
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mindy George-Weinstein | -- | 3650 | 2023-07-14 14:17:27 | | | |
| 2 | Camila Xu | Meta information modification | 3650 | 2023-07-17 07:33:14 | | |
Video Upload Options
Myo/Nog cells, discovered by their expression of the skeletal muscle specific transcription factor MyoD, bone morphogenetic protein inhibitor Noggin, and brain specific angiogenesis inhibitor 1, are integrated into the eye during early stages of embryonic development. While their release of Noggin is critical normal eye morphogenesis, wounding may stimulate Myo/Nog cells to form contractile myofibroblasts that cause secondary cataracts and retinal detachment.
1. Discovery of Myo/Nog Cells in the Embryo
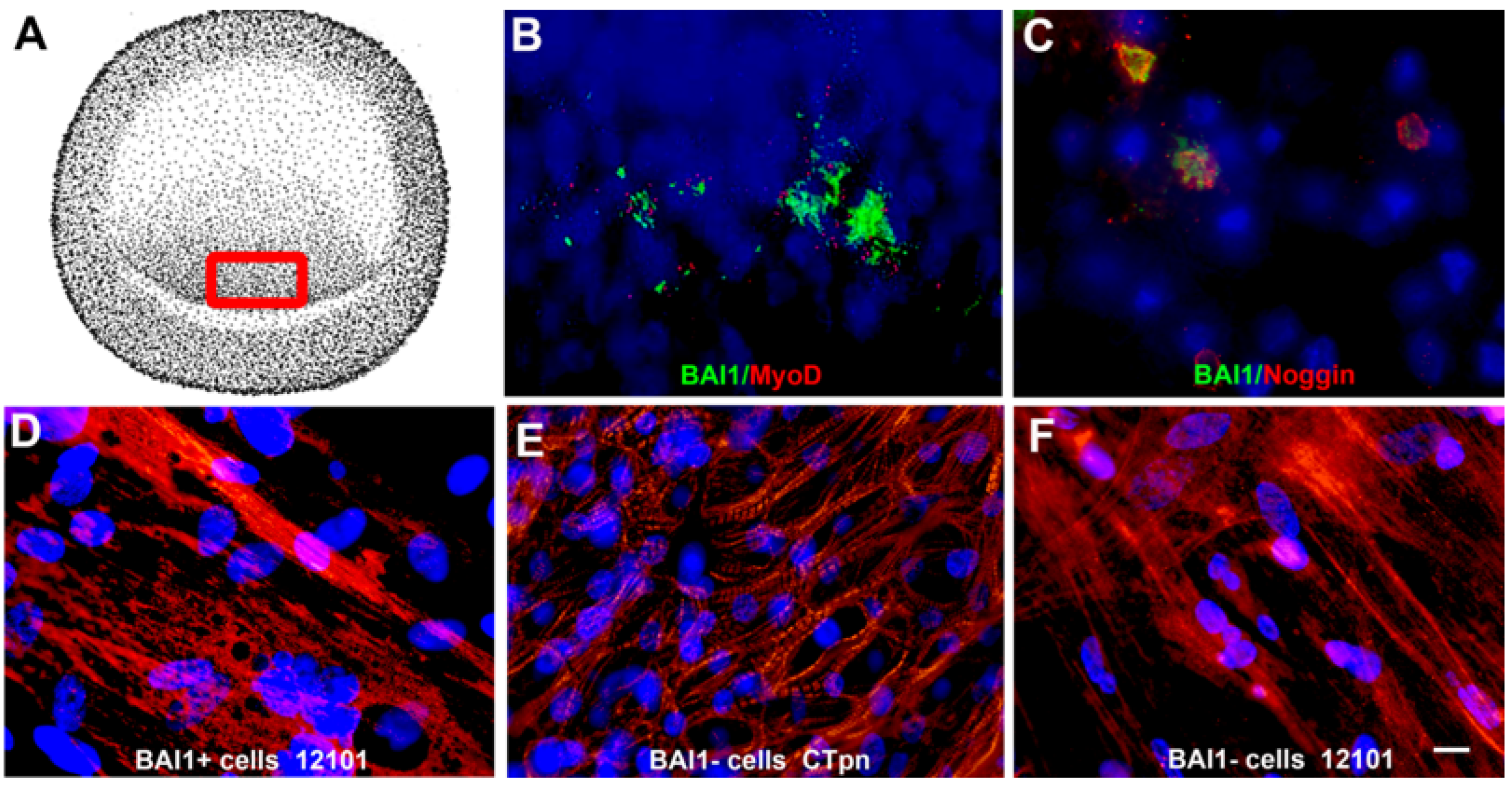
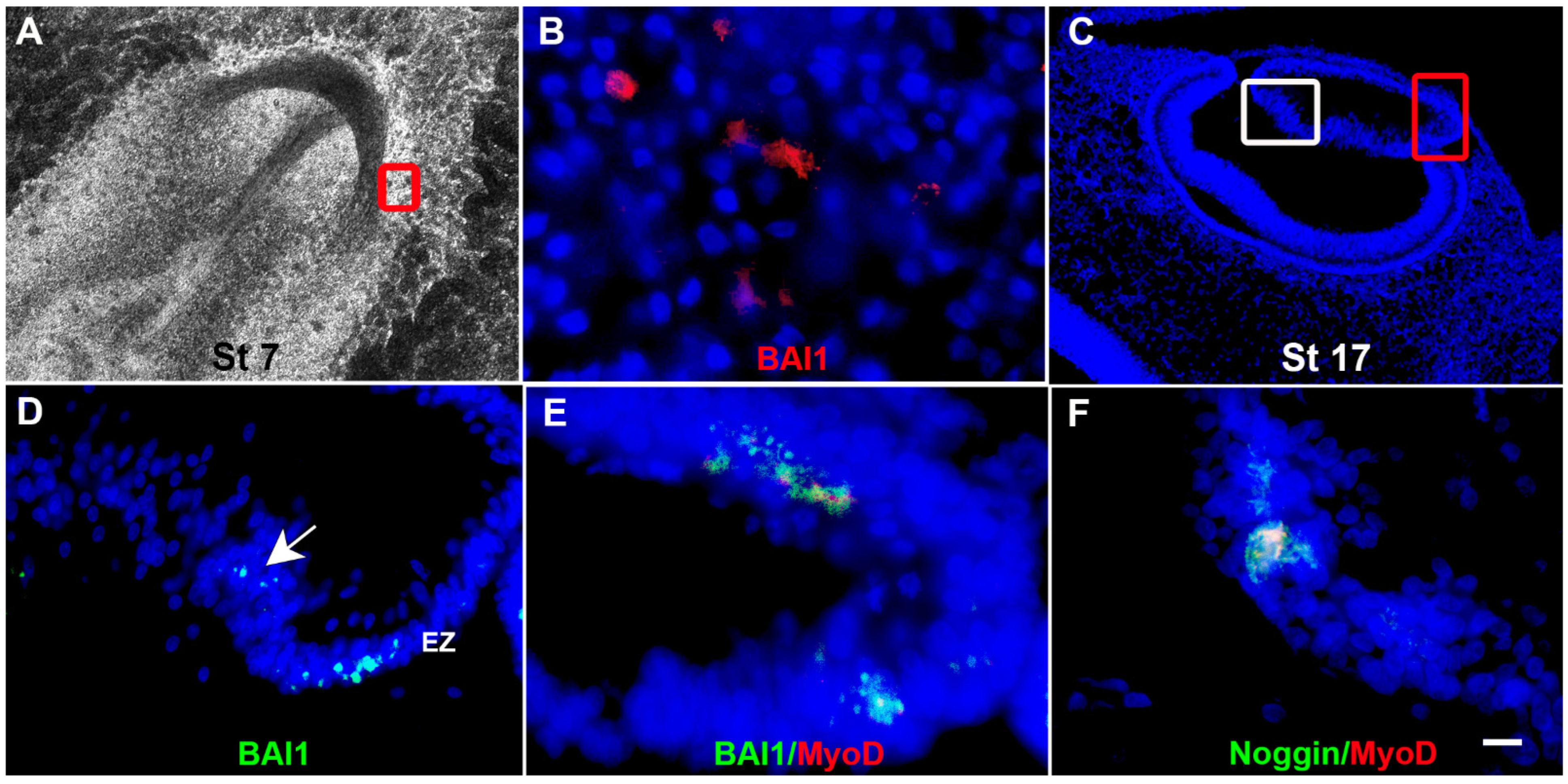
2. Roles of Myo/Nog Cells in the Developing Embryo
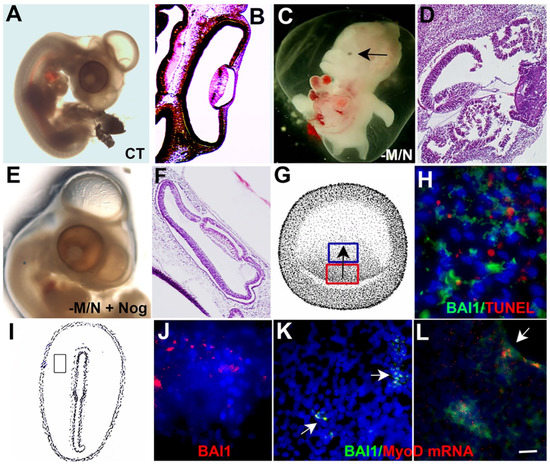
3. Myo/Nog Cells in the Adult Eye
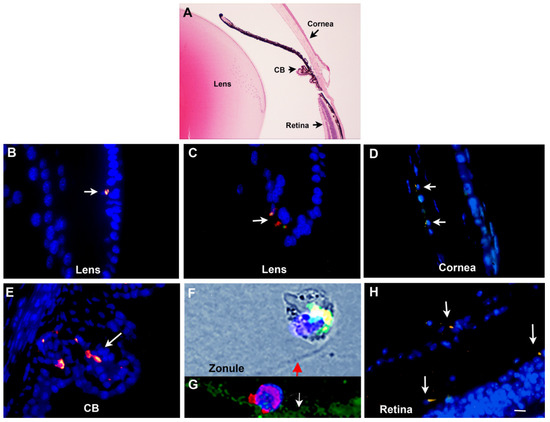
Behavior of Myo/Nog Cells in Explant Cultures of Anterior Human Lens Tissue
