You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dongmei Ren | -- | 1165 | 2023-07-16 14:14:38 | | | |
| 2 | Conner Chen | Meta information modification | 1165 | 2023-07-18 05:49:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, S.; Li, L.; Ren, D. Regulation of EMT Process in Cancer Progression. Encyclopedia. Available online: https://encyclopedia.pub/entry/46848 (accessed on 27 December 2025).
Liu S, Li L, Ren D. Regulation of EMT Process in Cancer Progression. Encyclopedia. Available at: https://encyclopedia.pub/entry/46848. Accessed December 27, 2025.
Liu, Shuangyu, Lingyu Li, Dongmei Ren. "Regulation of EMT Process in Cancer Progression" Encyclopedia, https://encyclopedia.pub/entry/46848 (accessed December 27, 2025).
Liu, S., Li, L., & Ren, D. (2023, July 16). Regulation of EMT Process in Cancer Progression. In Encyclopedia. https://encyclopedia.pub/entry/46848
Liu, Shuangyu, et al. "Regulation of EMT Process in Cancer Progression." Encyclopedia. Web. 16 July, 2023.
Copy Citation
A biological process called epithelial-mesenchymal transition (EMT) allows epithelial cells to change into mesenchymal cells and acquire some cancer stem cell properties. EMT contributes significantly to the metastasis, invasion, and development of treatment resistance in cancer cells. Current research has demonstrated that phytochemicals are emerging as a potential source of safe and efficient anti-cancer medications. Phytochemicals could disrupt signaling pathways related to malignant cell metastasis and drug resistance by suppressing or reversing the EMT process.
epithelial-mesenchymal transition
phytochemicals
cancer therapy
1. Introduction
Cancer has become a significant global public health problem with the continuous aging of the population. Although advancements in the treatment of cancer have been achieved due to the discovery of new targets and technologies, tumor metastasis remains the primary cause of mortality for cancer patients, and more than 90% of cancer-related deaths occur as a result of cancer metastasis [1]. Even if early detection and intervention can greatly increase the survival rate of patients with metastatic cancers, the development of treatments targeting metastatic cancers is still urgent [2].
Epithelial-mesenchymal transition (EMT) is a well-defined, reversible process in which epithelial cells lose their epithelial phenotype and acquire mesenchymal-like features [3]. The origin of EMT is associated with the loss of apical-basal polarity in epithelial cells. During the EMT program, epithelial cells gradually lose their cell-cell contacts due to the disassembly and deconstruction of cell junctions, and then they gain certain characteristics of mesenchymal cells [4]. These mesenchymal cells display enhanced migratory ability and resistance to cell death signals. EMT is a necessary physiological process in embryonic development, tissue repair, and cellular stemness maintenance. However, in a pathological process, improperly regulated EMT is hijacked by cancer cells and plays an important role in carcinogenesis and fibrosis [5][6]. Cancer cells undergoing EMT change in both morphology and motion, accompanied by increased invasion and metastasis potential as well as therapy resistance [7]. Therefore, EMT has emerged as a prospective target for the treatment of cancer in recent years. Preventing the EMT process of cancer cells has become an attractive strategy in cancer therapy.
Some phytochemicals, especially those that originate from plants, have been demonstrated as EMT modulators targeting multiple stages of the process. As a consequence, these phytochemicals provide advantages for controlling cancer cells spreading throughout the body and overcoming treatment resistance. This makes phytochemicals valuable candidates for novel anti-cancer drugs.
2. The Regulation of the EMT Process and Its Roles in Cancer Progression
2.1. The Regulation of the EMT Process
EMT is a dynamic process in which epithelial cells go through multiple biochemical changes leading to their conversion into a mesenchymal phenotype [8]. During this process, cells undergo morphological changes associated with the repression of epithelial markers and the acquirement of mesenchymal marker proteins. The most notable marker of epithelial cells is E-cadherin, while N-cadherin is the most representative marker of mesenchymal-type cells [9]. The switch from E-cadherin to N-cadherin is often used for the identification of EMT processes. Downregulation of E-cadherin destabilizes adherens junctions, resulting in a loss of affinity for epithelial cells; upregulation of N-cadherin mediates a greater affinity for mesenchymal cells. Other epithelial markers, including claudins and occludins, and mesenchymal markers, including vimentin and fibronectin, participate in altering cell-cell affinity together [10]. These markers can also be used for the identification of EMT processes.
The exchange of gene expression from epithelial to mesenchymal phenotypes is initiated by at least four layers of regulation: transcriptional control, small non-coding RNAs, differential splicing, translational control, and post-translational control [11]. Undoubtedly, transcriptional control is the most extensively studied network. EMT-inducing transcription factors (EMT-TFs) are the core of the transcriptional control network of EMT, and multiple signaling pathways lead to the regulation of EMT-TFs.
