Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jian Wu | -- | 2638 | 2023-07-14 10:18:24 | | | |
| 2 | Fanny Huang | Meta information modification | 2638 | 2023-07-17 08:10:05 | | | | |
| 3 | Fanny Huang | Meta information modification | 2638 | 2023-07-17 10:51:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Song, J.; Lv, J.; Jin, J.; Jin, Z.; Li, T.; Wu, J. Antibacterial Activities of 1,2,3-Triazolium Salts. Encyclopedia. Available online: https://encyclopedia.pub/entry/46806 (accessed on 07 February 2026).
Song J, Lv J, Jin J, Jin Z, Li T, Wu J. Antibacterial Activities of 1,2,3-Triazolium Salts. Encyclopedia. Available at: https://encyclopedia.pub/entry/46806. Accessed February 07, 2026.
Song, Jia, Jie Lv, Jiamiao Jin, Zhichao Jin, Tingting Li, Jian Wu. "Antibacterial Activities of 1,2,3-Triazolium Salts" Encyclopedia, https://encyclopedia.pub/entry/46806 (accessed February 07, 2026).
Song, J., Lv, J., Jin, J., Jin, Z., Li, T., & Wu, J. (2023, July 14). Antibacterial Activities of 1,2,3-Triazolium Salts. In Encyclopedia. https://encyclopedia.pub/entry/46806
Song, Jia, et al. "Antibacterial Activities of 1,2,3-Triazolium Salts." Encyclopedia. Web. 14 July, 2023.
Copy Citation
1,2,3-Triazolium salts have demonstrated significant potential in the fields of medicine and agriculture, exhibiting exceptional antibacterial, antifungal, anticancer, and antileishmanial properties. Moreover, these salts can be utilized as additives or components to produce nano- and fiber-based materials with antibacterial properties.
1,2,3-triazolium salts
synthesis
antibacterial
antibacterial activities
1. Introduction
1,2,3-Triazoles are widely used in the fields of pesticides and medicines due to their broad-spectrum bioactive properties [1]. However, the increasing problem of drug resistance associated with the frequent use of triazole drugs needs to be addressed through the development of novel pharmacophores [2]. The 1,2,3-triazolium unit is a significant bioactive group, which has attracted much attention in recent years [3][4]. Studies have shown that the triazolium transformated from triazole have good bioactivities [5][6][7][8][9][10][11][12]. Molecules containing 1,2,3-triazolium units have been proven to exhibit a variety of biological activities, including antibacterial [13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34], antifungal [5][6][7][8][9][35], anticancer [10][11][36][37][38][39][40][41][42], and antileishmanial [12][43][44][45][46] properties.
1,2,3-Triazolium salts possess unique chemical properties, such as high stability, tunable reactivity, and distinct electronic characteristics [4][47][48]. They are valuable building blocks in organic synthesis, and also have been employed in the development of novel catalysts, antimicrobial agents, fluorescent dyes, and bioactive molecules [47][48][49][50]. For example, functionalized biopolymers containing 1,2,3-triazolium units have shown excellent potential to produce fibers with antibacterial properties, dental materials, and medical antibacterial devices [32][33]. In general, 1,2,3-triazolium salts could be obtained through the copper-catalyzed 1,3-dipolar azide-alkyne cycloaddition (CuAAC) reaction, N-alkylation, and salt metathesis processes [47][48]. 1,2,3-Triazolium salts have three active sites, including N-1, N-3 and C-4 positions, which can be linked with different functional groups in a regioselective manner to regulate their biological activity and cytotoxicity [4].
2. Synthesis of 1,2,3-Triazolium Salts
In the early 21st century, a breakthrough in triazole chemistry occurred when the Sharpless group first proposed a new concept known as “click” chemistry [51]. “Click” chemistry is a powerful synthetic strategy that aims to efficiently and selectively generate chemical bonds under mild reaction conditions [47][48][49][50][51][52]. One of the most versatile click reactions is the synthesis of 1,2,3-triazolium salts [52][53]. The click chemistry for the synthesis of 1,2,3-triazolium salts generally includes three steps using azide and alkyne as the starting materials: cycloaddition reaction of 1,2,3-triazole, N-alkylation, and salt metathesis [47][48][50].