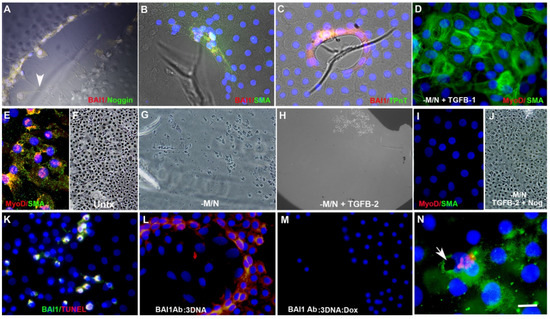
4. Role of Myo/Nog Cells in Posterior Capsule Opacification
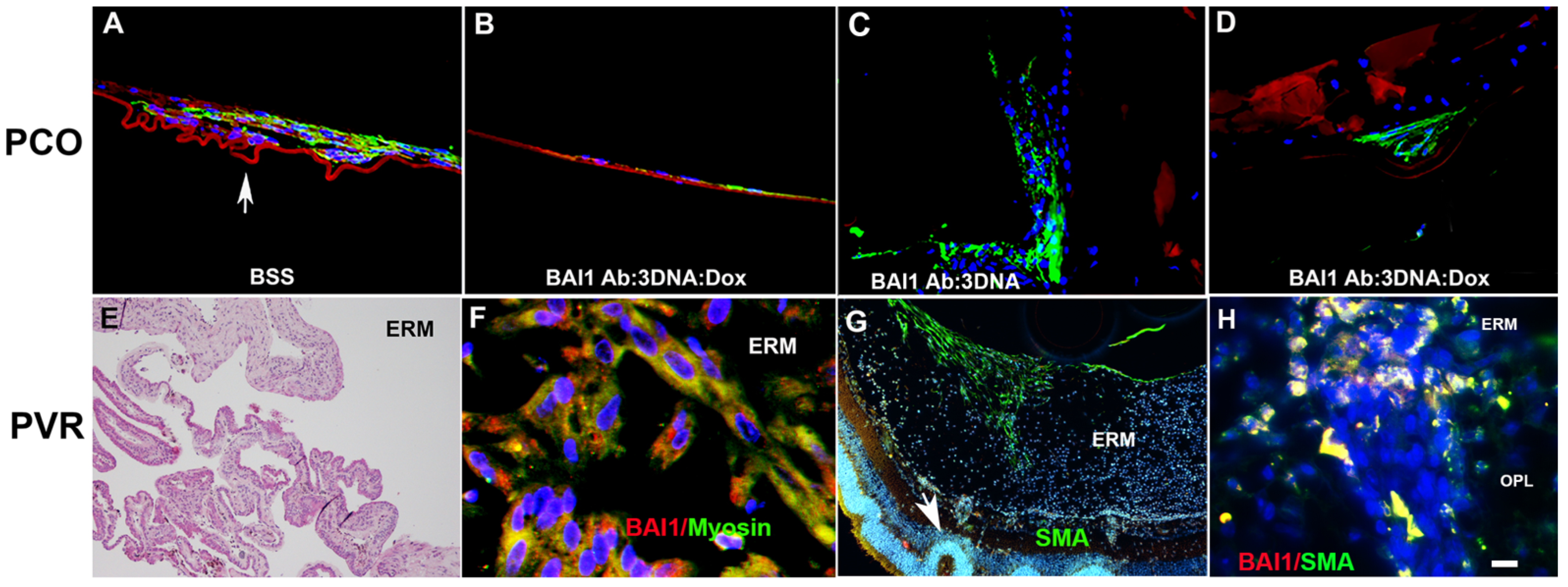
Having identified Myo/Nog cells as the progenitors of myofibroblasts in the lens, attention turned to PVR, a fibrotic disease that most often occurs in response to the repair of rhegmatogenous retinal detachment [99][100][101][102][103][104]. PVR is characterized by the formation of membranes on the inner surface of the retina (epiretinal), and/or behind or within the retina [99][100][101]. Myofibroblasts accumulate within the membranes and their contractions apply traction on the retina that may cause re-detachment [99][100][101][105]. In human epiretinal membranes, greater than 99% of cells with α-SMA, MyoD and striated muscle myosin II heavy chain co-expressed BAI1 and Noggin [67] (Figure 6F).
To examine the behavior of Myo/Nog cells during ERM formation, researchers utilized a mouse model in which PVR is induced by injecting SF6 gas and human retinal pigmented epithelial cells (ARPE-19) into the vitreous [106]. This model recapitulates disease progression seen in humans and other species. Myo/Nog cells were present on the inner surface of the retina early in the process of PVR induction and were the predominant cell type in ERMs [70]. They were the only cells that synthesized α-SMA apart from vascular smooth muscle and striated muscle myosin II [70]. Differentiated Myo/Nog cells overlaid retinal folds and areas of detachment (Figure 6G) and were present throughout the retina with disease progression (Figure 6H). Few leukocytes were present in the eye at all stages of PVR, indicating that inflammation did not appear to be a significant factor in Myo/Nog cell activation in this model. However, subpopulations of Myo/Nog cells expressed CD18 and CD45, markers for all leukocytes, and CD68 that is present in cells of the monocyte lineage [70]. It can be concluded that the death of human RPE cells was the likely stimulus for proliferation and migration of Myo/Nog cells and their engulfment of cell corpses [70], as occurs in other tissues, including the lens [68].
