [34].
2.4. 3D Ln–Organic Frameworks
Due to the high coordination number of lanthanide ions and their strong coordination ability with the oxygen atoms of the carboxylate ligand, the fluorescent lanthanide–organic framework with a 3D structure accounts for a large proportion of research. Owing to the advantages of the 3D microporous structure, the fluorescence-sensing performance of 3D lanthanide–organic frameworks is much better than those of 0D, 1D and 2D lanthanide–organic complexes; therefore, 3D lanthanide–organic frameworks are used not only for the detection of nitrobenzene, p-nitroaniline and 4-nitophenol, but also for 3,4-dinitrotoluene and 2,4,6-trinitrophenol.
The hydrothermal reaction of lanthanide ions (Ln3+) and 2,5-di(1H-1,2,4-triazol-1-yl)terephthalic acid (H2dttpa) formed lanthanide metal coordination polymers {[Ln(dttpa)1.5(H2O)2]·H2O}n (Ln–CP, Ln = La3+, Ce3+, Nd3+, Sm3+, Eu3+) and {[Ln(dttpa)1.5(H2O)]·0.75H2O}n (Ln = Tb3+, Er3+) [38], in which Eu–CP effectively sensitizes the visible emission of Tb3+ and shows high selectivity for Tb3+ and stable and high sensitive response with a minimal detection limit of 0.00988 μM; furthermore, Tb-CP acts as a good luminescence sensor to detect nitrobenzene (NB) with a detection limit of 0.0125 μM. Moreover, the fluorescence quenching of Tb-CP for NB can be attributed to the competitive absorption mechanism and the photo-induced electronic transfer mechanism of the excited-state interaction between the luminescent material and NB.
3. Detection Effect of Ln–Organic Complexes on Different Nitro Pollutants
3.1. Nitrobenzene (NB)
Nitrobenzene (NB), as the simplest nitroaromatic compound, is widely used in the synthesis of many products in industry, and leads to air, water, and soil pollution, as well as serious safety problems. Moreover, since NB is highly toxic, difficult to degrade, carcinogenic, and easy to settle, once it is exposed to groundwater and soil, it will have adverse effects on human health and the environment. The rapid and sensitive detection of NB is a crucial task; thus, many approaches have been developed for NB detection, such as chromatography, spectrophotometry, electrochemical method, and so on [21]. However, these methods are complex, expensive, and time-consuming. Many lanthanide complexes can be used as fluorescence materials to detect NB through the alteration in fluorescence properties and, usually, show high sensitivity and selectivity, due to NB inducing fluorescence quenching of lanthanide complexes in various organic solvents.
However, there are few in-depth studies on the fluorescence quenching mechanism of lanthanide–organic complexes induced by adding NB. To investigate the mechanism of fluorescence quenching with NB, Liu et al. designed and prepared a Tb–FDA complex, {[Tb(FDA)1.5(DMF)]⋅DMF}n, using the reaction of Tb3+ and 2,5-furandicarboxylic acid (H2FDA) under solvothermal conditions, and used it as a highly selective and sensitive fluorescent probe for nitrobenzene and Fe3+. The fluorescence quenching mechanism was explained by the lowest unoccupied molecular orbital (LUMO) energy level of NB (−2.437 eV), which is lower than that of the H2FDA ligand (−1.954 eV) [39].
3.2. Nitrophenol (4-NP or 2-NP)
Nitrophenol (4-NP or 2-NP) is one of the smaller nitroaromatics with one electron-withdrawing nitro group and one electron-donating hydroxyl group on a benzene ring. It is mainly used as intermediate of pesticides, medicines, dyes and other fine chemicals, and, therefore, causes serious pollution due to its high toxicity. The highly sensitive detection of NP in water is very important for water environment protection, human health and ecological environment safety. GC, AAS, MS, etc., are usually used for the detection of NP, but some potential disadvantages limit their applications [36]. Therefore, the use of efficient sensors for detecting NP is extremely convenient and significant; in particular, the development of Ln–organic complex-based fluorescence sensors with high sensitivity for NP is of pivotal practical significance.