2.1. Non-Selective Huisgen Reaction
The utilization and synthesis of 1,2,3-triazoles have experienced significant growth in recent decades [52]. The existence of triazoles has been recognized since the 1960s when Huisgen discovered the thermal cycloaddition reaction involving azides and alkynes (Scheme 1) [54]. Under high temperatures, the Huisgen 1,3-dipolar cycloaddition reaction between azides and terminal or internal alkynes leads to the formation of triazoles [54]. However, this method is limited due to its requirement for prolonged heating and its purification from the mixture of 1,4- and 1,5-regioisomers [52][55].
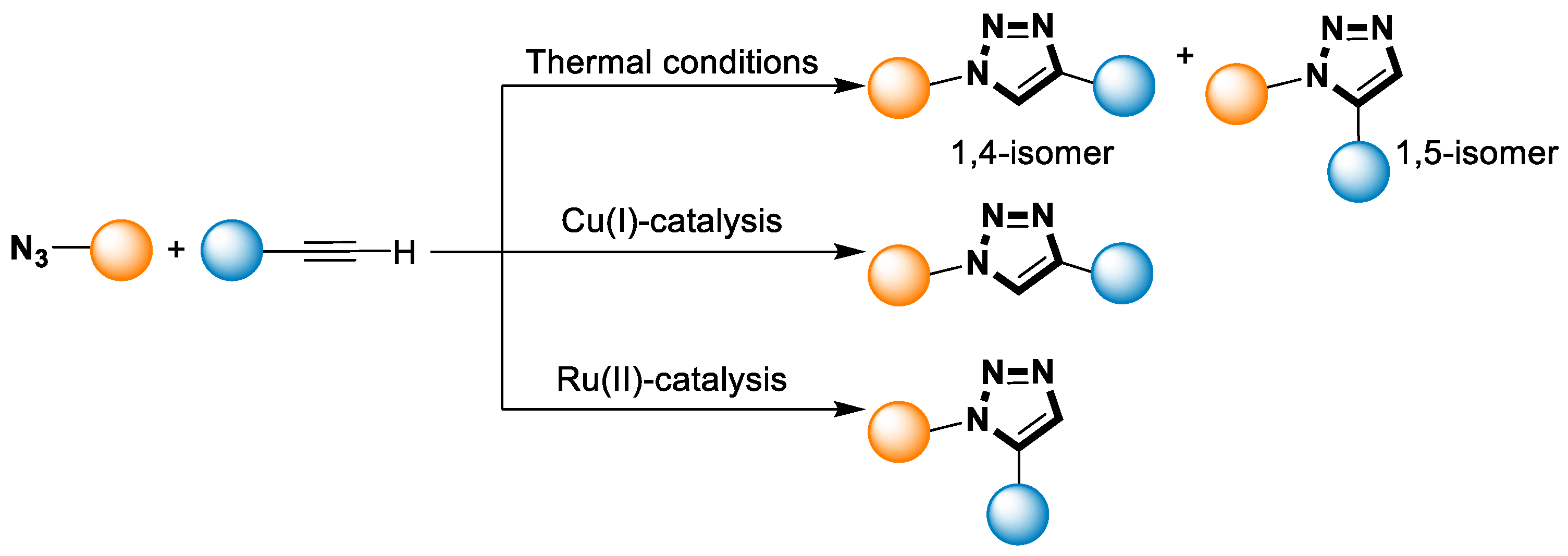
Scheme 1. Thermal and catalytic [3+2] azide-alkyne cycloaddition reaction of 1,2,3-triazole.