Myofibroblasts also appear in other ocular diseases [87]. Myo/Nog cells are present throughout the eye, and therefore, may be a source of myofibroblasts in the cornea, as well as the lens and retina. Behaviors of Myo/Nog cells in glaucoma are also worthy of exploration. Related questions are whether they become activated in response to increased intraocular pressure and if their differentiation and contraction narrow the outflow passages. The phagocytic function of Myo/Nog cells highlights the importance of examining their role in maintaining the patency of the trabecular meshwork and Schlemm’s canal for outflow of the aqueous humor. In the lens, Myo/Nog cells’ appearance among lens fiber cells raises the possibility that they engage in phagocytosis that may be important for maintaining transparency. The relative contributions of Myo/Nog cells, macrophages and microglia to clearance in the eye are likely to vary between tissues and the extent of inflammation.
The research has identified Myo/Nog cells as sole expressors of BAI1, MyoD and Noggin in the eye, mediators of phagocytosis and the source of myofibroblasts in the lens and retina. Myo/Nog cells are expected to be progenitors of myofibroblasts in other organs such as the heart, lung, kidney and liver whose functions are also compromised by fibrosis. Defining the molecular stimuli that activate Myo/Nog cells and trigger their proliferation, migration and differentiation may lead to additional therapeutic strategies to prevent and treat fibrotic disease. Their roles in regulating BMP signaling, neuroprotection and angiogenesis could also be leveraged for therapeutic purposes.
References
- Pownall, M.E.; Gustafsson, M.K.; Emerson, C.P., Jr. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 2002, 18, 747–783.
- Buckingham, M.; Vincent, S.D. Distinct and dynamic myogenic populations in the vertebrate embryo. Curr. Opin. Genet. Dev. 2009, 19, 444–453.
- Chal, J.; Pourquie, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122.
- Scaal, M.; Marcelle, C. Chick muscle development. Int. J. Dev. Biol. 2018, 62, 127–136.
- Choi, J.; Schultheiss, T.; Lu, M.; Wachtler, F.; Kuruc, N.; Franke, W.W.; Bader, D.; Fischman, D.A.; Holtzer, H. Founder cells for the cardiac and skeletal myogenic lineages. In precursors are critical components of cell fate analyses. In Cellular and Molecular Biology of Muscle Development; Stockdale, L.H.K.a.F.E., Ed.; A. R. Liss: New York, NY, USA, 1989; pp. 27–36.
- Chen, Y.; Solursh, M. The determination of myogenic and cartilage cells in the early chick embryo and the modifying effect of retinoic acid. Rouxs Arch. Dev. Biol. 1991, 200, 162–171.
- George-Weinstein, M.; Gerhart, J.V.; Foti, G.J.; Lash, J.W. Maturation of myogenic and chondrogenic cells in the presomitic mesoderm of the chick embryo. Exp. Cell Res. 1994, 211, 263–274.
- George-Weinstein, M.; Gerhart, J.; Reed, R.; Flynn, J.; Callihan, B.; Mattiacci, M.; Miehle, C.; Foti, G.; Lash, J.W.; Weintraub, H. Skeletal myogenesis: The preferred pathway of chick embryo epiblast cells in vitro. Dev. Biol. 1996, 173, 279–291.
- Gerhart, J.; Baytion, M.; DeLuca, S.; Getts, R.; Lopez, C.; Niewenhuis, R.; Nilsen, T.; Olex, S.; Weintraub, H.; George-Weinstein, M. DNA dendrimers localize MyoD mRNA in presomitic tissues of the chick embryo. J. Cell Biol. 2000, 149, 825–834.
- Buckingham, M.; Rigby, P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238.
- Hernandez-Hernandez, J.M.; Garcia-Gonzalez, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18.
- Lassar, A.B. Finding MyoD and lessons learned along the way. Semin. Cell Dev. Biol. 2017, 72, 3–9.
- Gerhart, J.; Bast, B.; Neely, C.; Iem, S.; Amegbe, P.; Niewenhuis, R.; Miklasz, S.; Cheng, P.F.; George-Weinstein, M. MyoD-positive myoblasts are present in mature fetal organs lacking skeletal muscle. J. Cell Biol. 2001, 155, 381–392.
- Gerhart, J.; Neely, C.; Stewart, B.; Perlman, J.; Beckmann, D.; Wallon, M.; Knudsen, K.; George-Weinstein, M. Epiblast cells that express MyoD recruit pluripotent cells to the skeletal muscle lineage. J. Cell Biol. 2004, 164, 739–746.
- Strony, R.; Gerhart, J.; Tornambe, D.; Perlman, J.; Neely, C.; Dare, J.; Stewart, B.; George-Weinstein, M. NeuroM and MyoD are expressed in separate subpopulations of cells in the pregastrulating epiblast. Gene Expr. Patterns 2005, 5, 387–395.
- Gerhart, J.; Bowers, J.; Gugerty, L.; Gerhart, C.; Martin, M.; Abdalla, F.; Bravo-Nuevo, A.; Sullivan, J.T.; Rimkunas, R.; Albertus, A.J.; et al. Brain-specific angiogenesis inhibitor 1 is expressed in the Myo/Nog cell lineage. PLoS ONE 2020, 15, e0234792.
- Stephenson, J.R.; Purcell, R.H.; Hall, R.A. The BAI subfamily of adhesion GPCRs: Synaptic regulation and beyond. Trends Pharmacol. Sci. 2014, 35, 208–215.
- Nishimori, H.; Shiratsuchi, T.; Urano, T.; Kimura, Y.; Kiyono, K.; Tatsumi, K.; Yoshida, S.; Ono, M.; Kuwano, M.; Nakamura, Y.; et al. A novel brain-specific p53-target gene, BAI1, containing thrombospondin type 1 repeats inhibits experimental angiogenesis. Oncogene 1997, 15, 2145–2150.