3.3. 2,4,6-Trinitrophenol (TNP)
Picric acid (PA), or 2,4,6-Trinitrophenol (TNP), possesses three electron-withdrawing nitro groups and one electron-donating hydroxyl group on a benzene ring. As a common and typical malignant organic pollutant, TNP is used widely in industries for dyes, explosives, pharmaceuticals, fireworks, firecrackers and leather. High toxicity, and harmfulness to eyes, skin and the respiratory system inspired many researchers to efficiently detect TNP. The traditional detection methods, including HPLC, RS, IMS, GC, MS and MIT, could be used to detect explosives, such as TNP; some potential limitations of these technologies restrict their application [40]. TNP remains in a dissociated form in solution and can interact with the positive center on the fluorophore due to its low pKa value (pKa = 0.42) [41][42]; thus, the fluorescence sensing method is suitable for the detection of TNP in solution.
The Ln–organic complexes-based sensors can effectively detect TNP with high sensitivity and selectivity in aqueous solution. Xu et al. prepared a 3D [Tb(TCBA)(H2O)2]2·DMF (H3TCBA = tris(3′-carboxybiphenyl)amine; DMF = dimethylformamide) by the solvothermal reaction of Tb3+ with a propeller-like H3TCBA ligand. The resultant Tb–organic complex features 1D triangular channels. Such a structure indicated high selectivity, with an extremely low detection limit about 1.64 ppb implied a high sensitivity for TNP explosive [43]. The lowest detection limit means the highest sensitivity of the sensor, compared with the previously reported Ln-based sensors [23][25][26][29][30][33][35]. The strong host–guest interactions between the Tb metal–organic framework (Tb-MOF) and TNP are captured and accurately determined by online microcalorimetry, which provides a distinctive thermodynamic perspective to understand the heterogeneous sensing behaviors.
4. Fluorescence Detection Mechanisms of Ln–Organic Complexes for Nitroaromatics
4.1. Resonance Energy Transfer (RET)
Resonance energy transfer, as a photoluminescence-sensing mechanism, is a short-range non-radiative energy transfer process which can significantly improve quenching efficiency and sensitivity. When the excited donor (fluorophore) induces the acceptor (analyte) to fluoresce, the fluorescence resonance energy is transferred, while the fluorescence intensity of the donor is reduced. Energy transfer is a distance-dependent physical process, and the efficiency of energy transfer usually hinges on (i) spectral overlap extent, the emission spectrum and the absorption spectrum of the host and guest; and (ii) dipole–dipole interaction, and the distance and relative orientation of the host and guest [44]. The efficiency and rate of energy transfer depends mainly on the degree of spectral overlap between the donor’s emission spectrum and the acceptor absorption spectrum. If the energies of the donor and acceptor are close, the energy transfer is significantly likely to occur. The degree of spectral overlap can be determined by experiment.
Both [Eu(BDPO)(H2O)4] (CUST-623) and [Tb(BDPO)(H2O)4] (CUST-624) based on N,N′-bis(3,5-dicarboxyphenyl)-oxalamide (BDPO) could be used as fluorescent sensors for detecting TNP, and the sensing mechanism could be attributed to RET because their fluorescence spectra overlap with the ultraviolet visible absorption spectra of TNP [29]. An Eu/Tb bifunctional metal–organic framework, [EuxTb1−x(L)(H2O)3]n (H4LCl = 3-bis(3,5-dicarboxyphenyl)imidazolium chloride), was synthesized successfully through a solvothermal reaction and used as a luminescent sensor to detect 4-NP in DMF and the quenching efficiency was affected by RET [43].
4.2. Competition Absorption (CA)
Competition absorption is the overlap between the excitation spectrum of an Ln–organic complex and the UV-vis absorption spectrum of the analyte. When the excitation spectrum of the complex overlaps greatly with the UV-vis absorption spectrum of the analyte, the complex and the analyte may competitively absorb the excitation light, thereby reducing the total available energy of the complex, resulting in a decrease in the excited state of the Ln–organic complex. As a result, the fluorescence property of the complex is quenched. For instance, a Dy organic complex [Dy(L)(NO3)(DMF)3]n (H2L = 2,5-di(1H-1,2,4-triazol-1-yl) terephthalic acid) with multi-emission peaks can be used as a luminescent ratio sensor for 4-NP, and the sensing mechanism is attributed to a competitive absorption of excitation energies [32].