2.2. Selective Huisgen Reaction Mediated by Metal Catalysis
In 1974, Huisgen and coworkers found that Cu(I) cations could catalyze the cyclization reaction between an azide and an alkyne, leading to the formation of 1,2,3-triazoles [4]. They demonstrated that under catalytic conditions, the CuAAC reaction could selectively generate the 1,4-disubstituted isomer as the main product [4]. The key step in the Huisgen synthesis is the formation of a copper(I) acetylide intermediate from the alkyne and the copper catalyst [55][56][57]. This intermediate then reacts with the azide to generate a highly stable 1,2,3-triazole product. The reaction exhibits high regioselectivities under mild reaction conditions, giving the 1,4-disubstituted triazole product as the major isomer (Scheme 1) [55][56][57]. The selective Huisgen synthesis offers several advantages over traditional methods for triazole synthesis [55]. It proceeds rapidly, often achieving complete conversion within minutes to hours. It also tolerates a wide range of functional groups, enabling the incorporation of diverse molecular entities into the triazole scaffold. Additionally, the reaction can be performed in water or bio-orthogonal conditions, making it suitable for applications in biological systems [55].
In 2008, Fokin’s group chose Ruthenium (II) as a catalyst to promote the reaction between a series of organic azides and terminal alkynes containing various functional groups. 1,5-disubstitued 1,2,3-triazole products were selectively generated at a high temperature (Scheme 1) [58]. Ru(II) catalysts possess advantages over copper catalysts due to their easy synthesis and air stability, which are suitable for ambient temperature cycloaddition reactions involving internal alkynes, aryl azides, and other thermally labile reactants [58]. Nonetheless, both catalysts exhibit excellent activities as well as chemo- and regio-selectivities. In addition to the CuAAC reaction, the new RuAAC process provides easier access to all 1H-1,2,3-triazole regioisomers [57][58].
2.3. N-Alkylation and Salt Metathesis
The highly efficient Cu(I)-catalyzed azide-alkyne “click” cycloaddition (CuAAC) can provide facile access to 1,4-disubstituted 1,2,3-triazoles and make them widely-used scaffolds in the field of synthetic chemistry [55][56]. Structurally complex 1,2,3-triazolium salts can be readily prepared from the corresponding 1,2,3-triazoles using alkyl halides, tosylate, trifluoromethane sulfonate, or tri-alkyloxoniums (Scheme 2) [49][50]. The alkylation process typically involves the use of a base to deprotonate the 1,2,3-triazole, followed by a reaction with the alkylating agent. This transformation generally tolerates a wide variety of functional groups at the N-1 and C-4 positions of the triazole ring and often occurs with generally N-3 regioselectivity [49][50].
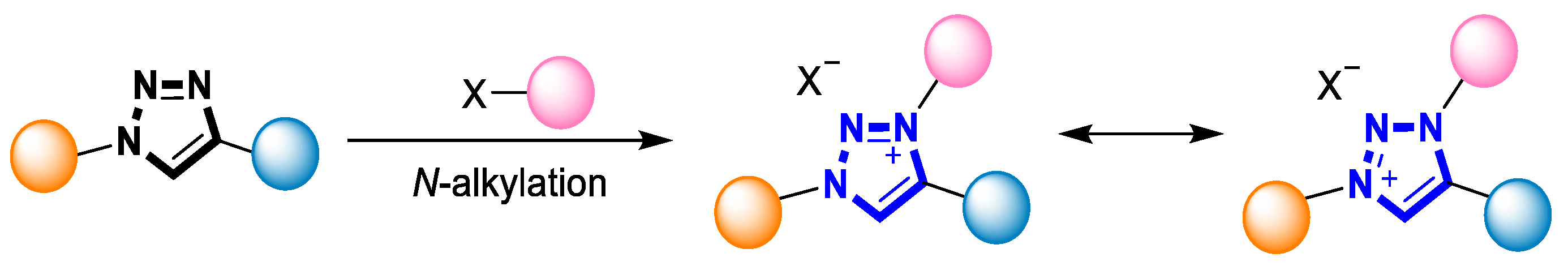
Scheme 2. N-alkylation of 1,2,3-triazole.
