
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Angeles Juarranz | -- | 3169 | 2023-07-18 11:16:59 | | | |
| 2 | Conner Chen | Meta information modification | 3169 | 2023-07-21 03:52:14 | | |
Video Upload Options
Exposure to sun radiation leads to higher risk of sunburn, pigmentation, immunosuppression, photoaging and skin cancer. In addition to ultraviolet radiation (UVR), recent research indicates that infrared radiation (IR) and visible light (VIS) can play an important role in the pathogenesis of some of these processes. Detrimental effects associated with sun exposure are well known, but new studies have shown that DNA damage continues to occur long after exposure to solar radiation has ended. Regarding photoprotection strategies, natural substances are emerging for topical and oral photoprotection. In this sense, Fernblock®, a standardized aqueous extract of the fern Polypodium Leucotomos (PLE), has been widely administered both topically and orally with a strong safety profile. Thus, this extract has been used extensively in clinical practice, including as a complement to photodynamic therapy (PDT) for treating actinic keratoses (AKs) and field cancerization.
1. Introduction
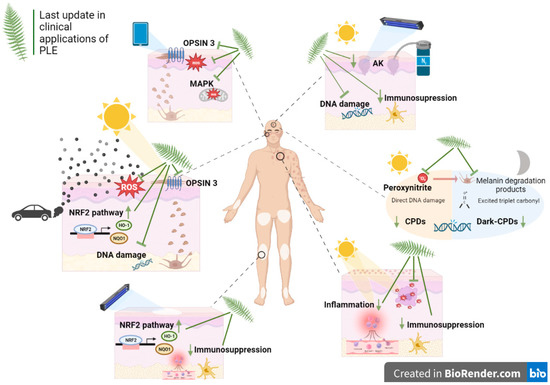
2. Oncodermatology
| Oncodermatology | |||
|---|---|---|---|
| Design | Pathology/Focus | Summary/Outcome | Study Reference |
| Review | General oncodermatology | This review reports the mechanisms through which Polypodium leucotomos acts to evaluate its uses in oncodermatology with references to in vitro and in vivo studies. | [8] |
| Review and book chapter |
Continuing medical education about skin cancer and sunscreen use | These reviews provide evidence-based recommendations for the use of sunscreen as a preventive strategy against skin cancer while also considering potential risks and environmental impacts associated with the use of some chemical sunscreen filters. PLE is included as a reference oral sunscreen technology for prevention of photodamage. | [28][29][30][31][32] |
| Reviews and book chapter | Botanical interventions for photoprotection and skin cancer | These works review the main actives derived from plants with scientific evidence as treatment in photoprotection and offer an overview of cancer and phytotherapy. Specifically, they review the existing literature on the properties of PLE and its potential therapeutic effects in preventing skin damage. These reviews include studies conducted in vitro, in vivo and clinical trials. | [33][34][35][36][37] |
| Review | Preventive interventions for keratinocyte carcinoma | This manuscript examines the potential of pharmaceuticals, plant-derived phytochemicals and vitamins for preventing keratinocyte carcinoma. One such reference photoprotectant is PLE, which has been shown to inhibit the development of tumors and acute UV-induced damage in humans. | [38] |
| Clinical study | Field cancerization | This clinical study suggests that a new medical device treatment containing Fernblock® (NMD) is a useful treatment method for improving the precancerous field and preventing the development of new AKs. | [16] |
| Clinical study | Oral cancer | The findings indicate that PLE has the ability to suppress oral cancer cell growth in vitro and prevent tumor development in vivo. Thus, PLE could be a promising natural therapeutic approach for preventing and treating oral cancer. | [27] |
| Preclinical study | Skin cancer markers | This in vitro study suggests that FB could be a promising candidate to complement traditional sunscreens in providing long-lasting skin protection against dark-CPDs formation after irradiation. | [21] |
| Preclinical study | Melanoma | This in vitro research suggests that supplements containing sulforaphane/FB could be used to prevent skin aging and help treat advanced melanoma. | [20] |
| Preclinical study | Skin cancer induced by photopollution | This preclinical study demonstrates the efficacy of PLE in preventing changes in cellular structure, viability, oxidative stress and activation of the melanogenic signaling pathway caused by exposure to both BaP and UVA light. | [23] |
| Actinic keratosis | |||
| Review | Actinic keratosis | This review article examines in vitro experiments and clinical trials that utilize evidence-based therapeutic methods before or after photodynamic therapy (PDT). Specifically, the effectiveness of topical treatments and oral supplementation, such as diclofenac, imiquimod and PLE, among others, as well as mechanical-physical treatments, are evaluated. | [39] |
| Review | Actinic keratosis | In this article, the authors offer expert opinions and practical insights into the treatment of actinic keratosis and field cancerization using monotherapy or a combination of therapies among which PLE is cited. The primary objective is to achieve improved, quicker and more tolerable clinical outcomes. | [40] |
| Review | Actinic keratosis | This review discusses various physical ablative techniques and drug preparations available for treatment. It emphasizes the need for careful evaluation of efficacy, toxicity and tolerability data, as well as practical considerations such as treatment protocols and patient preferences, to achieve maximal adherence and prevent treatment failure. It includes PLE as a chemopreventive treatment tool against the development of AK. | [41] |
| Xeroderma pigmentosum | |||
| Review | Xeroderma pigmentosum | The purpose of this review is to present the symptoms, diagnosis, and treatment of XP. It also includes oral PLE as a treatment adjuvant due to its chemoprotective, antioxidative, anti-inflammatory and immunomodulatory properties. All these effects have the potential to lessen the phototoxic effects of UVR and thus reduce UVR-induced skin damage and cancer. | [42] |
References
- Yeager, D.G.; Lim, H.W. What’s New in Photoprotection: A Review of New Concepts and Controversies. Dermatol. Clin. 2019, 37, 149–157.