- Park, D.; Tosello-Trampont, A.C.; Elliott, M.R.; Lu, M.; Haney, L.B.; Ma, Z.; Klibanov, A.L.; Mandell, J.W.; Ravichandran, K.S. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 2007, 450, 430–434.
- Hochreiter-Hufford, A.E.; Lee, C.S.; Kinchen, J.M.; Sokolowski, J.D.; Arandjelovic, S.; Call, J.A.; Klibanov, A.L.; Yan, Z.; Mandell, J.W.; Ravichandran, K.S. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 2013, 497, 263–267.
- Kaur, B.; Brat, D.J.; Devi, N.S.; Van Meir, E.G. Vasculostatin, a proteolytic fragment of brain angiogenesis inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene 2005, 24, 3632–3642.
- Zhu, D.; Li, C.; Swanson, A.M.; Villalba, R.M.; Guo, J.; Zhang, Z.; Matheny, S.; Murakami, T.; Stephenson, J.R.; Daniel, S.; et al. BAI1 regulates spatial learning and synaptic plasticity in the hippocampus. J. Clin. Investig. 2015, 125, 1497–1508.
- Billings, E.A.; Lee, C.S.; Owen, K.A.; D’Souza, R.S.; Ravichandran, K.S.; Casanova, J.E. The adhesion GPCR BAI1 mediates macrophage ROS production and microbicidal activity against Gram-negative bacteria. Sci. Signal. 2016, 9, ra14.
- Elliott, M.R.; Zheng, S.; Park, D.; Woodson, R.I.; Reardon, M.A.; Juncadella, I.J.; Kinchen, J.M.; Zhang, J.; Lysiak, J.J.; Ravichandran, K.S. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature 2010, 467, 333–337.
- Hirsinger, E.; Duprez, D.; Jouve, C.; Malapert, P.; Cooke, J.; Pourquie, O. Noggin acts downstream of Wnt and Sonic Hedgehog to antagonize BMP4 in avian somite patterning. Development 1997, 124, 4605–4614.
- Dietrich, S.; Schubert, F.R.; Healy, C.; Sharpe, P.T.; Lumsden, A. Specification of the hypaxial musculature. Development 1998, 125, 2235–2249.
- Reshef, R.; Maroto, M.; Lassar, A.B. Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev. 1998, 12, 290–303.
- Tonegawa, A.; Takahashi, Y. Somitogenesis controlled by Noggin. Dev. Biol. 1998, 202, 172–182.
- Gerhart, J.; Elder, J.; Neely, C.; Schure, J.; Kvist, T.; Knudsen, K.; George-Weinstein, M. MyoD-positive epiblast cells regulate skeletal muscle differentiation in the embryo. J. Cell Biol. 2006, 175, 283–292.
- Gerhart, J.; Neely, C.; Elder, J.; Pfautz, J.; Perlman, J.; Narciso, L.; Linask, K.K.; Knudsen, K.; George-Weinstein, M. Cells that express MyoD mRNA in the epiblast are stably committed to the skeletal muscle lineage. J. Cell Biol. 2007, 178, 649–660.
- Gerhart, J.; Pfautz, J.; Neely, C.; Elder, J.; DuPrey, K.; Menko, A.S.; Knudsen, K.; George-Weinstein, M. Noggin producing, MyoD-positive cells are crucial for eye development. Dev. Biol. 2009, 336, 30–41.
- Gerhart, J.; Scheinfeld, V.L.; Milito, T.; Pfautz, J.; Neely, C.; Fisher-Vance, D.; Sutter, K.; Crawford, M.; Knudsen, K.; George-Weinstein, M. Myo/Nog cell regulation of bone morphogenetic protein signaling in the blastocyst is essential for normal morphogenesis and striated muscle lineage specification. Dev. Biol. 2011, 359, 12–25.
- Anderson, R.M.; Lawrence, A.R.; Stottmann, R.W.; Bachiller, D.; Klingensmith, J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development 2002, 129, 4975–4987.
- Bachiller, D.; Klingensmith, J.; Kemp, C.; Belo, J.A.; Anderson, R.M.; May, S.R.; McMahon, J.A.; McMahon, A.P.; Harland, R.M.; Rossant, J.; et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 2000, 403, 658–661.
- Brunet, L.J.; McMahon, J.A.; McMahon, A.P.; Harland, R.M. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 1998, 280, 1455–1457.
- Choi, M.; Stottmann, R.W.; Yang, Y.P.; Meyers, E.N.; Klingensmith, J. The bone morphogenetic protein antagonist noggin regulates mammalian cardiac morphogenesis. Circ. Res. 2007, 100, 220–228.
- McMahon, J.A.; Takada, S.; Zimmerman, L.B.; Fan, C.M.; Harland, R.M.; McMahon, A.P. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998, 12, 1438–1452.
- Stottmann, R.W.; Berrong, M.; Matta, K.; Choi, M.; Klingensmith, J. The BMP antagonist Noggin promotes cranial and spinal neurulation by distinct mechanisms. Dev. Biol. 2006, 295, 647–663.
- Gazzerro, E.; Canalis, E. Bone morphogenetic proteins and their antagonists. Rev. Endocr. Metab. Disord. 2006, 7, 51–65.