4.3. Photoinduced Electron Transfer (PET)
The photoinduced electron transfer (PET) mechanism could be used to explain the luminescence quenching process of Ln–organic complexes caused by nitroaromatics. The luminescence of a lanthanide–organic complex is caused by the antenna effect, in which the energy is transferred from chromogenic organic ligands (sensors) to Ln3+ ions. For the PET mechanism, the conduction band (CB) energy levels of the chemosensors are higher than the LUMO levels of the explosives. Thus, the excitation energy of the chemosensors may be consumed by their electron transfer to the electron-deficient nitro explosives, resulting in the luminescence quenching of the lanthanide–organic complexes at the lowest LUMO energy value.
Various interactions in the metal coordination polymer (such as hydrogen bonding or π-π packing) with the host and guest of the analyte may facilitate the photoelectron transfer process during photoexcitation. PET is a redox process in which excited photoelectrons are transferred from the donor to the electron-deficient ground-state receptor. When the lowest unoccupied molecular orbital (LUMO) energy of the donor (fluorophores) is higher than the LUMO energy of the acceptor (analytical compound), photoelectrons may be transferred from the excited donor to the ground-state receptor, resulting in the fluorescence quenching of the donor. Correspondingly, when the LUMO orbital energy of the donor is lower than the LUMO orbital energy of the recipient, photoelectrons may be transferred from the recipient to the donor, resulting in the enhanced fluorescence of the donor.
4.4. Structural Transformation Mechanism (ST)
The structural transformation mechanism is when guest molecules enter the pore skeleton of a metal–organic complex (host) to generate guest–host interactions, thereby leading to the alteration in the coordination environment of metal ions and even structural deformation, resulting in significant changes in the luminescence of the metal–organic complex. This case can be applied to the selective detection of analytes. The host–guest interaction promotes or interrupts the sensitization pathways of the lanthanide ion and, therefore, leads to the enhancement or quenching of the luminescence, respectively [45]. An investigation elucidated the detection principle of luminescence quenching in a [Tb(BTTA)1.5(H2O)4.5]n sensor based on 2,5-bis(1H-1,2,4-triazol-1-yl) terephthalic acid (BTTA) for NB. Through multireference CASSCF/NEVPT2 calculations, it demonstrated the value of host–guest-interaction simulations and the rate constants of the radiative and nonradiative processes in understanding and elucidating the sensing mechanism in Ln-MOF sensors [46].
4.5. Static or Dynamic Quenching Mechanism (SY)
Static quenching is the formation of a non-fluorescent ground-state complex between the complex and the analyte, which absorbs light but does not emit photons and immediately returns to the ground state. Dynamic quenching is the transfer of electrons between the quencher and the sensor through excited-state collisions. Both are generally distinguished by changes in fluorescence decay lifetime and two quenching processes. Fluorescence lifetime spectroscopy is a potential technique to distinguish between static quenching and dynamic quenching. The fluorescence lifetime spectra of static quenching before and after the introduction of an analyte are similar, and dynamic quenching decreases before and after the introduction of the analyte and, thereby, results in the fluorescence-quenching phenomenon of the complexes. Under the static mechanism, a non-fluorescent complex is formed between the complex and the analyte, and fluorescence derived from the same complex. However, the fluorescence lifetime was found to decrease in dynamic quenching, because the collisions between the complex and the analyte reduced the excited states.
References
- Silver, B. Atomic Absorption Spectroscopy Reveals Anomalous Transfer of Heavy Metal across a Water/1,2-DCE Interface. Port. Electrochim. Acta 2021, 39, 415–420.
- Ruella Oliveira, S.; Tuttis, K.; Rita Thomazela Machado, A.; Cristina de Souza Rocha, C.; Maria Greggi Antunes, L.; Barbosa, F. Cell-to-cell heterogeneous association of prostate cancer with gold nanoparticles elucidated by single-cell inductively coupled plasma mass spectrometry. Microchem. J. 2022, 177, 107275.
- Lamei, N.; Ezoddin, M.; Kakavandi, N.R.; Abdi, K.; Ghazi-khansari, M. Ultrasound-Assisted Switchable Solvent in Determination of Quaternary Ammonium Herbicide Paraquat in Biological, Environmental Water, and Apple Juice Samples Using Chemical Reduction Process Coupled to GC–MS Detection. Chromatographia 2018, 81, 923–930.
- Gruznov, V.M.; Baldin, M.N.; Makas, A.L.; Titov, B.G. Progress in methods for the identification of explosives in Russia. J. Anal. Chem. 2011, 66, 1236–1246.
