Food samples are complex heterogenous matrices, where all analytes are distributed in a random manner. Food analysis involves sampling, homogenization, and sample preparation that increase the analytical accuracy and precision. Focusing on sample preparation it usually involves storage, particle size reduction, homogenization, weighting, dilution, filtration, extraction, clean-up, and derivatization. Proper sample preparation protocols result in matrix interference elimination and analyte preconcentration, thus affecting the selectivity, sensitivity, detection capability, and the overall performance of an analytical technique. The most time-consuming step in analytical method development is the optimization of the sample preparation protocol that includes analyte extraction and clean-up. Some of the most common sample preparation techniques used in food analysis are liquid-liquid extraction (LLE), solid-liquid extraction (SLE), solid-phase extraction (SPE), solid-phase microextraction (SPME), stir bar sorptive extraction (SBSE), supercritical fluid extraction (SFE), accelerated solvent extraction (ASE), ultrasonic, and Soxhlet extraction.
1. Liquid-Liquid Extraction (LLE)
LLE is one of the most used sample preparation techniques, along with SPE, and probably the oldest. In LLE the analytes are extracted from an aqueous sample into a water immiscible solvent, according to relative solubility. Despite the wide use of LLE, there are many disadvantages, such as increased time and solvent requirements, analyte lose, sample contamination, and low sensitivity, that reduce LLE applications in modern analytical chemistry. Liquid-phase microextraction (LPME) is an LLE-based microextraction technique that has been developed in order to overcome LLE disadvantages. Extraction takes place between the aqueous sample phase (donor phase) and a water immiscible solvent extraction phase (acceptor phase). LPME can be divided into single-drop microextraction (SMDE), hollow fiber liquid-phase microextraction (HF-LPME), and dispersive liquid-liquid microextraction (DLLME). DLLME is a ternary extraction system that involves the aqueous sample, an extraction solvent, and a disperser solvent. Microliters of the organic extraction solvent and the dispersive solvent are injected into the sample solution and extraction solvent droplets are formed with the help of the dispersive solvent, resulting in a cloudy solution. After the extraction equilibrium between the sample and the extraction solvent is achieved, the cloudy solution is centrifuged and the lower organic phase is collected for analysis. The extraction solvent should be water immiscible, such as chloroform, carbon tetrachloride, and dichloromethane, while the dispersive solvent should be miscible in both aqueous solution and organic solvent, such as ethanol, methanol, acetonitrile, and acetone. Following the principles of green analytical chemistry, conventional extraction solvent can be replaced by ionic liquids that are liquid organic salts, combinations of organic cations with inorganic anions with unique physicochemical properties. Ionic liquids are characterized by low volatility and high density, thus forming more stable droplets and better phase separation than the conventional organic solvents
[1].
2. Solid-Liquid Extraction (SLE)
The principals of SLE are similar to LLE, except that the analytes are extracted from solid samples. The solid sample is mixed with extraction solvent, the two phases interact, and the soluble sample components diffuse into the extraction phase. Various organic solvents can be used in SLE, however, SLE is usually laborious and has analogous disadvantages as LLE, such as increased solvent requirements, partial extraction, solvent impurities, and emulsion formation. In order to increase the extraction efficiency of the organic solvent, heating and pressure or ultrasounds can be applied during the extraction. In pressurized liquid extraction (PLE), also known as ASE, the solid sample and the extraction solvent are transferred into an extraction cell and the extraction takes place under high temperature (40–200 °C) and pressure (500–3000 psi) for 5–15 min. After the extraction is complete, the sample extract is collected and purged. The increased temperature and pressure applied in PLE result in reduced extraction time, enhanced analyte’s solubility and mass transfer, better solvent penetration, and overall improved extraction yields. However, PLE requires expensive equipment and high temperature solvent reduces the extraction selectivity and applicability of PLE for the extraction of less thermal stable compounds. Ultrasound-assisted extraction (UAE) is a less extreme extraction approach in which extraction is assisted by the application of ultrasounds. Compared with other sample preparation techniques, UAE is relatively faster with reduced solvent requirements, and at the same time enables the extraction of analytes in room temperature. However, this technique lacks selectivity and enrichment capability when extraction of trace amounts is required and is usually combined with other clean-up techniques for improved extraction efficiency
[2].