- Zimmerman, L.B.; De Jesus-Escobar, J.M.; Harland, R.M. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 1996, 86, 599–606.
- Shu, D.Y.; Lovicu, F.J. Insights into Bone Morphogenetic Protein-(BMP-) Signaling in Ocular Lens Biology and Pathology. Cells 2021, 10, 2604.
- Furuta, Y.; Hogan, B.L. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998, 12, 3764–3775.
- Dudley, A.T.; Lyons, K.M.; Robertson, E.J. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995, 9, 2795–2807.
- Jena, N.; Martin-Seisdedos, C.; McCue, P.; Croce, C.M. BMP7 null mutation in mice: Developmental defects in skeleton, kidney, and eye. Exp. Cell Res. 1997, 230, 28–37.
- Wawersik, S.; Purcell, P.; Rauchman, M.; Dudley, A.T.; Robertson, E.J.; Maas, R. BMP7 acts in murine lens placode development. Dev. Biol. 1999, 207, 176–188.
- Faber, S.C.; Robinson, M.L.; Makarenkova, H.P.; Lang, R.A. Bmp signaling is required for development of primary lens fiber cells. Development 2002, 129, 3727–3737.
- Beebe, D.; Garcia, C.; Wang, X.; Rajagopal, R.; Feldmeier, M.; Kim, J.Y.; Chytil, A.; Moses, H.; Ashery-Padan, R.; Rauchman, M. Contributions by members of the TGFbeta superfamily to lens development. Int. J. Dev. Biol. 2004, 48, 845–856.
- Chang, B.; Smith, R.S.; Peters, M.; Savinova, O.V.; Hawes, N.L.; Zabaleta, A.; Nusinowitz, S.; Martin, J.E.; Davisson, M.L.; Cepko, C.L.; et al. Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure. BMC Genet. 2001, 2, 18.
- Solursh, M.; Langille, R.M.; Wood, J.; Sampath, T.K. Osteogenic protein-1 is required for mammalian eye development. Biochem. Biophys. Res. Commun. 1996, 218, 438–443.
- de Iongh, R.U.; Lovicu, F.J.; Overbeek, P.A.; Schneider, M.D.; Joya, J.; Hardeman, E.D.; McAvoy, J.W. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development 2001, 128, 3995–4010.
- Belecky-Adams, T.L.; Adler, R.; Beebe, D.C. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development 2002, 129, 3795–3802.
- Boswell, B.A.; Overbeek, P.A.; Musil, L.S. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev. Biol. 2008, 324, 202–212.
- Sjodal, M.; Edlund, T.; Gunhaga, L. Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev. Cell 2007, 13, 141–149.
- Hung, F.C.; Zhao, S.; Chen, Q.; Overbeek, P.A. Retinal ablation and altered lens differentiation induced by ocular overexpression of BMP7. Vis. Res. 2002, 42, 427–438.
- Huang, J.; Liu, Y.; Filas, B.; Gunhaga, L.; Beebe, D.C. Negative and positive auto-regulation of BMP expression in early eye development. Dev. Biol. 2015, 407, 256–264.
- Liu, C.S.; Wormstone, I.M.; Duncan, G.; Marcantonio, J.M.; Webb, S.F.; Davies, P.D. A study of human lens cell growth in vitro. A model for posterior capsule opacification. Investig. Ophthalmol. Vis. Sci. 1996, 37, 906–914.
- Wormstone, I.M.; Liu, C.S.; Rakic, J.M.; Marcantonio, J.M.; Vrensen, G.F.; Duncan, G. Human lens epithelial cell proliferation in a protein-free medium. Investig. Ophthalmol. Vis. Sci. 1997, 38, 396–404.
- Saxby, L.; Rosen, E.; Boulton, M. Lens epithelial cell proliferation, migration, and metaplasia following capsulorhexis. Br. J. Ophthalmol. 1998, 82, 945–952.
- Walker, J.L.; Zhai, N.; Zhang, L.; Bleaken, B.M.; Wolff, I.; Gerhart, J.; George-Weinstein, M.; Menko, A.S. Unique precursors for the mesenchymal cells involved in injury response and fibrosis. Proc. Natl. Acad. Sci. USA 2010, 107, 13730–13735.
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503.
- Gerhart, J.; Hayes, C.; Scheinfeld, V.; Chernick, M.; Gilmour, S.; George-Weinstein, M. Myo/Nog cells in normal, wounded and tumor-bearing skin. Exp. Dermatol. 2012, 21, 466–468.
- Gerhart, J.; Greenbaum, M.; Scheinfeld, V.; Fitzgerald, P.; Crawford, M.; Bravo-Nuevo, A.; Pitts, M.; George-Weinstein, M. Myo/Nog cells: Targets for preventing the accumulation of skeletal muscle-like cells in the human lens. PLoS ONE 2014, 9, e95262.
- Brandli, A.; Gerhart, J.; Sutera, C.K.; Purushothuman, S.; George-Weinstein, M.; Stone, J.; Bravo-Nuevo, A. Role of Myo/Nog Cells in Neuroprotection: Evidence from the Light Damaged Retina. PLoS ONE 2017, 12, e0169744.
- Gerhart, J.; Withers, C.; Gerhart, C.; Werner, L.; Mamalis, N.; Bravo-Nuevo, A.; Scheinfeld, V.; FitzGerald, P.; Getts, R.; George-Weinstein, M. Myo/Nog cells are present in the ciliary processes, on the zonule of Zinn and posterior capsule of the lens following cataract surgery. Exp. Eye Res. 2018, 171, 101–105.
