Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Maria Moitzi | -- | 2907 | 2023-07-18 09:40:59 | | | |
| 2 | Catherine Yang | Meta information modification | 2907 | 2023-07-18 09:44:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Moitzi, A.M.; König, D. The Glycaemic Index and Substrate Metabolism in Athletes. Encyclopedia. Available online: https://encyclopedia.pub/entry/46907 (accessed on 08 February 2026).
Moitzi AM, König D. The Glycaemic Index and Substrate Metabolism in Athletes. Encyclopedia. Available at: https://encyclopedia.pub/entry/46907. Accessed February 08, 2026.
Moitzi, Anna Maria, Daniel König. "The Glycaemic Index and Substrate Metabolism in Athletes" Encyclopedia, https://encyclopedia.pub/entry/46907 (accessed February 08, 2026).
Moitzi, A.M., & König, D. (2023, July 18). The Glycaemic Index and Substrate Metabolism in Athletes. In Encyclopedia. https://encyclopedia.pub/entry/46907
Moitzi, Anna Maria and Daniel König. "The Glycaemic Index and Substrate Metabolism in Athletes." Encyclopedia. Web. 18 July, 2023.
Copy Citation
Nutrition has a decisive influence on athletic performance. However, it is not only the nutrient intake during exercise that is important, but the daily diet must also be adapted to the requirements of physical activity in order to optimally promote training adaptations. The targeted modification of macronutrient intake is a common method of influencing substrate metabolism, fuel selection, and performance.
glycaemic index
long-term effects
sport nutrition
endurance performance
1. Introduction
Endurance athletes should not only pay attention to sufficient carbohydrate intake during exercise, but also to the carbohydrate content of their daily diet, in order to optimally replenish glycogen stores. The recommended amount of daily CHO intake depends on several factors, including training frequency, duration, and intensity. Therefore, the recommendation for CHO intake varies from 3 to 12 g·kg body weight−1·day−1. Depending on which metabolic or structural adaptation is to be the focus of the current training load, it must be decided whether more or less carbohydrates should be supplied [1][2].
Furthermore, it is important for athletes to be mindful of the nutritional and physiological effects associated with their dietary intake. The quality of carbohydrates, and in particular the glycaemic index, can significantly influence the metabolic processes after ingestion. The glycaemic index (GI) categorizes carbohydrates according to their impact on blood glucose concentration and the extent to which they stimulate insulin secretion, usually compared to glucose (GI = 100) or white bread (GI = 70) [3][4]. Therefore, the GI reflects the availability of the consumed CHO in the blood. So, for the same amount of carbohydrates, a low-GI food (GI ≤ 55) will not raise blood glucose to the same extent as a high-GI food (GI ≥ 70). Low-GI foods are therefore digested and absorbed more slowly compared to high-GI foods [5][6]. Among other factors, the relative ratio of carbohydrate to fat oxidation is decisively co-regulated by the level of insulin concentration in the blood. The higher the GI, the faster the blood glucose rises, the higher the insulin secretion, and the lower the fat oxidation. This relationship has already been demonstrated in athletes during endurance activity [7][8].
For improving performance, an athlete needs a combination of adequate fuel stores to provide enough ATP for muscle work and metabolic flexibility. Metabolic flexibility refers to the capacity to effectively utilize various pathways to optimize ATP regeneration, while also enabling the utilization of all muscle fuel sources to meet the specific demands of exercise at different intensities [9]. So far, it has been believed that a low-carbohydrate diet has beneficial effects on fat oxidation. However, the disadvantage of these so-called low-carbohydrate diets is that performance at higher intensities deteriorates because metabolic flexibility is worsened by the absence of carbohydrates [10]. In theory, with the help of the GI and its influence on postprandial insulin secretion and fat oxidation, it should be possible to improve metabolic flexibility by improving fat oxidation and having sufficient carbohydrates for energy provision at high intensities.
2. Mechanism of Substrate Oxidation during Endurance Exercise and Influencing Factors
During prolonged endurance exercise, the main source of ATP fuel for muscle work is derived from the oxidative phosphorylation of both fat and carbohydrates (CHO). The primary substrates utilized by the muscles, include muscle and liver glycogen, blood glucose, and fatty acids derived from muscle and adipose tissue triglyceride stores [11][12]. The oxidation of proteins for energy production is less important compared to the primary sources—carbohydrates and fats—and they contribute only about 5% to ATP supply [12]. As the intensity of exercise increases, there is a greater reliance on glucose and glycogen as fuel sources, surpassing the oxidation of fat. This shift occurs because carbohydrates provide a greater energy output per unit of time, leading to an increased reliance on glucose and glycogen [13][14][15]. In healthy individuals, maximal rates of fat oxidation can be expected at intensities of 60–65% of maximal oxygen uptake (VO2max) [16] and vary from 0.18 to 1.01 g·min−1 [17]. With glycogen stores in the muscles and liver limited to approximately 2000 kcal, they represent one of the most important limiting factors for prolonged endurance exercise at higher intensities. Research over recent decades has shown that the most effective diet is the one that is able to augment and preserve CHO fuel stores (i.e., muscle and liver glycogen) for the decisive phases of a race [18]. Fat stores, on the other hand, are normally present in the body in sufficient quantities to theoretically supply the body with fuel for several days [12]. Half a kilogram of fat provides around 4500 kcal and the high storage capacity makes fat the energy source of choice for low to moderate intensities when enough oxygen is available [19][20][21]. Combined with exercise, a high rate of fat oxidation modulates insulin sensitivity and glucose tolerance. Hence, enhanced fat oxidation is not only a goal for athletes but also for the general population as it might improve performance at first glance and health in the longer-term speaking [22][23].