The resulting alkylated product can be further converted into the various 1,2,3-triazolium salts through protonation or appropriate transformations [4][50]. For example, the anionic counterion X− of the triazolium salt can be easily exchanged by simply washing with excess inorganic salt or using an exchange resin [17] (Scheme 3).
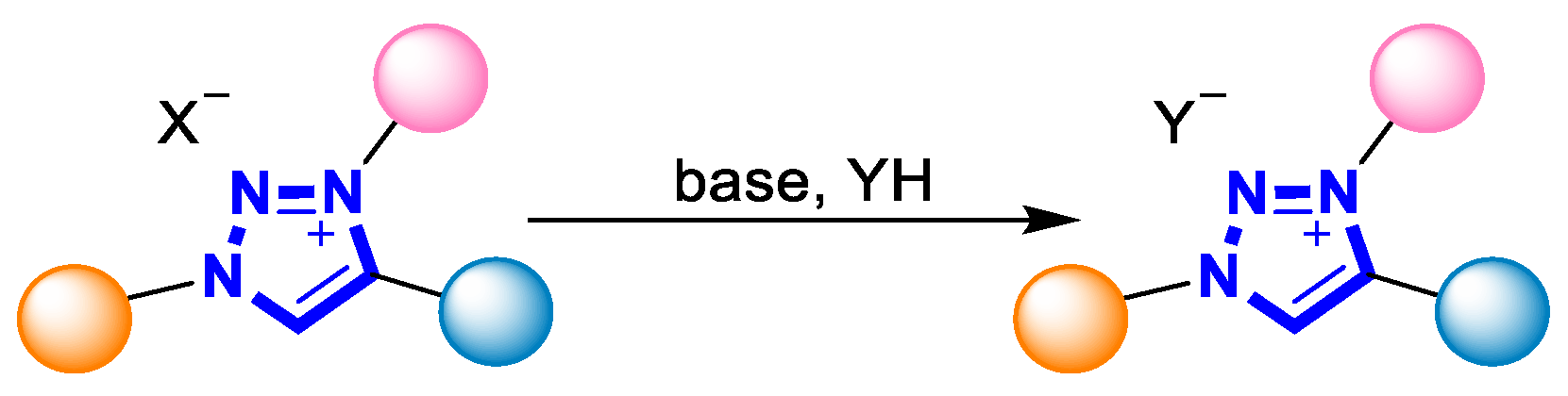
Scheme 3. Salt metathesis of 1,2,3-triazolium salt.
3. Antibacterial Activities of 1,2,3-Triazolium Salts
Quaternary ammonium compounds (QACs) are a kind of cationic biocide with broad-spectrum antibacterial activity which are used in various fields from household cleaning and agriculture to medicine and industry [59]. QACs possess unique amphiphilic characteristics that allow them to disrupt the phospholipid bilayer of the cell membrane [59]. Consequently, they are often used as bactericides, such as benzalkonium chloride [60].
3.1. Antibacterial 1,3,4-Trisubstituted-1,2,3-triazolium Salts
Analogues of the functional 1,3,4-trisubstituted-1,2,3-triazolium bromide salts have demonstrated excellent antibiotic properties. In 2018, Fletcher et al. synthesized a series of 1,3,4-trisubstituted-1,2,3-triazolium bromide salts through a two-step process involving azide-alkyne cycloaddition and benzyl substitution [13]. Several of these compounds exhibited good antibacterial activities against gram-positive (G+) strains and gram-negative (G-) strains. Compound 1 demonstrated the best antibacterial activity, with minimum inhibitory concentration (MIC) values of less than 8 µM. In addition, it displayed a good inhibitory effect against yeast, with a MIC value of 8 µM against Saccharomyces cerevisiae (S. cerevisiae) (Figure 1).