- Brown, K.E.; Greenfield, M.T.; McGrane, S.D.; Moore, D.S. Advances in explosives analysis–part I: Animal, chemical, ion, and mechanical methods. Anal. Bioanal. Chem. 2016, 408, 35–47.
- Brown, K.E.; Greenfield, M.T.; McGrane, S.D.; Moore, D.S. Advances in explosives analysis–part II: Photon and neutron methods. Anal. Bioanal. Chem. 2016, 408, 49–65.
- Liu, Y.-Y.; Xu, X.; Xin, J.-W.; Ghulamb, M.; Fan, J.; Dong, X.; Qiu, L.-L.; Xue, M.; Meng, Z.-H. Molecularly imprinted colloidal array for the high-throughput screening of explosives. Chin. J. Anal. Chem. 2023, 51, 100215.
- Bairy, G.; Dey, A.; Dutta, B.; Ray, P.P.; Sinha, C. 2D Cd(II)-MOF of Pyridyl-Imidazoquinazoline: Structure, Luminescence, and Selective Detection of TNP and Fabrication of Semiconducting Devices. Cryst. Growth Des. 2022, 22, 3138–3147.
- Razavi, S.A.A.; Morsali, A.; Piroozzadeh, M. A Dihydrotetrazine-Functionalized Metal–Organic Framework as a Highly Selective Luminescent Host–Guest Sensor for Detection of 2,4,6-Trinitrophenol. Inorg. Chem. 2022, 61, 7820–7834.
- Goswami, R.; Mandal, S.C.; Pathak, B.; Neogi, S. Guest-Induced Ultrasensitive Detection of Multiple Toxic Organics and Fe3+ Ions in a Strategically Designed and Regenerative Smart Fluorescent Metal–Organic Framework. ACS Appl. Mater. Interfaces 2019, 11, 9042–9053.
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401.
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Design of fluorescent sensors based on azaheterocyclic push-pull systems towards nitroaromatic explosives and related compounds: A review. Dye. Pigment. 2020, 180, 108414.
- Ahmed, R. Review: Fluorophores for detecting Nitroaromatic Compounds, Picric Acid. Int. J. Multidiscip. Res. Anal. 2023, 6, 1119–1131.
- Svalova, T.S.; Saigushkina, A.A.; Verbitskiy, E.V.; Chistyakov, K.A.; Varaksin, M.V.; Rusinov, G.L.; Charushin, V.N.; Kozitsina, A.N. Rapid and sensitive determination of nitrobenzene in solutions and commercial honey samples using a screen-printed electrode modified by 1,3-/1,4-diazines. Food Chem. 2022, 372, 131279.
- Zhao, P.; Liu, Y.; He, C.; Duan, C. Synthesis of a Lanthanide Metal–Organic Framework and Its Fluorescent Detection for Phosphate Group-Based Molecules Such as Adenosine Triphosphate. Inorg. Chem. 2022, 61, 3132–3140.
- Sun, Y.-Q.; Cheng, Y.; Yin, X.-B. Dual-Ligand Lanthanide Metal–Organic Framework for Sensitive Ratiometric Fluorescence Detection of Hypochlorous Acid. Anal. Chem. 2021, 93, 3559–3566.
- Wang, G.-D.; Li, Y.-Z.; Shi, W.-J.; Zhang, B.; Hou, L.; Wang, Y.-Y. A robust cluster-based Eu-MOF as multi-functional fluorescence sensor for detection of antibiotics and pesticides in water. Sens. Actuators B Chem. 2021, 331, 129377.
- Xu, M.-Y.; Liang, G.-Y.; Feng, J.-S.; Liang, G.-M.; Wang, X.-J. Temperature-Induced Structural Transformations of Lanthanide Coordination Polymers Based on a Semirigid Tricarboxylic Acid Ligand: Crystal Structures and Luminescence Properties. Cryst. Growth Des. 2022, 22, 1583–1593.
- Xiao, J.; Liu, M.; Tian, F.; Liu, Z. Stable Europium-based Metal–Organic Frameworks for Naked-eye Ultrasensitive Detecting Fluoroquinolones Antibiotics. Inorg. Chem. 2021, 60, 5282–5289.
- Sahoo, S.; Mondal, S.; Sarma, D. Luminescent Lanthanide Metal-Organic Frameworks (Ln MOFs): A Versatile Platform towards Organomolecule Sensing. Coord. Chem. Rev. 2022, 470, 214707.