Substrate utilization can be influenced through the intake of exogenous carbohydrates during exercise. Since the beginning of the 20th century, the effects of CHO intake during prolonged endurance exercise have been of interest for researchers [24][25][26]. Dill, et al. [27] for example, found as early as 1932 that the blood sugar level in dogs could be kept constant during prolonged exercise by administering 20 g of carbohydrates per hour and, furthermore, the dogs could maintain a certain speed for a longer time-period compared to when consuming water. Moreover, it was found that by supplying carbohydrates before and during exercise, symptoms of hypoglycaemia could be avoided and endogenous carbohydrate stores were spared [28][29][30]. Controlled studies have shown that carbohydrate supplementation can prolong performance during endurance exercise because CHO oxidation can be maintained for a longer period of time compared to placebo administration [31][32][33][34]. The understanding that carbohydrates exert an influence on performance was established many years ago. Since then, numerous studies have been conducted to find the optimal amount, type, and timing of carbohydrate intake during exercise [35]. The current recommendations suggest ingesting up to 60 g·h−1 of rapidly available carbohydrates such as glucose or glucose-fructose mixes for activities that are not longer than 2.5 h. When mixtures of glucose and fructose are ingested, this amount could be increased up to 90 g·h−1 for prolonged exercise, because of different intestinal transport pathways [1][2][36].
As shown in Figure 1, both CHO via glycolysis and fat via beta-oxidation can supply acetyl CoA for the tricarboxylic acid cycle (TCA, also known as the Krebs cycle or citric acid cycle) [37][38]. Therefore, these two mechanisms are closely related. Thus, the mnemonic that fat burns in the fire of carbohydrates was presented. On the one hand, it has been observed that the complete oxidation of fatty acids is facilitated by the simultaneous oxidation of carbohydrates. Early studies have shown that the intermediates from the TCA have an igniting effect on fat oxidation by accumulating adenosine diphosphate (ADP) and forming an acetyl-acceptor in the form of oxaloacetate [38]. On the other hand, however, research with isotopes has shown that carbohydrate metabolism can limit the oxidation of fatty acids by limiting the production of ketone bodies [39]. Early studies conducted on isolated cells have shown that acetyl CoA derived from carbohydrates is accessible for forming acylcarnitine, while acetyl CoA from beta-oxidation is more readily available for the TCA cycle. However, the rate of fatty acid oxidation is influenced by the acetyl CoA derived from the pyruvate metabolism [40]. Because of the steps in the metabolism of carbohydrates and lipids that occur before the TCA cycle, it is difficult to draw conclusions from studies with isolated cells or where only the isolated substrate was added to start the TCA cycle.
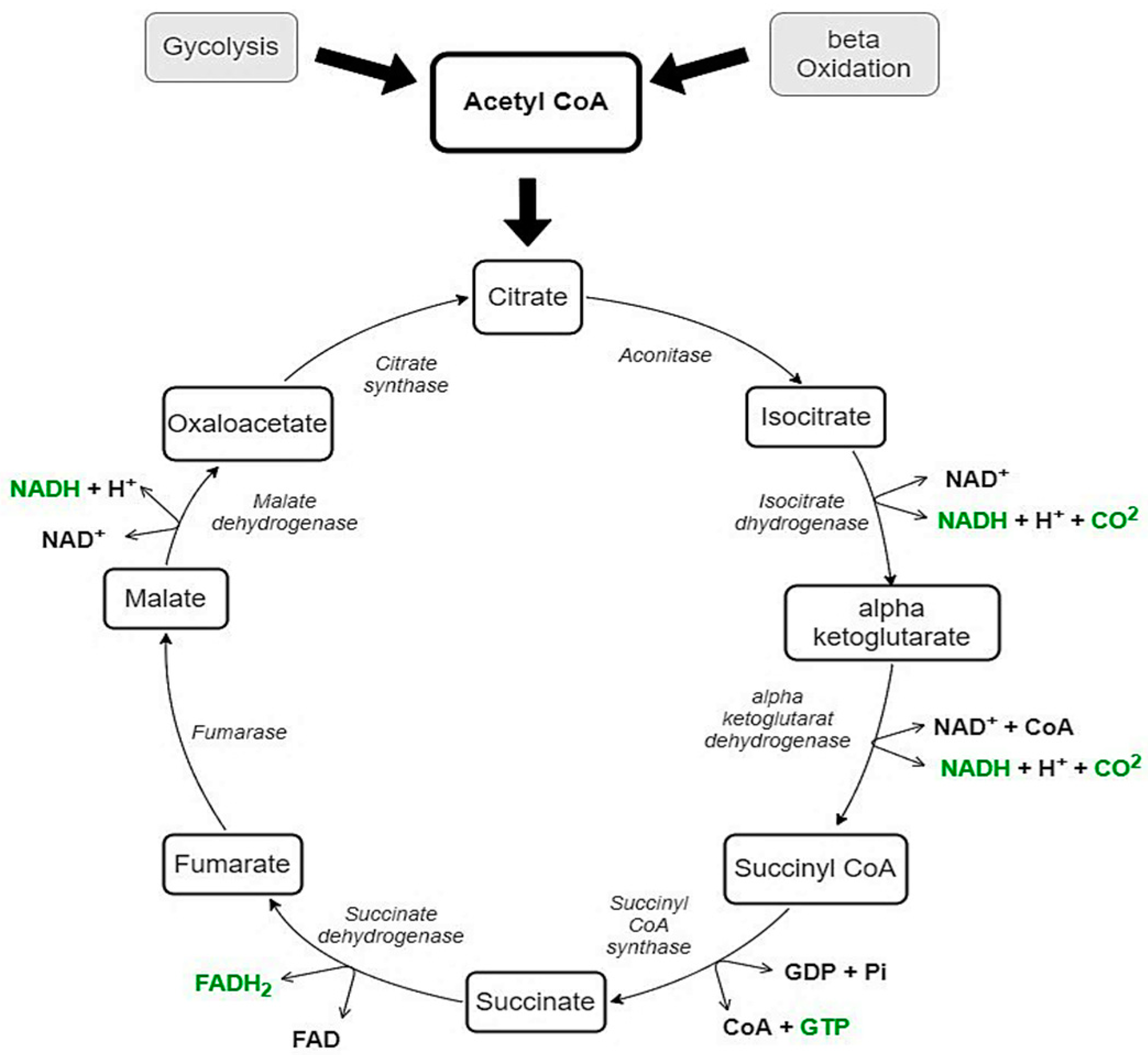
Figure 1. The TCA cycle with all intermediates (black), energy providing products (green), and enzymes (italic). The formation of acetyl CoA is carried out either by glycolysis or beta-oxidation. NADH = nicotinamide adenine dinucleotide; FADH2 = flavin adenine dinucleotide; GDP = guanosine diphosphate; GTP = guanosine triphosphate.