3. Salting-Out Extraction
The salting-out effect has been exploited in sample preparation techniques, such as Quick Easy Cheap Effective Rugged Safe (QuEChERS) and salting-out liquid-liquid extraction (SALLE), in order to improve analyte’s extraction from aqueous samples. The salting-out effect is based on the decrease of solubility of water soluble organic analytes when salt concentration in the aqueous sample solution is increased. This effect favors the partition of the analytes into a water-miscible organic solvent and separation between the two phases. Various water-miscible solvents can be used, but acetonitrile is the most convenient water-miscible organic solvent for the application of the salting-out effect due to being chemically inert with organic analytes and the most common mobile phase component in liquid chromatography, and at the same time, acetonitrile has the ability to precipitate matrix proteins. Salting-out agents are usually inorganic and organic salts that provide cations (Mg
2+, Sr
2+, Ca
2+, Ba
2+, K
+, Na
+, NH
4+, Li
+) and anions (SO
42−, CH
3COO
−, Cl
−, NO
3−, Br
−, I
−, CNS
−), such as MgSO
4, NaCl, CH
3COONH
4, CH
3COONa, and (NH
4)
2SO
4, which promote the transfer of the hydrophilic compounds to the organic phase. These salts should be soluble in the aqueous sample but have negligible solubility in the organic phase
[3]. In either QuEChERS and SALLE procedures, a water-miscible solvent and a salting-out agent are sequentially added to the sample and the mixture is shaken and centrifuged. The formed organic phase is then collected and can be either injected directly for analysis or further treated for water removal and analyte isolation
[2]. Aqueous two-phase system (ATPS) extraction is another alternative based on the partitioning of the analytes between two phases and is used for the extraction of analytes from aqueous samples. The most common biphasic system in food analysis is polymer/salt, while ionic liquid/salt systems were also reported. Polyethylene glycol is usually the polymer of choice combined with phosphate, sulfate, or citrate salts. Parameters that affect the extraction include the molecular weight and concentration of the polymeric phase, the solution pH, and temperature. ATPS does not require the use of organic solvents in contrast with other sample preparation techniques, while sample deproteinization or defatting is not always required
[4].
4. Solid-Phase Extraction (SPE)
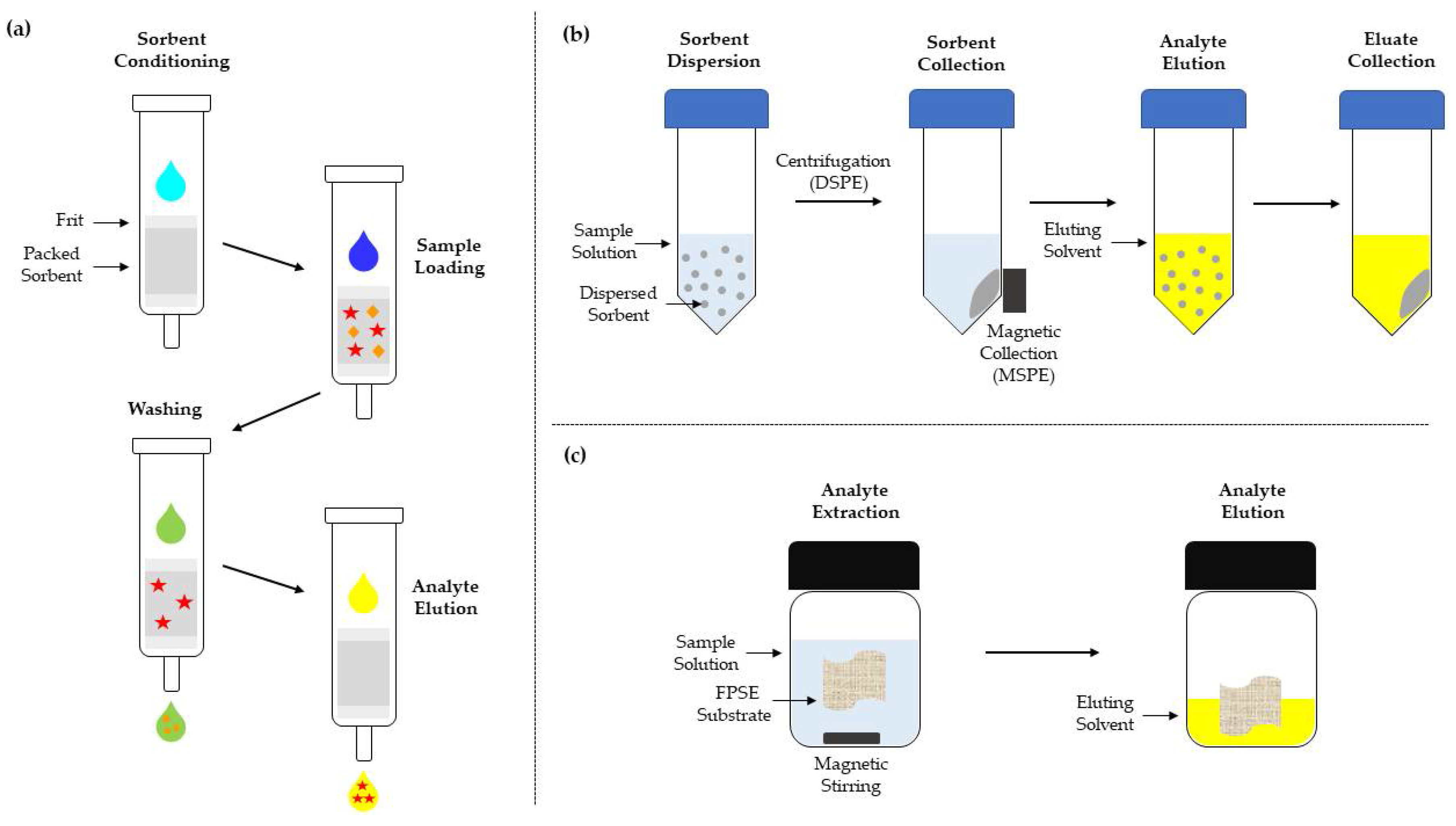