- Chai, H.M.; Yan, J.L.; Zhang, G.Q.; Wang, J.X.; Ren, Y.X.; Gao, L.J. Five Mesoporous Lanthanide Metal-Organic Frameworks: Syntheses, Structures, and Fluorescence Sensing of Fe3+, Cr2O72−, and H2O2 and Electrochemical Sensing of Trinitrophenol. Inorg. Chem. 2022, 61, 7286–7295.
- Gao, R.C.; Guo, F.S.; Bai, N.N.; Wu, Y.L.; Yang, F.; Liang, J.Y.; Li, Z.J.; Wang, Y.Y. Two 3D Isostructural Ln(III)-MOFs: Displaying the Slow Magnetic Relaxation and Luminescence Properties in Detection of Nitrobenzene and Cr2O72−. Inorg. Chem. 2016, 55, 11323–11330.
- Gao, T.; Ma, Z.; Ren, Y.; Wang, Z.; Zhang, M.; Chen, X.; Wang, J. High sensitive fluorescent sensing and photocatalytic degradation performance of two-dimensional Tb-organic network. J. Rare Earths 2023, in press.
- Chen, F.; Wang, J.; Dong, M.W.; Zhang, S.L.; Wu, C.P.; Liu, J.Q. Five lanthanide supramolecular frameworks based on mixed 3-(4-hydroxyphenyl)propanoic acid and 1,10-phenanthroline tectons: Crystal structures and luminescent properties. J. Mol. Struct. 2019, 1177, 117–123.
- Ma, Y.; Yang, X.; Niu, M.; Hao, W.; Shi, D.; Schipper, D. High-Nuclearity Cd(II)–Nd(III) Nanowheel with NIR Emission Sensing of Metal Cations and Nitro-Based Explosives. Cryst. Growth Des. 2021, 21, 2821–2827.
- Ma, Y.; Yang, X.; Niu, M.; Shi, D.; Schipper, D. One high-nuclearity Yb(III) nanoring with NIR luminescent sensing towards antibiotics and explosives. J. Lumin. 2022, 241, 118494.
- Shi, D.; Yang, X.; Chen, H.; Jiang, D.; Liu, J.; Ma, Y.; Schipper, D.; Jones, R.A. Large Ln42 coordination nanorings: NIR luminescence sensing of metal ions and nitro explosives. Chem. Comm. 2019, 55, 13116–13119.
- Shi, D.; Yang, X.; Chen, H.; Ma, Y.; Schipper, D.; Jones, R.A. Self-assembly of luminescent 42-metal lanthanide nanowheels with sensing properties towards metal ions and nitro explosives. J. Mater. Chem. C 2019, 7, 13425–13431.
- Wang, S.; Sun, B.; Su, Z.; Hong, G.; Li, X.; Liu, Y.; Pan, Q.; Sun, J. Lanthanide-MOFs as multifunctional luminescent sensors. Inorg. Chem. Front. 2022, 9, 3259–3266.
- Liu, Y.; Sun, Q.; Zhou, H.; Gao, H.; Li, D.; Li, Y. One-dimensional Europium-coordination polymer as luminescent sensor for highly selective and sensitive detection of 2,4,6-trinitrophenol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120303.
- Li, J.J.; Fan, T.T.; Qu, X.L.; Han, H.L.; Li, X. Temperature-induced 1D lanthanide polymeric frameworks based on Lnn (n = 2, 2, 4, 6) cores: Synthesis, crystal structures and luminescence properties. Dalton Trans. 2016, 45, 2924–2935.
- Liu, W.; Liu, G.; Gao, G.; Gao, Z.; Zhang, Y.; Wu, S.; Gao, E.; Zhu, M. A new dysprosium (III)-Organic framework as a ratiometric luminescent sensor for Nitro-compounds and antibiotics in aqueous solutions. Inorg. Chem. Commun. 2021, 133, 108952.
- Smith, J.A.; Singh-Wilmot, M.A.; Carter, K.P.; Cahill, C.L.; Ridenour, J.A. Supramolecular assembly of lanthanide-2,3,5,6-tetrafluoroterephthalic acid coordination polymers via fluorine⋯fluorine interactions: A platform for luminescent detection of Fe3+ and nitroaromatic compounds. New J. Chem. 2020, 44, 12317–12330.