When both carbohydrates and fats are present for fuel, the muscle tends to prioritize the oxidation of carbohydrates. This preference for carbohydrates is due to their higher glycolytic flux and the resulting increased production of acetyl CoA. Consequently, carbohydrates hinder the oxidation of fats by inhibiting the production of acetyl CoA from fat and impeding the transport of long-chain fatty acids into the mitochondria [41]. However, the oxidation of fatty acids also regulates the rate and fate of glucose metabolism in the muscle. This reciprocal relationship between the oxidation of the two fuels is known as the glucose/fatty acid cycle [42].
Insulin counts as a key regulator in this complex interplay. It is known as an anabolic hormone, which regulates the storage of energy in the form of glycogen and fat [43]. During the process of energy production, insulin has various effects on fat oxidation. Firstly, insulin stimulates the activity of acetyl-CoA carboxylase (ACC), an enzyme responsible for the conversion of acetyl CoA into malonyl-CoA. Malonyl-CoA, in turn, hinders the function of carnitine palmitoyltransferase (CPT), which is responsible for transporting fatty acids into the mitochondria for energy generation. Monitoring the concentration of malonyl-CoA can provide insights into the availability of carbohydrates as a substrate. When carbohydrates are oxidized (CHO oxidation), there is an increase in glycolytic flow and a greater production of pyruvate. Consequently, the concentration of acetyl CoA, and hence malonyl-CoA, rises, leading to a decrease in fatty acid oxidation. This decrease occurs as CPT’s activity is inhibited, reducing the transportation of long-chain fatty acids into the mitochondria [44][45]. Secondly, insulin is a potent inhibitor of lipolysis, working by reducing non-esterified free fatty acid (NEFA) availability. During the process of fatty acid release from adipose tissue, insulin hinders the activity of hormone-sensitive lipase in adipose tissue. This inhibition leads to a decrease in the levels of non-esterified fatty acids (NEFAs) in the bloodstream. Consequently, the muscle is compelled to utilize glucose to a greater extent. When plasma insulin increases, after the administration of carbohydrates [46][47], a reduction in total fat oxidation could be observed [41][48][49][50]. During periods of low CHO availability, insulin secretion is suffocated and the organism relies on fat as a fuel [43].
Overall, insulin has two significant effects on substrate oxidation, ultimately resulting in the inhibition of fat oxidation and the promotion of glucose utilization: (1) increasing malonyl-CoA concentration through activated ACC and thereby decreasing the rate of fatty acid oxidation via inhibited CPT activity; and (2) reducing NEFA availability via the effect on adipose tissue lipolysis. In the fed state (i.e., when insulin secretion is stimulated), insulin regulates energy storage and limits fat oxidation, whilst in the fasted state, fat oxidation is promoted.
In summary, since the GI reflects the insulinemic response of a CHO, low-GI foods can attenuate the suppression of fat oxidation compared to high-GI foods. A low-GI CHO results in a lower postprandial increase in blood glucose and insulin and consequently a mitigated inhibition of fat oxidation.