- Gerhart, J.; Behling, K.; Paessler, M.; Milton, L.; Bramblett, G.; Garcia, D.; Pitts, M.; Hurtt, R.; Crawford, M.; Lackman, R.; et al. Rhabdomyosarcoma and Wilms tumors contain a subpopulation of noggin producing, myogenic cells immunoreactive for lens beaded filament proteins. PLoS ONE 2019, 14, e0214758.
- Gerhart, J.; Werner, L.; Mamalis, N.; Infanti, J.; Withers, C.; Abdalla, F.; Gerhart, C.; Bravo-Nuevo, A.; Gerhart, O.; Getts, L.; et al. Depletion of Myo/Nog Cells in the Lens Mitigates Posterior Capsule Opacification in Rabbits. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1813–1823.
- Gerhart, J.; Morrison, N.; Gugerty, L.; Telander, D.; Bravo-Nuevo, A.; George-Weinstein, M. Myo/Nog cells expressing muscle proteins are present in preretinal membranes from patients with proliferative vitreoretinopathy. Exp. Eye Res. 2020, 197, 108080.
- Gerhart, J.; Gugerty, L.; Lecker, P.; Abdalla, F.; Martin, M.; Gerhart, O.; Gerhart, C.; Johal, K.; Bernstein, J.; Spikes, J.; et al. Myo/Nog cells are nonprofessional phagocytes. PLoS ONE 2020, 15, e0235898.
- Joseph-Pauline, S.; Morrison, N.; Braccia, M.; Payne, A.; Gugerty, L.; Mostoller, J.; Lecker, P.; Tsai, E.J.; Kim, J.; Martin, M.; et al. Acute Response and Neuroprotective Role of Myo/Nog Cells Assessed in a Rat Model of Focal Brain Injury. Front. Neurosci. 2021, 15, 780707.
- Crispin, M.; Gerhart, J.; Heffer, A.; Martin, M.; Abdalla, F.; Bravo-Nuevo, A.; Philp, N.J.; Kuriyan, A.E.; George-Weinstein, M. Myo/Nog Cells Give Rise to Myofibroblasts during Epiretinal Membrane Formation in a Mouse Model of Proliferative Vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2023, 64, 1.
- Gerhart, J.; Greenbaum, M.; Casta, L.; Clemente, A.; Mathers, K.; Getts, R.; George-Weinstein, M. Antibody-Conjugated, DNA-Based Nanocarriers Intercalated with Doxorubicin Eliminate Myofibroblasts in Explants of Human Lens Tissue. J. Pharmacol. Exp. Ther. 2017, 361, 60–67.
- Bravo-Nuevo, A.; Brandli, A.A.; Gerhart, J.; Nichols, J.; Pitts, M.; Sutera, C.K.; Assali, S.; Scheinfeld, V.; Prendergast, G.C.; Stone, J.; et al. Neuroprotective effect of Myo/Nog cells in the stressed retina. Exp. Eye Res. 2016, 146, 22–25.
- Sappino, A.P.; Schurch, W.; Gabbiani, G. Differentiation repertoire of fibroblastic cells: Expression of cytoskeletal proteins as marker of phenotypic modulations. Lab. Investig. 1990, 63, 144–161.
- Schaart, G.; Pieper, F.R.; Kuijpers, H.J.; Bloemendal, H.; Ramaekers, F.C. Baby hamster kidney (BHK-21/C13) cells can express striated muscle type proteins. Differentiation 1991, 46, 105–115.
- Ogata, I.; Saez, C.G.; Greenwel, P.; Ponce, M.d.L.; Geerts, A.; Leinwand, L.A.; Rojkind, M. Rat liver fat-storing cell lines express sarcomeric myosin heavy chain mRNA and protein. Cell Motil. Cytoskelet. 1993, 26, 125–132.
- Mayer, D.C.; Leinwand, L.A. Sarcomeric gene expression and contractility in myofibroblasts. J. Cell Biol. 1997, 139, 1477–1484.
- Wormstone, I.M.; Tamiya, S.; Anderson, I.; Duncan, G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2301–2308.
- Liu, J.; Hales, A.M.; Chamberlain, C.G.; McAvoy, J.W. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor beta. Investig. Ophthalmol. Vis. Sci. 1994, 35, 388–401.
- Kurosaka, D.; Muraki, Y.; Inoue, M.; Katsura, H. TGF-beta 2 increases alpha-smooth muscle actin expression in bovine retinal pigment epithelial cells. Curr. Eye Res. 1996, 15, 1144–1147.
- Gordon-Thomson, C.; de Iongh, R.U.; Hales, A.M.; Chamberlain, C.G.; McAvoy, J.W. Differential cataractogenic potency of TGF-beta1, -beta2, and -beta3 and their expression in the postnatal rat eye. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1399–1409.
- Richiert, D.M.; Ireland, M.E. TGF-beta elicits fibronectin secretion and proliferation in cultured chick lens epithelial cells. Curr. Eye Res. 1999, 18, 62–71.
- Lee, E.H.; Joo, C.K. Role of transforming growth factor-beta in transdifferentiation and fibrosis of lens epithelial cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2025–2032.
- Marcantonio, J.M.; Rakic, J.M.; Vrensen, G.F.; Duncan, G. Lens cell populations studied in human donor capsular bags with implanted intraocular lenses. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1130–1141.