- Smith, J.A.; Singh-Wilmot, M.A.; Carter, K.P.; Cahill, C.L.; Ridenour, J.A. Lanthanide-2,3,5,6-Tetrabromoterephthalic Acid Metal–Organic Frameworks: Evolution of Halogen···Halogen Interactions across the Lanthanide Series and Their Potential as Selective Bifunctional Sensors for the Detection of Fe3+, Cu2+, and Cryst. Growth Des. 2019, 19, 305–319.
- Gao, T.; Ren, Y.; Wang, Z.; Zhang, M.; Cao, J.; Wang, J. A multi-functional Tb-organic network featuring high selectivity fluorescent sensing for Fe3+, Cr2O72−, tetracycline and 2,4,6-trinitrophenol in aqueous solution. J. Solid State Chem. 2022, 309, 123002.
- Cheng, X.; Hu, J.; Li, J.; Zhang, M. Tunable emission and selective luminescence sensing for nitro- pollutants and metal ions based on bifunctional lanthanide metal-organic frameworks. J. Lumin. 2020, 221, 117100.
- Zhang, S.-R.; Du, D.-Y.; Qin, J.-S.; Li, S.-L.; He, W.-W.; Lan, Y.-Q.; Su, Z.-M. 2D Cd(II)–Lanthanide(III) Heterometallic–Organic Frameworks Based on Metalloligands for Tunable Luminescence and Highly Selective, Sensitive, and Recyclable Detection of Nitrobenzene. Inorg. Chem. 2014, 53, 8105–8113.
- Wang, A.; Zhang, Y.; Lu, L.; Zhu, M.; Yuan, C.; Feng, S. Seven Ln(III) coordination polymers with two kinds of geometric coordination but the same 3D topological property: Luminescence sensing and magnetic property. Dalton Trans. 2022, 51, 12324–12333.
- Liu, W.; Li, D.; Wang, F.; Chen, X.; Wang, X.; Tian, Y. A luminescent lanthanide MOF as highly selective and sensitive fluorescent probe for nitrobenzene and Fe3+. Opt. Mater. 2022, 123, 111895.
- Kim, D.; Lynch, V.; Nielsen, K.; Johnsen, C.; Jeppesen, J.; Sessler, J. A chloride-anion insensitive colorimetric chemosensor for trinitrobenzene and picric acid. Anal. Bioanal. Chem. 2009, 395, 393–400.
- Bodman, S.E.; Butler, S.J. Advances in anion binding and sensing using luminescent lanthanide complexes. Chem. Sci. 2021, 12, 2716–2734.
- Herrera-Chacon, A.; Gonzalez-Calabuig, A.; del Valle, M. Dummy Molecularly Imprinted Polymers Using DNP as a Template Molecule for Explosive Sensing and Nitroaromatic Compound Discrimination. Chemosensors 2021, 9, 255.
- Xu, W.; Chen, H.; Xia, Z.; Ren, C.; Han, J.; Sun, W.; Wei, Q.; Xie, G.; Chen, S. A Robust TbIII-MOF for Ultrasensitive Detection of Trinitrophenol: Matched Channel Dimensions and Strong Host–Guest Interactions. Inorg. Chem. 2019, 58, 8198–8207.
- Zhao, Y.F.; Zeng, H.; Zhu, X.W.; Lu, W.G.; Li, D. Metal–organic frameworks as photoluminescent biosensing platforms: Mechanisms and applications. Chem. Soc. Rev. 2021, 50, 4484–4513.
- Ma, W.P.; Yan, B. Lanthanide Functionalized MOF Thin Films as Effective Luminescent Materials and Chemical Sensors for Ammonia. Dalton Trans. 2020, 49, 15663–15671.
- Hidalgo-Rosa, Y.; Mena-Ulecia, K.; Treto-Suárez, M.A.; Schott, E.; Páez-Hernández, D.; Zarate, X. Expanding the Knowledge of the Selective-Sensing Mechanism of Nitro Compounds by Luminescent Terbium Metal–Organic Frameworks through Multiconfigurational ab Initio Calculations. J. Phys. Chem. C 2022, 126, 7040–7050.
More
Information
Subjects:
Chemistry, Inorganic & Nuclear
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
570
Revisions:
2 times
(View History)
Update Date:
20 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