Modifying substrate metabolism through dietary and exercise interventions has been a subject of interest for several years. For prolonged low-intensity endurance exercise, it is beneficial to promote high fat oxidation as it helps to reduce lactate concentrations and conserve limited carbohydrate stores. Various approaches and dietary plans, such as ketogenic or low-carbohydrate, high-fat diets (LCHF), have been suggested to alter substrate metabolism and, consequently, enhance endurance performance. During a low-carbohydrate, high-fat (LCHF) diet, the intake of carbohydrates (<20 E-% carbohydrates per day, >50 E-% fat per day) is drastically reduced. These metabolic changes facilitate an increase in the availability of free fatty acids, resulting in a reduced utilization of muscle glycogen and a reduction in CHO oxidation during physical activity [16][47][51]. After two to three weeks on a LCHF diet, the body enters a ketogenic state, which is thought to enhance performance during prolonged exercise by improving substrate utilization in favor of fat and conserving muscle glycogen [52][53]. The process of oxidizing non-esterified fatty acids in the liver produces ketone bodies (such as acetoacetate, acetone, etc.), leading to ketosis. In situations when carbohydrate availability is limited, muscles oxidize ketone bodies to generate energy [16][54]. Volek, et al. [55] demonstrated that a long-term LCHF diet can increase maximal fat oxidation to 1.5 g/min and the intensity at which it occurs to 70% VO2max. Phinney, Bistrian, Evans, Gervino and Blackburn [53] additionally found that after four months on the LCHF diet, CHO oxidation decreases and muscle glycogen is conserved. The same was observed by McSwiney, et al. [56] After following a ketogenic diet for a week, the maximum rate of fat oxidation increased by a factor of two.
So far, there are mixed results on the influence of LCHF on VO2max, a marker for endurance capacity [19]. Possible explanations for changes in VO2max include changes in oxidative metabolic processes [57][58], the production of certain metabolic by-products [16], such as tryptophan and ammonium, or reduced energy intake [52]. By promoting an elevation in intramuscular triglycerides, lipase activity, and the expression of fatty acid translocase FAT/CD36 protein and CPT [57], a low-carbohydrate, high-fat (LCHF) diet can potentially enhance performance at the aerobic threshold [59][60][61].
However, as far as competitive endurance performance is concerned, a high-fat diet could even have a negative impact on performance, particularly at higher intensities. This occurs due to the low ability of the muscles to efficiently activate glycogenolysis and pyruvate dehydrogenase due to the absence of sufficient carbohydrates during prior training or exercise sessions. [62]. An ergogenic effect of a LCHF diet could therefore not be convincingly proven, as substrate deficits are present especially in the high-intensity range and thus training optimizations are more difficult [63].
3. GI and Its Relevance in Sport Nutrition
The GI indicates the impact that a carbohydrate has on blood glucose levels. The GI can therefore be seen as an indicator of how quickly or slowly the body digests and absorbs the CHO present in the food consumed [64]. The GI is calculated by measuring the area under the curve (AUC) of blood glucose for 2 h after consumption of the test food, containing 50 g of available carbohydrates and a reference food that also contains 50 g of available carbohydrates [65]. The AUC of the test food is then compared to the reference food. The GI is expressed as a percentage of the quotient of AUC from the tested foods (Figure 2) [4]. Foods can then be classified based upon their GI. A list of GI values of common foods can be found at Atkinson, et al. [66].
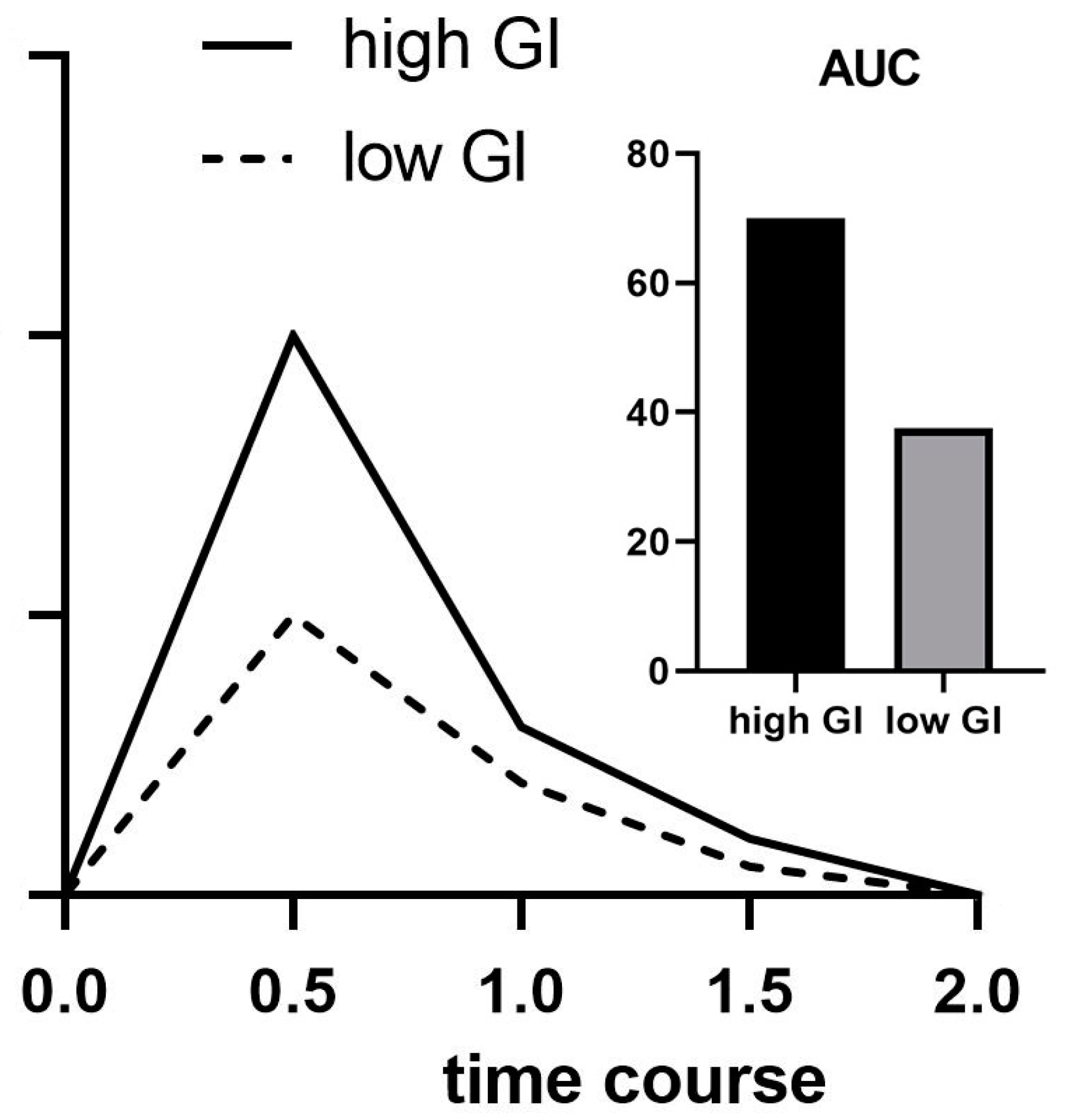
Figure 2. Schematic presentation of high- and low-GI foods and their respective area under the curve (AUC).