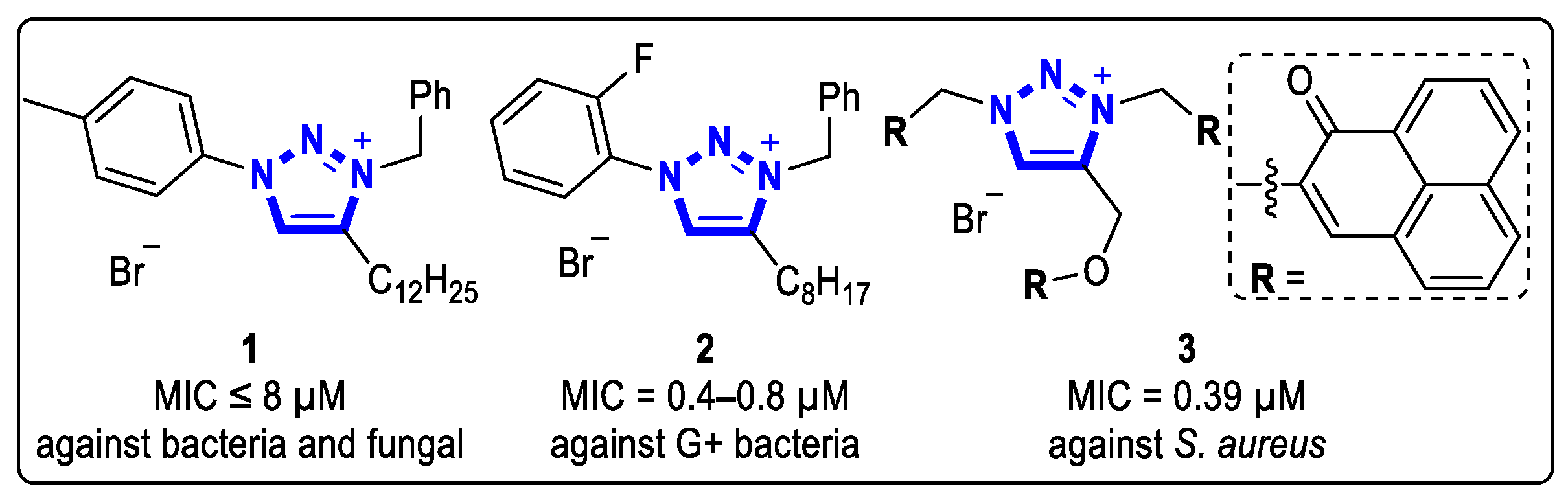
Figure 1. Structures of 1,3,4-trisubstituted 1,2,3-triazolium salts with antibacterial activities.
Four years later, they synthesized more 1,3,4-trisubstituted-1,2,3-triazolium bromide salts via click reactions [15]. Compounds with 2-fluorenyl, 1-naphthyl, 2-naphthyl, 2-anthracenyl, or 1-pyrenyl at the N-1 position of the triazolium salt exhibited emission properties and good antibacterial activities. Compound 2 showed similar inhibitory activities against G+ strains as compound 1, with MIC values of 0.4–0.8 μM (Figure 1).
In 2021, Sol and coworkers prepared a variety of 1,2,3-triazolium-containing phenolic ketone derivatives with moderate to good water solubility [14]. Among them, compound 3 exhibited good resistance to bacteria in the absence of light, and its antibacterial activity could be further increased under light irradiation through the light-induced activation of the highly conjugated R group. The MIC values of compound 3 against Staphylococcus aureus (S. aureus) CIP76.25 and Staphylococcus epidermidis (S. epidermidis) CIP109.562 under light conditions were 0.39 µM and 0.78 µM, respectively (Figure 1).