- Parrado, C.; Philips, N.; Gilaberte, Y.; Juarranz, A.; González, S. Oral Photoprotection: Effective Agents and Potential Candidates. Front. Med. 2018, 5, 188.
- García, F.; Pivel, J.P.; Guerrero, A.; Brieva, A.; Martínez-Alcázar, M.P.; Caamaño-Somoza, M.; González, S. Phenolic Components and Antioxidant Activity of Fernblock, an Aqueous Extract of the Aerial Parts of the Fern Polypodium Leucotomos. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 157–160.
- González, S.; Lucena, S.R.; Delgado, P.; Juarranz, A. Comparison of Several Hydrophilic Extracts of Polypodium Leucotomos Reveals Different Antioxidant Moieties and Photoprotective Effects in Vitro. J. Med. Plants Res. 2018, 13, 336–345.
- Del Rosso, J.Q. Polypodium Leucotomos Extract (PLE): New Study Gives Evidence-Based Insight into Ain’t Nothing Like the Real Thing. J. Clin. Aesthet. Dermatol. 2019, 12, 45.
- Parrado, C.; Nicolas, J.; Juarranz, A.; Gonzalez, S. The Role of the Aqueous Extract Polypodium Leucotomos in Photoprotection. Photochem. Photobiol. Sci. 2020, 19, 831–843.
- Pourang, A.; Dourra, M.; Ezekwe, N.; Kohli, I.; Hamzavi, I.; Lim, H.W. The Potential Effect of Polypodium Leucotomos Extract on Ultraviolet- and Visible Light-Induced Photoaging. Photochem. Photobiol. Sci. 2021, 20, 1229–1238.
- Calzari, P.; Vaienti, S.; Nazzaro, G. Uses of Polypodium Leucotomos Extract in Oncodermatology. J. Clin. Med. 2023, 12, 673.
- Villa, A.; Viera, M.H.; Amini, S.; Huo, R.; Perez, O.; Ruiz, P.; Amador, A.; Elgart, G.; Berman, B. Decrease of Ultraviolet A Light-Induced “Common Deletion” in Healthy Volunteers after Oral Polypodium Leucotomos Extract Supplement in a Randomized Clinical Trial. J. Am. Acad. Dermatol. 2010, 62, 511–513.
- Rodríguez-Yanes, E.; Juarranz, Á.; Cuevas, J.; Gonzalez, S.; Mallol, J. Polypodium Leucotomos Decreases UV-Induced Epidermal Cell Proliferation and Enhances P53 Expression and Plasma Antioxidant Capacity in Hairless Mice. Exp. Dermatol. 2012, 21, 638–640.
- Philips, N.; Smith, J.; Keller, T.; Gonzalez, S. Predominant Effects of Polypodium Leucotomos on Membrane Integrity, Lipid Peroxidation, and Expression of Elastin and Matrixmetalloproteinase-1 in Ultraviolet Radiation Exposed Fibroblasts, and Keratinocytes. J. Dermatol. Sci. 2003, 32, 1–9.
- Philips, N.; Conte, J.; Chen, Y.J.; Natrajan, P.; Taw, M.; Keller, T.; Givant, J.; Tuason, M.; Dulaj, L.; Leonardi, D.; et al. Beneficial Regulation of Matrixmetalloproteinases and Their Inhibitors, Fibrillar Collagens and Transforming Growth Factor-β by Polypodium Leucotomos, Directly or in Dermal Fibroblasts, Ultraviolet Radiated Fibroblasts, and Melanoma Cells. Arch. Dermatol. Res. 2009, 301, 487–495.