EMT-TFs directly or indirectly contribute to the regulatory network of the EMT process. Some TFs, including SNAIL1, SNAIL2, ZEB1, ZEB2, E47 (also known as transcription factor-3, TCF-3), kruppel-like factor 8 (KLF8), and Brachyury, repress the expression of E-cadherin through direct binding to the CDH1 promoter, which encodes E-cadherin. Simultaneously, these TFs also repress other junctional proteins, such as claudins. Some other TFs, including TWIST1, hepatocyte nuclear factor 3 (FOXC2), gooseciod, E2-2, SIX1, and paired mesoderm homeobox protein 1 (PRRX1), regulate the EMT process without direct binding to the CDH1 promoter. Among these TFs, some are very common in most studies about EMT, particularly the nuclear factors of the SNAIL, ZEB, and TWIST families. This collection of TFs seems to be enough for the regulation of EMT [12]. Most of the other TFs, which have only been mentioned in a few studies, may just have an assisting role [13].
A variety of signaling pathways collaboratively regulate EMT progression, mainly including the transforming growth factor β (TGF-β), wingless-type MMTV integration site family (Wnt), Hedgehog, and epidermal growth factor receptor (EGFR) pathways. Some other signaling pathways, such as Notch, Hippo, and nuclear factor kappa-B (NF-κB), also regulate EMT in certain kinds of cancer. All of these pathways eventually converge at the level of transcription factors such as ZEB, SNAIL, and TWIST to regulate the EMT process [8].
TGF-β, a multifunctional cytokine, is the best-known EMT inducer. When TGF-β ligands bind to TGF-β receptors, signals are transmitted into cells through its intracellular transducers, Smads, or factors other than Smads, such as phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinases (MAPKs), and c-Jun N-terminal kinase (JNK). Both the Smads-dependent and Smads-independent pathways are involved in the regulation of EMT [14][15]. The Wnt signaling pathway is another multifunctional pathway to induce EMT. β-Catenin is the downstream factor of the Wnt receptor. The Wnt signaling pathway induces EMT through the interaction of β-catenin with some transcription factors [16]. The hedgehog signaling pathway exerts its role by terminating at a transcription factor, glioma-associated oncogenes (GLI), and aberrant activation of Hedgehog/GLI induces EMT [17][18]. EGF is also a well-known EMT inducer. Through binding to its receptor EGFR, EGF-induced signals are transmitted by PI3K, focal adhesion kinase (FAK), or rat sarcoma (RAS) pathways [19][20] (Figure 1).
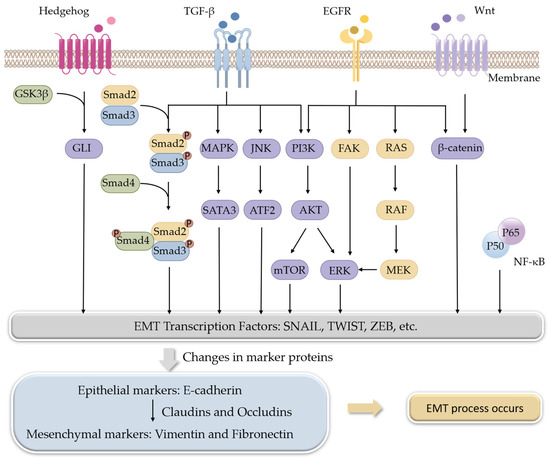
Figure 1. Signaling pathways involved in the regulation of EMT.
2.2. EMT Modulates Cancer Progression
Because abnormal activation of EMT gets involved in many stages of cancer progression, it is easy to conclude that the pathways and molecular targets of EMT are related to the poor prognosis of cancers. Generally, metastasis and chemotherapeutic resistance in cancer are major consequences of EMT activation.
At the initial stage of EMT, malignant epithelial cells in the primary tumor obtain the ability to lose their cell-cell connections and detach from the primary tumor. The detached cells or cell clusters acquire characteristics of mesenchymal-like cells, break through the basement membrane, invade surrounding tissue, and gain access to blood vessels to achieve spread and dissemination [21]. The acquired mesenchymal properties assist the survival of cancer cells in the circulation and, in addition, provide resistance to cell death signals. When the circulating tumor cells arrive in a distant position of the body with a suitable micro-environment, the spreading cells pass through the blood vessels again and colonize to form metastases [22] (Figure 2). As a consequence, suppressing EMT in cancer cells or developing anti-EMT adjuvants emerge as attractive strategies for cancer treatment.
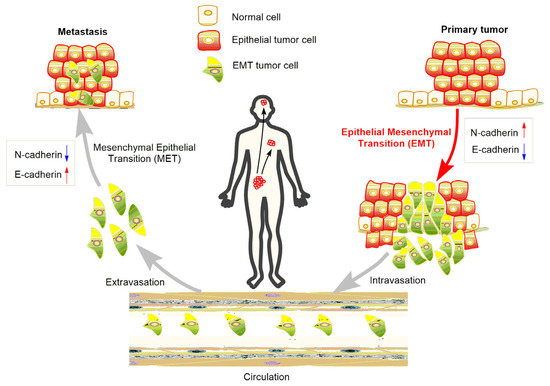
Figure 2. Role of EMT in the metastasis of cancer progression.