The importance of the GI has increased steadily in recent years. In the last 12 years, the number of scientific publications involving the GI or glycaemic load has tripled, from about 2500 to 7500 [67]. There are still concerns about the variation in published GI values for apparently identical foods. These variations derive either from methodological issues or differences in the physical and chemical characteristics of the food. This is also the reason why the GI cannot be calculated and must be measured. The exact composition of the ingredients and the processing can have an important influence on the rate of CHO digestion and hence the GI. Furthermore, different testing methods may cause variations in GI values [68]. Nevertheless, published GI values show the mean of the reported values from a number of studies conducted in different laboratories [66].
Since the GI indicates how quickly a CHO affects blood glucose, it seems reasonable that eating foods with different GI values before, during, and after exercise has an impact on athletic performance [64]. As the concept of the GI in sports is relatively new, there are still many unanswered questions regarding its role in sport nutrition [69].
In particular, the interest in low GI carbohydrates and their effects in athletic performance and substrate metabolism has increased in recent years. The slow absorption and gradual release of these carbohydrates provide a steady supply of energy during prolonged exercise and promote fat oxidation [7][8][70][71]. Consequently, because low GI carbohydrates result in a reduced blood glucose and insulin response, they help maintain normal blood sugar levels (i.e., euglycemia) and preserve muscle glycogen, which in turn affects the utilization of substrates during exercise [72]. The acute effects of low-GI carbohydrates in endurance performance have already been investigated in some studies. For example, in Achten, Jentjens, Brouns and Jeukendrup [70], supplementation with isomaltulose, a low-GI sugar, was most likely to increase fat oxidation compared to sucrose, based on measured differences in plasma insulin. The same observations were made by Oosthuyse, Carstens and Millen [8]. When isomaltulose was administered before exercise, blood glucose levels were more stable during exercise, and fat oxidation was higher [7][73]. Miyashita, et al. [74] also found that taking isomaltulose before exercise resulted in a lower postprandial glucose concentration. By affecting blood glucose and insulin, the GI shifts substrate metabolism towards higher fat oxidation [73][75][76][77][78][79][80][81], even though not all studies have demonstrated this [82][83].
Moreover, there have already been numerous studies examining the impact of consuming a low-glycemic-index (GI) meal before training on performance during the training session. Burdon, Spronk, Cheng and O’Connor [84] conducted a systematic review with meta-analysis of these studies and concluded that consuming a low-GI meal does not result in a significant improvement in endurance performance. However, they did observe a small, non-significant increase in performance when no exogenous carbohydrates were provided during exercise, which may be attributed to the maintenance of carbohydrate availability. Another meta-analysis showed a weak, but positive, effect of a low-GI meal on subsequent endurance exercise [85]. Inconsistent findings may also derive from methodological differences (e.g., meal content and GI, timing, amount of carbohydrates, exercise protocol, …) between studies. In terms of substrate metabolism, a low-GI compared to a high-GI meal before exercise leads to a reduced blood glucose and insulin response [6][79], which could help increase fat oxidation and maintain euglycemia during exercise [86].
References
- Thomas, D.; Burke, L.; Erdman, K. Nutrition and Athletic Performance. Med. Sci. 2016, 48, 543–568.
- Podlogar, T.; Wallis, G.A. New Horizons in Carbohydrate Research and Application for Endurance Athletes. Sport. Med. 2022, 52, 5–23.
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366.
- Wolever, T.M.; Jenkins, D.J.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854.
- Carneiro, L.; Leloup, C. Mens sana in corpore sano: Does the Glycemic Index Have a Role to Play? Nutrients 2020, 12, 2989.
- Cocate, P.G.; Pereira, L.G.; Marins, J.C.; Cecon, P.R.; Bressan, J.; Alfenas, R.C. Metabolic responses to high glycemic index and low glycemic index meals: A controlled crossover clinical trial. Nutr. J. 2011, 10, 1.