- Kohli, I.; Shafi, R.; Isedeh, P.; Griffith, J.L.; Al-Jamal, M.S.; Silpa-archa, N.; Jackson, B.; Athar, M.; Kollias, N.; Elmets, C.A.; et al. The Impact of Oral Polypodium Leucotomos Extract on Ultraviolet B Response: A Human Clinical Study. J. Am. Acad. Dermatol. 2017, 77, 33–41.e1.
- Middelkamp-Hup, M.A.; Pathak, M.A.; Parrado, C.; Goukassian, D.; Rius-Díaz, F.; Mihm, M.C.; Fitzpatrick, T.B.; González, S. Oral Polypodium Leucotomos Extract Decreases Ultraviolet-Induced Damage of Human Skin. J. Am. Acad. Dermatol. 2004, 51, 910–918.
- Auriemma, M.; Di Nicola, M.; Gonzalez, S.; Piaserico, S.; Capo, A.; Amerio, P. Polypodium Leucotomos Supplementation in the Treatment of Scalp Actinic Keratosis. Dermatol. Surg. 2015, 41, 898–902.
- De Unamuno Bustos, B.; Aguilera, N.C.; Azorín García, I.; Andrino, A.C.; Ros, M.L.; Rodrigo, R.; Vitale, M.; González, S.; Botella Estrada, R. Long-Term Efficacy of a New Medical Device Containing Fernblock ® and DNA Repair Enzyme Complex in the Treatment and Prevention of Cancerization Field in Patients with Actinic Keratosis. J. Clin. Exp. Dermatol. Res. 2019, 10, 499.
- Lamberti, A.; Cartocci, A.; Donelli, C.; Cortonesi, G.; Trovato, E.; Milani, M.; Rubegni, P.; Cinotti, E. Prevention Strategies in Patients Affected by Actinic Keratosis of the Head: A 12-Month, Prospective, Assessor-Blinded, Controlled Study with Lesion-Directed Treatment Associated with Medicalized Photoprotection; Longdom Publishing SL: Barcelona, Spain, 2022; Volume 13, p. 5.
- Pellacani, G.; Peris, K.; Ciardo, S.; Pezzini, C.; Tambone, S.; Farnetani, F.; Longo, C.; Chello, C.; Gonzalez, S. The Combination of Oral and Topical Photoprotection with a Standardized Polypodium Leucotomos Extract Is Beneficial against Actinic Keratosis. Photodermatol. Photoimmunol. Photomed. 2023, 1–8.
- Aguilera, P.; Carrera, C.; Puig-Butille, J.A.; Badenas, C.; Lecha, M.; González, S.; Malvehy, J.; Puig, S. Benefits of Oral Polypodium Leucotomos Extract in MM High-Risk Patients. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1095–1100.
- Serini, S.; Guarino, R.; Vasconcelos, R.O.; Celleno, L.; Calviello, G. The Combination of Sulforaphane and Fernblock® XP Improves Individual Beneficial Effects in Normal and Neoplastic Human Skin Cell Lines. Nutrients 2020, 12, 1608.
- Portillo-Esnaola, M.; Rodríguez-Luna, A.; Nicolás-Morala, J.; Gallego-Rentero, M.; Villalba, M.; Juarranz, Á.; González, S. Formation of Cyclobutane Pyrimidine Dimers after UVA Exposure (Dark-CPDs) Is Inhibited by an Hydrophilic Extract of Polypodium Leucotomos. Antioxidants 2021, 10, 1961.
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Brash, D.E. Chemiexcitation of Melanin Derivatives Induces DNA Photoproducts Long after UV Exposure. Science 2015, 347, 842–847.
- Gallego-Rentero, M.; Nicolás-Morala, J.; Alonso-Juarranz, M.; Carrasco, E.; Portillo-Esnaola, M.; Rodríguez-Luna, A.; González, S. Protective Effect of the Hydrophilic Extract of Polypodium Leucotomos, Fernblock®, against the Synergistic Action of UVA Radiation and BenzoPyrene Pollutant. Antioxidants 2022, 11, 2185.
- Schalka, S.; Donato, L.C. Evaluation of Effectiveness of a Sunscreen Containing Polypodium Leucatomos Extract in Reducing the Sun Damage to the Skin. Surg. Cosmet. Dermatol. 2019, 11, 310–318.
- Aguilera, J.; Vicente-Manzanares, M.; de Gálvez, M.V.; Herrera-Ceballos, E.; Rodríguez-Luna, A.; González, S. Booster Effect of a Natural Extract of Polypodium Leucotomos (Fernblock®) That Improves the UV Barrier Function and Immune Protection Capability of Sunscreen Formulations. Front. Med. 2021, 8, 684665.
- González-Morán, A.; Piquero-Casals, J. Use of a Topical Film-Forming Medical Device Containing Repairsomes® in a Patient with Xeroderma Pigmentosum to Avoid Progression to Skin Cancerization. Clin. Cosmet. Investig. Dermatol. 2020, 13, 677–681.