References
- Jonckheere, S.; Adams, J.; De Groote, D.; Campbell, K.; Berx, G.; Goossens, S. Epithelial-Mesenchymal Transition (EMT) as a therapeutic target. Cells Tissues Organs 2022, 211, 157–182.
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, 9040–9054.
- Sha, Y.; Haensel, D.; Gutierrez, G.; Du, H.; Dai, X.; Nie, Q. Intermediate cell states in epithelial-to-mesenchymal transition. Phys. Biol. 2019, 16, 1–8.
- Illam, S.P.; Narayanankutty, A.; Mathew, S.E.; Valsalakumari, R.; Jacob, R.M.; Raghavamenon, A.C. Epithelial Mesenchymal Transition in cancer progression: Preventive phytochemicals. Recent Pat. Anticancer Drug Discov. 2017, 12, 234–246.
- Basu, B.; Ghosh, M.K. Ubiquitination and deubiquitination in the regulation of epithelial-mesenchymal transition in cancer: Shifting gears at the molecular level. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119261–119276.
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in wound healing, tissue regeneration and organ fibrosis. Cells 2021, 10, 1587.
- Xu, Z.; Zhang, Y.; Dai, H.; Han, B. Epithelial-Mesenchymal Transition-mediated tumor therapeutic resistance. Molecules 2022, 27, 4750.
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. Embo J. 2021, 40, 108647–108669.
- Pouliquen, D.L.; Boissard, A.; Henry, C.; Coqueret, O.; Guette, C. Curcuminoids as modulators of EMT in invasive cancers: A review of molecular targets with the contribution of malignant mesothelioma studies. Front. Pharmacol. 2022, 13, 934534–934559.
- Ikenouchi, J.; Matsuda, M.; Furuse, M.; Tsukita, S. Regulation of tight junctions during the epithelium-mesenchyme transition: Direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 2003, 116, 1956–1967.
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45.
- Mittal, V. Epithelial Mesenchymal Transition in tumor metastasis. Mol. Oncol. 2018, 11, 28–39.
- Kang, E.; Seo, J.; Yoon, H.; Cho, S. The post-translational tegulation of Epithelial-Mesenchymal Transition-inducing transcription factors in cancer metastasis. Int. J. Mol. Sci. 2021, 22, 3591.
- Yuki, R. Aberrant activation mechanism of TGF-β signaling in Epithelial-mesenchymal Transition. Yakugaku Zasshi 2021, 141, 1229–1234.
- Song, M.Y.; Lee, D.Y.; Yun, S.M.; Kim, E.H. GLUT3 promotes Epithelial-Mesenchymal transition via TGF-β/JNK/ATF2 signaling pathway in colorectal cancer cells. Biomedicines 2022, 10, 1837.
- Zhang, J.; Tian, X.-J.; Xing, J. Signal transduction pathways of EMT induced by TGF-β, SHH, and WNT and their crosstalks. J. Clin. Med. 2016, 5, 41.
- Khatra, H.; Bose, C.; Sinha, S. Discovery of Hedgehog antagonists for cancer therapy. Curr. Med. Chem. 2017, 24, 2033–2058.
- Neelakantan, D.; Zhou, H.; Oliphant, M.U.J.; Zhang, X.; Simon, L.M.; Henke, D.M.; Shaw, C.A.; Wu, M.-F.; Hilsenbeck, S.G.; White, L.D.; et al. EMT cells increase breast cancer metastasis via paracrine GLI activation in neighbouring tumour cells. Nat. Commun. 2017, 8, 15773–15787.
- Xie, J.; Zhou, J.; Xia, J.; Zeng, Y.; Huang, G.; Zeng, W.; Fan, T.; Li, L.; Zeng, X.; Tao, Q. Phospholipase C delta 1 inhibits WNT/β-catenin and EGFR-FAK-ERK signaling and is disrupted by promoter CpG methylation in renal cell carcinoma. Clin. Epigenet. 2023, 15, 30–45.
- Martinelli, E.; Morgillo, F.; Troiani, T.; Ciardiello, F. Cancer resistance to therapies against the EGFR-RAS-RAF pathway: The role of MEK. Cancer Treat. Rev. 2017, 53, 61–69.
- Majidpoor, J.; Mortezaee, K. Steps in metastasis: An updated review. Med. Oncol. 2021, 38, 3–20.
- He, S.J.; Xiang, C.Q.; Zhang, Y.; Lu, X.T.; Chen, H.W.; Xiong, L.X. Recent progress on the effects of microRNAs and natural products on tumor epithelial-mesenchymal transition. Onco Targets Ther. 2017, 10, 3435–3451.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
898
Revisions:
2 times
(View History)
Update Date:
18 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