- König, D.; Zdzieblik, D.; Holz, A.; Theis, S.; Gollhofer, A. Substrate Utilization and Cycling Performance Following Palatinose™ Ingestion: A Randomized, Double-Blind, Controlled Trial. Nutrients 2016, 8, 390.
- Oosthuyse, T.; Carstens, M.; Millen, A.M. Ingesting Isomaltulose Versus Fructose-Maltodextrin During Prolonged Moderate-Heavy Exercise Increases Fat Oxidation but Impairs Gastrointestinal Comfort and Cycling Performance. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 427–438.
- Burke, L.M. Ketogenic low-CHO, high-fat diet: The future of elite endurance sport? J. Physiol. 2021, 599, 819–843.
- Burke, L.M. Re-Examining High-Fat Diets for Sports Performance: Did We Call the ‘Nail in the Coffin’ Too Soon? Sport. Med. 2015, 45, 33–49.
- Howard, E.E.; Margolis, L.M. Intramuscular Mechanisms Mediating Adaptation to Low-Carbohydrate, High-Fat Diets during Exercise Training. Nutrients 2020, 12, 2496.
- Alghannam, A.F.; Ghaith, M.M.; Alhussain, M.H. Regulation of Energy Substrate Metabolism in Endurance Exercise. Int. J. Environ. Res. Public Health 2021, 18, 4963.
- Spriet, L.L. New Insights into the Interaction of Carbohydrate and Fat Metabolism During Exercise. Sport. Med. 2014, 44, 87–96.
- Hargreaves, M.; Spriet, L.L. Exercise Metabolism: Fuels for the Fire. Cold Spring Harb. Perspect. Med. 2018, 8, a029744.
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391.
- Chang, C.-K.; Borer, K.; Lin, P.-J. Low-Carbohydrate-High-Fat Diet: Can it Help Exercise Performance? J. Hum. Kinet. 2017, 56, 81–92.
- Venables, M.C.; Achten, J.; Jeukendrup, A.E. Determinants of fat oxidation during exercise in healthy men and women: A cross-sectional study. J. Appl. Physiol. 2005, 98, 160–167.
- Ormsbee, M.; Bach, C.; Baur, D. Pre-Exercise Nutrition: The Role of Macronutrients, Modified Starches and Supplements on Metabolism and Endurance Performance. Nutrients 2014, 6, 1782–1808.
- Bailey, C.P.; Hennessy, E. A review of the ketogenic diet for endurance athletes: Performance enhancer or placebo effect? J. Int. Soc. Sport. Nutr. 2020, 17, 1–11.
- Jeukendrup, A.; Saris, W.; Wagenmakers, A. Fat Metabolism During Exercise: A Review—Part II: Regulation of Metabolism and the Effects of Training. Int. J. Sport. Med. 1998, 19, 293–302.
- Jeukendrup, A.; Saris, W.; Wagenmakers, A. Fat Metabolism During Exercise: A Review. Part I: Fatty Acid Mobilization and Muscle Metabolism. Int. J. Sport. Med. 1998, 19, 231–244.
- Bonen, A.; Dohm, G.L.; van Loon, L.J. Lipid metabolism, exercise and insulin action. Essays Biochem. 2006, 42, 47–59.
- Ni, C.; Jia, Q.; Ding, G.; Wu, X.; Yang, M. Low-Glycemic Index Diets as an Intervention in Metabolic Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 307.
- Christensen, E.H.; Hansen, O. II. Hypoglykämie, Arbeitsfähigkeit und Ermüdung. Skand. Arch. Für Physiol. 1939, 81, 172–179.
- Christensen, E.H.; Hansen, O. III. Arbeitsfähigkeit und Ernährung. Skand. Arch. Für Physiol. 1939, 81, 160–171.
- Krogh, A.; Lindhard, J. The Relative Value of Fat and Carbohydrate as Sources of Muscular Energy: With Appendices on the Correlation between Standard Metabolism and the Respiratory Quotient during Rest and Work. Biochem. J. 1920, 14, 290–363.
- Dill, D.B.; Edwards, H.T.; Talbott, J.H. Studies in muscular activity: VII. Factors limiting the capacity for work. J. Physiol. 1932, 77, 49–62.
- Gordon, B.; Kohn, L.A.; Levine, S.A.; Matton, M.; Scriver, W.M.; Whiting, W.B. Sugar content of the blood in runners following a marathon race: With special reference to the prevention of hypoglycemia: Further observations. J. Am. Med. Assoc. 1925, 85, 508–509.
- Levine, S.A.; Grodon, B.; Derick, C.L. Some changes in the chemical constituents of the blood following a marathon race: With special reference to the development of hypoglycemia. J. Am. Med. Assoc. 1924, 82, 1778–1779.
- McConell, G.; Snow, R.J.; Proietto, J.; Hargreaves, M. Muscle metabolism during prolonged exercise in humans: Influence of carbohydrate availability. J. Appl. Physiol. 1999, 87, 1083–1086.
- Febbraio, M.A.; Chiu, A.; Angus, D.J.; Arkinstall, M.J.; Hawley, J.A. Effects of carbohydrate ingestion before and during exercise on glucose kinetics and performance. J. Appl. Physiol. 2000, 89, 2220–2226.