- Lacerda, P.A.; Oenning, L.C.; Bellato, G.C.; Lopes-Santos, L.; de Antunes, N.J.; Mariz, B.A.L.A.; Teixeira, G.; Vasconcelos, R.; Simões, G.F.; de Souza, I.A.; et al. Polypodium Leucotomos Targets Multiple Aspects of Oral Carcinogenesis and It Is a Potential Antitumor Phytotherapy against Tongue Cancer Growth. Front. Pharmacol. 2023, 13, 1098374.
- González, S.; De Gálvez, M.V.; De Troya, M.; Rodríguez-Luna, A.; Calzavara-Pinton, P. Personalized Medical Photoprotection: Determining Optimal Measures for Susceptible Patient Groups. Open Dermatol. J. 2023, 17, 1–7.
- González, S.; Aguilera, J.; Berman, B.; Calzavara-Pinton, P.; Gilaberte, Y.; Goh, C.L.; Lim, H.W.; Schalka, S.; Stengel, F.; Wolf, P.; et al. Expert Recommendations on the Evaluation of Sunscreen Efficacy and the Beneficial Role of Non-Filtering Ingredients. Front. Med. 2022, 9, 790207.
- Perez, M.; Abisaad, J.A.; Rojas, K.D.; Marchetti, M.A.; Jaimes, N. Skin Cancer: Primary, Secondary, and Tertiary Prevention. Part I. J. Am. Acad. Dermatol. 2022, 87, 255–268.
- Passeron, T.; Lim, H.W.; Goh, C.L.; Kang, H.Y.; Ly, F.; Morita, A.; Ocampo Candiani, J.; Puig, S.; Schalka, S.; Wei, L.; et al. Photoprotection According to Skin Phototype and Dermatoses: Practical Recommendations from an Expert Panel. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1460–1469.
- Sander, M.; Sander, M.; Burbidge, T.; Beecker, J. The Efficacy and Safety of Sunscreen Use for the Prevention of Skin Cancer. CMAJ 2020, 192, E1802–E1808.
- Philips, N.; Richardson, R.; Siomyk, H.; Bynum, D.; Gonzalez, S. “Skin Cancer, Polyphenols, and Oxidative Stress” or Coun-teraction of Oxidative Stress, Inflammation, Signal Transduction Pathways, and Extracellular Matrix Remodeling That Mediate Skin Carcinogenesis by Polyphenols. In Cancer; Academic Press: Cambridge, MA, USA, 2021; pp. 439–450.
- Araújo Lacerda, P.; Marinho Ottoni Costa, L.; Cuoghi Bellato, G.; Ayaka Yamashita, M.; Lopes-Santos, L.; Augusto, T.M.; Karla Cervigne, N. Perspectives on Cancer and Phytotherapy: An Overview Focusing on Polypodium Leucotomos Therapeutic Properties. J. Cancer Prev. Curr. Res. 2021, 12, 9–18.
- Subhadarshani, S.; Athar, M.; Elmets, C.A. Photocarcinogenesis. Curr. Dermatol. Rep. 2020, 9, 189–199.
- Bhatia, B.K.; Lim, H.W.; Hamzavi, I.H. Comprehensive Dermatologic Drug Therapy; Wolverton, E.S., Jashin, J., Wu, J.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 23, pp. 263–270.
- Zimmerman, C. Herbs for Low-Risk Skin Cancers and Precancers. Altern. Complement. Ther. 2019, 25, 163–166.
- Pihl, C.; Togsverd-Bo, K.; Andersen, F.; Haedersdal, M.; Bjerring, P.; Lerche, C.M. Keratinocyte Carcinoma and Photoprevention: The Protective Actions of Repurposed Pharmaceuticals, Phytochemicals and Vitamins. Cancers 2021, 13, 3684.
- Piaserico, S.; Mazzetto, R.; Sartor, E.; Bortoletti, C. Combination-Based Strategies for the Treatment of Actinic Keratoses with Photodynamic Therapy: An Evidence-Based Review. Pharmaceutics 2022, 14, 1726.
- Piquero-Casals, J.; Morgado-Carrasco, D.; Gilaberte, Y.; Del Rio, R.; Macaya-Pascual, A.; Granger, C.; López-Estebaranz, J.L. Management Pearls on the Treatment of Actinic Keratoses and Field Cancerization. Dermatol. Ther. 2020, 10, 903–915.
- Calzavara-Pinton, P.; Calzavara-Pinton, I.; Rovati, C.; Rossi, M. Topical Pharmacotherapy for Actinic Keratoses in Older Adults. Drugs Aging 2022, 39, 143–152.
- Leung, A.K.C.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Xeroderma Pigmentosum: An Updated Review. Drugs Context 2022, 11, 1–17.