- Saika, S.; Okada, Y.; Miyamoto, T.; Ohnishi, Y.; Ooshima, A.; McAvoy, J.W. Smad translocation and growth suppression in lens epithelial cells by endogenous TGFbeta2 during wound repair. Exp. Eye Res. 2001, 72, 679–686.
- Wormstone, I.M.; Tamiya, S.; Eldred, J.A.; Lazaridis, K.; Chantry, A.; Reddan, J.R.; Anderson, I.; Duncan, G. Characterisation of TGF-beta2 signalling and function in a human lens cell line. Exp. Eye Res. 2004, 78, 705–714.
- Dawes, L.J.; Eldred, J.A.; Anderson, I.K.; Sleeman, M.; Reddan, J.R.; Duncan, G.; Wormstone, I.M. TGF beta-induced contraction is not promoted by fibronectin-fibronectin receptor interaction, or alpha SMA expression. Investig. Ophthalmol. Vis. Sci. 2008, 49, 650–661.
- Shu, D.Y.; Lovicu, F.J. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Resarch 2017, 60, 44–65.
- Flokis, M.; Lovicu, F.J. FGF-2 Differentially Regulates Lens Epithelial Cell Behaviour during TGF-beta-Induced EMT. Cells 2023, 12, 827.
- Shu, D.Y.; Wojciechowski, M.C.; Lovicu, F.J. Bone Morphogenetic Protein-7 Suppresses TGFbeta2-Induced Epithelial-Mesenchymal Transition in the Lens: Implications for Cataract Prevention. Investig. Ophthalmol. Vis. Sci. 2017, 58, 781–796.
- Sharma, N.; Pushker, N.; Dada, T.; Vajpayee, R.B.; Dada, V.K. Complications of pediatric cataract surgery and intraocular lens implantation. J. Cataract Refract. Surg. 1999, 25, 1585–1588.
- Baratz, K.H.; Cook, B.E.; Hodge, D.O. Probability of Nd:YAG laser capsulotomy after cataract surgery in Olmsted County, Minnesota. Am. J. Ophthalmol. 2001, 131, 161–166.
- Boureau, C.; Lafuma, A.; Jeanbat, V.; Smith, A.F.; Berdeaux, G. Cost of cataract surgery after implantation of three intraocular lenses. Clin. Ophthalmol. 2009, 3, 277–285.
- Apple, D.J.; Solomon, K.D.; Tetz, M.R.; Assia, E.I.; Holland, E.Y.; Legler, U.F.; Tsai, J.C.; Castaneda, V.E.; Hoggatt, J.P.; Kostick, A.M. Posterior capsule opacification. Surv. Ophthalmol. 1992, 37, 73–116.
- Awasthi, N.; Guo, S.; Wagner, B.J. Posterior capsular opacification: A problem reduced but not yet eradicated. Arch. Ophthalmol. 2009, 127, 555–562.
- Moisseiev, J.; Bartov, E.; Schochat, A.; Blumenthal, M. Long-term study of the prevalence of capsular opacification following extracapsular cataract extraction. J. Cataract Refract. Surg. 1989, 15, 531–533.
- Wormstone, I.M.; Wang, L.; Liu, C.S. Posterior capsule opacification. Exp. Eye Res. 2009, 88, 257–269.
- McDonnell, P.J.; Zarbin, M.A.; Green, W.R. Posterior capsule opacification in pseudophakic eyes. Ophthalmology 1983, 90, 1548–1553.
- Gwon, A. The Rabbit in Cataract/IOL Surgery. In Animal Models in Eye Research; Academic Press: Cambridge, MA, USA, 2008; pp. 184–203.
- Pastor, J.C. Proliferative vitreoretinopathy: An overview. Surv. Ophthalmol. 1998, 43, 3–18.
- Idrees, S.; Sridhar, J.; Kuriyan, A.E. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019, 59, 221–240.
- Shahlaee, A.; Woeller, C.F.; Philp, N.J.; Kuriyan, A.E. Translational and clinical advancements in management of proliferative vitreoretinopathy. Curr. Opin. Ophthalmol. 2022, 33, 219–227.
- Pastor, J.C.; de la Rua, E.R.; Martin, F. Proliferative vitreoretinopathy: Risk factors and pathobiology. Prog. Retin. Eye Res. 2002, 21, 127–144.
- Morescalchi, F.; Duse, S.; Gambicorti, E.; Romano, M.R.; Costagliola, C.; Semeraro, F. Proliferative vitreoretinopathy after eye injuries: An overexpression of growth factors and cytokines leading to a retinal keloid. Mediat. Inflamm. 2013, 2013, 269787.
- Nagasaki, H.; Shinagawa, K.; Mochizuki, M. Risk factors for proliferative vitreoretinopathy. Prog. Retin. Eye Res. 1998, 17, 77–98.
- Glaser, B.M.; Cardin, A.; Biscoe, B. Proliferative vitreoretinopathy. The mechanism of development of vitreoretinal traction. Ophthalmology 1987, 94, 327–332.
- Heffer, A.; Wang, V.; Sridhar, J.; Feldon, S.E.; Libby, R.T.; Woeller, C.F.; Kuriyan, A.E. A Mouse Model of Proliferative Vitreoretinopathy Induced by Intravitreal Injection of Gas and RPE Cells. Transl. Vis. Sci. Technol. 2020, 9, 9.