- Wallis, G.A.; Yeo, S.E.; Blannin, A.K.; Jeukendrup, A.E. Dose-response effects of ingested carbohydrate on exercise metabolism in women. Med. Sci. Sport. Exerc. 2007, 39, 131–138.
- Carter, J.M.; Jeukendrup, A.E.; Mann, C.H.; Jones, D.A. The Effect of Glucose Infusion on Glucose Kinetics during a 1-h Time Trial. Med. Sci. Sport. Exerc. 2004, 36, 1543–1550.
- Wilber, R.L.; Moffatt, R.J. Influence of carbohydrate ingestion on blood glucose and performance in runners. Int. J. Sport Nutr. 1992, 2, 317–327.
- Jeukendrup, A.E. Carbohydrate and exercise performance: The role of multiple transportable carbohydrates. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 452–457.
- Vitale, K.; Getzin, A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients 2019, 11, 1289.
- Akram, M. Citric Acid Cycle and Role of its Intermediates in Metabolism. Cell Biochem. Biophys. 2014, 68, 475–478.
- Bowtell, J.L.; Marwood, S.; Bruce, M.; Constantin-Teodosiu, D.; Greenhaff, P.L. Tricarboxylic Acid Cycle Intermediate Pool Size. Sport. Med. 2007, 37, 1071–1088.
- Masoro, E.J.; Felts, J.M. Role of carbohydrate metabolism in promoting fatty acid oxidation. J. Biol. Chem. 1958, 231, 347–356.
- Abdel-aleem, S.; Nada, M.A.; Sayed-Ahmed, M.; Hendrickson, S.C.; St Louis, J.; Walthall, H.P.; Lowe, J.E. Regulation of fatty acid oxidation by acetyl-CoA generated from glucose utilization in isolated myocytes. J. Mol. Cell. Cardiol. 1996, 28, 825–833.
- Coyle, E.F.; Jeukendrup, A.E.; Wagenmakers, A.J.; Saris, W.H. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am. J. Physiol. 1997, 273, E268–E275.
- Randle, P.J. Regulatory interactions between lipids and carbohydrates: The glucose fatty acid cycle after 35 years. Diabetes Metab Rev. 1998, 14, 263–283.
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pr. 2011, 93 (Suppl. S1), S52–S59.
- Odland, L.M.; Heigenhauser, G.J.; Lopaschuk, G.D.; Spriet, L.L. Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am. J. Physiol. 1996, 270, E541–E544.
- McGarry, J.D.; Mannaerts, G.P.; Foster, D.W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J. Clin. Investig. 1977, 60, 265–270.
- DeFronzo, R.A.; Ferrannini, E.; Hendler, R.; Felig, P.; Wahren, J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes 1983, 32, 35–45.
- Costill, D.L.; Coyle, E.; Dalsky, G.; Evans, W.; Fink, W.; Hoopes, D. Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 43, 695–699.
- Campbell, P.J.; Carlson, M.G.; Hill, J.O.; Nurjhan, N. Regulation of free fatty acid metabolism by insulin in humans: Role of lipolysis and reesterification. Am. J. Physiol. 1992, 263, E1063–E1069.
- Ahlborg, G.; Felig, P. Influence of glucose ingestion on fuel-hormone response during prolonged exercise. J. Appl. Physiol. 1976, 41, 683–688.
- Horowitz, J.F.; Mora-Rodriguez, R.; Byerley, L.O.; Coyle, E.F. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am. J. Physiol. 1997, 273, E768–E775.
- Vukovich, M.D.; Costill, D.L.; Hickey, M.S.; Trappe, S.W.; Cole, K.J.; Fink, W.J. Effect of fat emulsion infusion and fat feeding on muscle glycogen utilization during cycle exercise. J. Appl. Physiol. (1985) 1993, 75, 1513–1518.
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015, 6, 27.
- Phinney, S.D.; Bistrian, B.R.; Evans, W.J.; Gervino, E.; Blackburn, G.L. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 1983, 32, 769–776.
- Devrim-Lanpir, A.; Hill, L.; Knechtle, B. Efficacy of Popular Diets Applied by Endurance Athletes on Sports Performance: Beneficial or Detrimental? A Narrative Review. Nutrients 2021, 13, 491.
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M.; et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism 2016, 65, 100–110.
- McSwiney, F.T.; Fusco, B.; McCabe, L.; Lombard, A.; Crowley, P.; Walsh, J.; Hone, M.; Egan, B. Changes in body composition and substrate utilization after a short-term ketogenic diet in endurance-trained males. Biol. Sport 2021, 38, 145–152.
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J. Physiol. 2017, 595, 2785–2807.
- McSwiney, F.T.; Wardrop, B.; Hyde, P.N.; Lafountain, R.A.; Volek, J.S.; Doyle, L. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism 2018, 81, 25–34.
- Helge, J.W. Long-term fat diet adaptation effects on performance, training capacity, and fat utilization. Med. Sci. Sport. Exerc. 2002, 34, 1499–1504.
- Langfort, J.; Pilis, W.; Zarzeczny, R.; Nazar, K.; Kaciuba-Uściłko, H. Effect of low-carbohydrate-ketogenic diet on metabolic and hormonal responses to graded exercise in men. J. Physiol. Pharmacol. 1996, 47, 361–371.
- Zajac, A.; Poprzecki, S.; Maszczyk, A.; Czuba, M.; Michalczyk, M.; Zydek, G. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Nutrients 2014, 6, 2493–2508.