3.2. Antibacterial Cationic Anthraquinone Analogs
Anthraquinone is the structural core of anthracycline and exhibits a wide range of biological activities [17]. It has played an important role in the discovery of new biological and pharmaceutical therapeutic drugs [18]. The analogs of anthraquinone fused with 1,2,3-triazolium units are called cationic anthraquinone analogs (CAAs), which have been reported to possess good antibacterial activities [17][18][19]. These compounds are composed of several popular antibacterial scaffolds, including anthraquinone, triazolium, and QAC cations.
Since 2011, the Chang group has systematically studied the biological activities of anthraquinone derivatives derived from 1-alkyl-1H- or 2-alkyl-2H-naphtho [2,3-d]triazole-4,9-diones to search for new bactericidal molecules against drug-resistant bacteria [16][18]. They found that anthraquinone derivatives with cationic triazolium units possessed good antibacterial activities. For example, compound 4 showed good activity against S. aureus, with MIC values of 0.032 to 0.064 μg/mL (Figure 2).
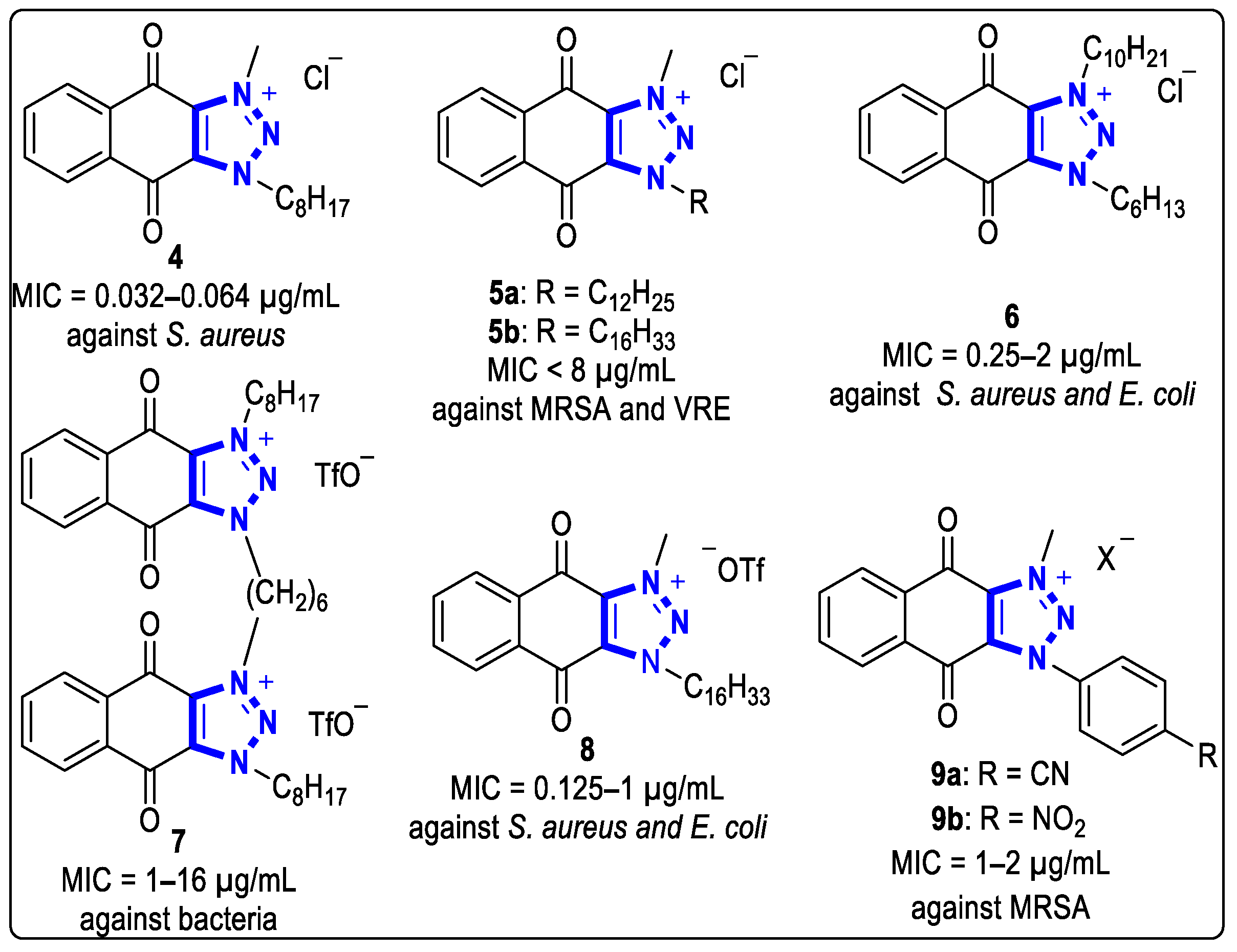
Figure 2. Structures of anthraquinone triazolium compounds with antibacterial activities.