SPE is a widely used sample preparation technique that provides high enrichment factors and recoveries with reduced sample volume requirements and automation capability. SPE can be used for the daily laboratory routine and in many cases SPE has replaced LLE. In SPE the components of the sample solution interfere with a solid phase (sorbent material) and separation between the target analytes and the matrix interferences can be achieved. The sorbent is usually packed into SPE cartridges between two frits. SPE cartridges are commercially available for specific applications or can be manually prepared with the selected sorbents. In a typical SPE procedure, the SPE cartridges are conditioned/activated with a solvent or a solvent mixture, the sample solution is loaded into the cartridge, the loaded cartridge is washed to remove the retained interreferences, and the retained analytes are eluted with an appropriate solvent (
Figure 1a). However, SPE applications are restricted by the type of the sorbent and the characteristics of the sample components, while the tightly packed SPE cartridges increase the extraction time and cause backpressure
[1]. These problems can be eliminated with dispersive solid-phase extraction (DSPE) where the sorbent is dispersed in the sample solution and not packed into a cartridge. After the dispersion, the solution is shaken, and when the extraction is complete, the sorbent is collected by centrifugation or filtration and the analytes are eluted by repeating the previous step with the use of an appropriate solvent (
Figure 1b)
[5]. C18 and OASIS
® HLB are two of the most widely applied commercially available materials for SPE. C18 is a nonpolar sorbent that consists of octadecylsilane bonded to silica particles and is suitable for the reversed-phase binding of hydrophobic analytes. OASIS
® HLB is a water-wettable hydrophilic-lipophilic-balanced polymeric reversed-phase sorbent that consists of N-vinylpyrrolidone and divinylbenzene and can be used for the binding of acidic, basic, or neutral analytes
[2]. Novel SPE sorbents include carbon nanotubes (CNTs)
[1], molecularly imprinted polymers (MIPs)
[6], and magnetic materials. Magnetic solid-phase extraction (MSPE) is a SPE-based technique that employs magnetic sorbent materials. The magnetic materials comprise of a magnetic metal oxide nanoparticle core, usually Fe
3O
4, coated with inorganic or organic materials, such as silica, alumina, chitosan, or polypyrrole, while the coating can be modified with functional groups for improved sorption capability. A MSPE application is similar to DSPE, with the difference that the sorbent can be collected by means of a magnet (
Figure 1b)
[7]. While solid-phase techniques can be used for aqueous samples or sample solutions, matrix solid-phase dispersion (MSPD) can be applied directly for the extraction of analytes from solid, semi-solid, and viscous samples. Typically, the sample is mechanically blended with solid support in order to achieve matrix disruption and the development of interactions between the analytes and the sorbent material. The mixture is then transferred and packed into a SPE cartridge and the analytes are eluted with an appropriate sorbent. Apart from the applicability on solid samples, MSDP provides a simple and selective approach for sample extraction and clean-up in a single step