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828.
- Murphy, N.E.; Carrigan, C.T.; Margolis, L.M. High-Fat Ketogenic Diets and Physical Performance: A Systematic Review. Adv. Nutr. 2021, 12, 223–233.
- Donaldson, C.M.; Perry, T.L.; Rose, M.C. Glycemic Index and Endurance Performance. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 154–165.
- Jenkins, D.J.A.; Wolever, T.M.S.; Jenkins, A.L.; Thorne, M.J.; Lee, R.; Kalmusky, J.; Reichert, R.; Wong, G.S. The glycaemic index of foods tested in diabetic patients: A new basis for carbohydrate exchange favouring the use of legumes. Diabetologia 1983, 24, 257–264.
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283.
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632.
- Flavel, M.; Jois, M.; Kitchen, B. Potential contributions of the methodology to the variability of glycaemic index of foods. World J. Diabetes 2021, 12, 108–123.
- Thomas, D.E.; Brotherhood, J.R.; Brand, J.C. Carbohydrate feeding before exercise: Effect of glycemic index. Int. J. Sport. Med. 1991, 12, 180–186.
- Achten, J.; Jentjens, R.L.; Brouns, F.; Jeukendrup, A.E. Exogenous oxidation of isomaltulose is lower than that of sucrose during exercise in men. J. Nutr. 2007, 137, 1143–1148.
- König, D.; Theis, S.; Kozianowski, G.; Berg, A. Postprandial substrate use in overweight subjects with the metabolic syndrome after isomaltulose (Palatinose™) ingestion. Nutrition 2012, 28, 651–656.
- Maresch, C.C.; Petry, S.F.; Theis, S.; Bosy-Westphal, A.; Linn, T. Low Glycemic Index Prototype Isomaltulose-Update of Clinical Trials. Nutrients 2017, 9, 381.
- Wu, C.L.; Williams, C. A low glycemic index meal before exercise improves endurance running capacity in men. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 510–527.
- Miyashita, M.; Hamada, Y.; Fujihira, K.; Namura, S.; Sakazaki, M.; Miyasaka, K.; Nagai, Y. The effects of isomaltulose ingestion on gastric parameters and cycling performance in young men. J. Exerc. Sci. Fit. 2019, 17, 101–107.
- Sun, F.-H.; O’Reilly, J.; Li, L.; Wong, S.H.-S. Effect of the glycemic index of pre-exercise snack bars on substrate utilization during subsequent exercise. Int. J. Food Sci. Nutr. 2013, 64, 1001–1006.
- Kirwan, J.P.; Cyr-Campbell, D.; Campbell, W.W.; Scheiber, J.; Evans, W.J. Effects of moderate and high glycemic index meals on metabolism and exercise performance. Metabolism 2001, 50, 849–855.
- Sparks, M.J.; Selig, S.S.; Febbraio, M.A. Pre-exercise carbohydrate ingestion: Effect of the glycemic index on endurance exercise performance. Med. Sci. Sport. Exerc. 1998, 30, 844–849.
- Wee, S.L.; Williams, C.; Gray, S.; Horabin, J. Influence of high and low glycemic index meals on endurance running capacity. Med. Sci. Sport. Exerc. 1999, 31, 393–399.
- Stevenson, E.; Williams, C.; Nute, M. The influence of the glycaemic index of breakfast and lunch on substrate utilisation during the postprandial periods and subsequent exercise. Br. J. Nutr. 2005, 93, 885–893.
- Moore, L.J.; Midgley, A.W.; Thurlow, S.; Thomas, G.; Mc Naughton, L.R. Effect of the glycaemic index of a pre-exercise meal on metabolism and cycling time trial performance. J. Sci. Med. Sport 2010, 13, 182–188.
- Stevenson, E.J.; Williams, C.; Mash, L.E.; Phillips, B.; Nute, M.L. Influence of high-carbohydrate mixed meals with different glycemic indexes on substrate utilization during subsequent exercise in women. Am. J. Clin. Nutr. 2006, 84, 354–360.
- Backhouse, S.H.; Williams, C.; Stevenson, E.; Nute, M. Effects of the glycemic index of breakfast on metabolic responses to brisk walking in females. Eur. J. Clin. Nutr. 2007, 61, 590–596.
- Bennard, P.; Doucet, É. Acute effects of exercise timing and breakfast meal glycemic index on exercise-induced fat oxidation. Appl. Physiol. Nutr. Metab. 2006, 31, 502–511.
- Burdon, C.A.; Spronk, I.; Cheng, H.L.; O’Connor, H.T. Effect of Glycemic Index of a Pre-exercise Meal on Endurance Exercise Performance: A Systematic Review and Meta-analysis. Sport. Med. 2017, 47, 1087–1101.
- Heung-Sang Wong, S.; Sun, F.H.; Chen, Y.J.; Li, C.; Zhang, Y.J.; Ya-Jun Huang, W. Effect of pre-exercise carbohydrate diets with high vs low glycemic index on exercise performance: A meta-analysis. Nutr. Rev. 2017, 75, 327–338.
- Stannard, S.R.; Constantini, N.W.; Miller, J.C. The effect of glycemic index on plasma glucose and lactate levels during incremental exercise. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 51–61.
More
Information
Subjects:
Sport Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
753
Revisions:
2 times
(View History)
Update Date:
18 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