In the same year, to clarify the effects of alkyl chain length on antibacterial activities, they synthesized linear alkyl chain anthraquinone triazolium salts with different sizes at the N-3 position and tested their activities against drug-resistant bacteria [18]. The results showed that compounds 5a and 5b had broad activities against methicillin-resistant S. aureus (MRSA) and vancomycin-resistant Enterococcus faecalis (VRE), and the MIC values were both less than 8 μg/mL (Figure 2).
Later, they used the same method to obtain a series of anthraquinone triazolium salts containing different alkyl chains connecting to the N-1 and N-3 positions. They found that compound 6 (Figure 2) had the best inhibitory activities against S. aureus and Escherichia coli (E. coli), with MIC values of 0.25 to 2 μg/mL [17].
In 2017, they developed a series of dimeric cationic anthraquinone analogs with antibacterial activities [19]. The MIC values of compound 7 against G+ bacteria and G- bacteria were 1 to 16 μg/mL. In the following year, anthraquinone triazolium compounds with different anions were also tested for antibacterial activities [20]. The MIC values of compound 8 with ¯OTf anions against S. aureus and E. coli were 0.125 to 1 μg/mL. In addition to the alkyl chain, the anthraquinone triazolium compounds connecting the aryl group at the N-3 position also showed good inhibitory activities against MRSA. The MIC values of Compounds 9a and 9b against MRSA were 1 to 2 μg/mL (Figure 2) [21].
3.3. Antibacterial 1,2,3-Triazolium-Based Peptoid Oligomers
Amphiphilic cationic peptides with N-substituted glycine showed great prospects as antimicrobial peptide mimetics. In 2018, Faure and coworkers obtained a series of 1,2,3-triazolium cationic amphiphilic peptide oligomers on a carrier [23]. Short hexamer 10 had a great inhibitory effect against G+ bacteria with MIC values of 6.3 μM and 3.1 μM against E. faecalis and S. aureus, respectively (Figure 3). Furthermore, this hexamer was found to be non-hemolytic and non-cytotoxic to Hela cells.