[2].
Figure 1. Schematic representation of the basic steps in (a) SPE, (b) DSPE and MSPE, and (c) FPSE.
Other solid-phase extraction variations include SPME and SBSE. In both techniques, the sorbent material is coated on a substrate, and extraction/analyte desorption follow the same principles. SPME employs silica or stainless-steel fibers coated with the sorbent material that can be used for the extraction of analytes from gaseous, liquid, and solid samples. A typical SPME procedure involves the partitioning of the analytes between the sorbent and the sample matrix and analyte desorption directly into the analytical instrument. SPME can be coupled with HPLC by means of a six-port injector combined with a desorption chamber, and desorption can be performed with an organic solvent or the mobile phase in static or dynamic mode. SMPE fibers can be coated with various sorbent materials, thus SMPE applicability can be expanded for the extraction of a wide range of analytes and sample matrices
[2]. SBSE employs magnetic stir bars coated with polydimethylsiloxane that is a polar polymeric material and develops hydrophilic interactions with the analytes, such as hydrogen bonds and van der Waals forces. In a typical SBSE procedure, a coated stir bar is introduced into the sample solution and the analytes are absorbed onto the coating by continuous stirring. After the extraction, the bar is collected, washed with deionized water, and dried, while analytes can be desorpted, either thermally by thermal or liquid desorption
[1]. Both SPME and SBSE are less time-consuming and have reduced sample and solvent requirements in comparison with SPE.
5. Fabric Phase Sorptive Extraction (FPSE)
Fabric phase sorptive extraction (FPSE) is a recently introduced novel microextraction technique that employs reusable cellulose or polyester fabric substrates homogenously coated with sol-gel hybrid sorbents. In a typical FPSE procedure, the coated fabric, along with a magnetic stir bar, are introduced into a vial that contains the sample or sample solution, and analyte extraction is conducted under stirring. The coated fabric is collected and placed for 4–10 min into a second vial that contains the eluting solvent (
Figure 1c). The eluate can be centrifuged prior to analysis. The coated fabric is washed with an appropriate organic solvent and rinsed with deionized water between extractions. Reported FPSE coatings include polydimethylsiloxane, poly(ethyleneglycol), C18, and graphene. The FPSE protocol is simple and fast, with reduced solvent requirements, while the coated fabric can be introduced directly to the liquid samples and is compatible with a wide range of organic solvents. FPSE substrates are characterized by increased sorbent loading and improved adsorption capacity in comparison with SPME, providing high analyte preconcentration
[8].