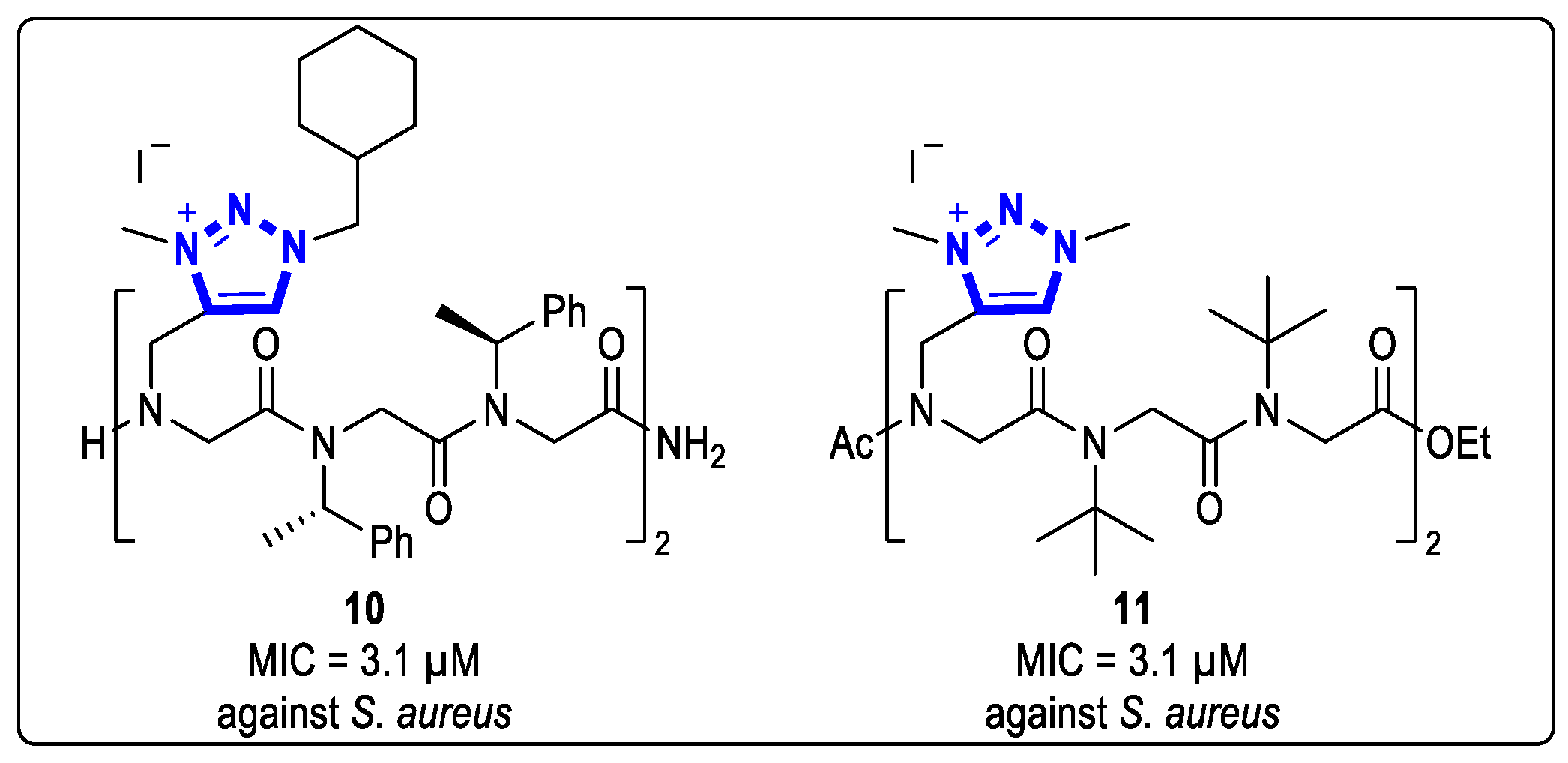
Figure 3. Structures of 1,2,3-triazolium-based peptoid oligomers with antibacterial activities.
In 2021, they reported that the hexamer 11, which contains a triazolium cationic side chain, exhibited good inhibition activities against G+ strains, with a MIC value of 3.1 μM against S. aureus CIP 6525 [24]. This hexamer was the first peptide reported to have a preventive effect on biofilm formation in P. aeruginosa and E. faecalis at sub-MIC concentrations. The hemolytic assay demonstrated that Compound 11 had good selectivity and was neither hemolytic nor cytotoxic to Hela cells, even at high concentrations (Figure 3).
3.4. Antibacterial Condensed-Heterocyclic 1,2,3-Triazolium Salts
In 1992, Yoshimura et al. synthesized a series of antibacterial compounds by transforming cephalosporins into heterocycles fused with triazolium [25]. Compound 12, which contains 1,2,3-triazolium cationic groups, showed effective antibacterial activities against S. aureus 308A-1, E. coli NIHJ JC-2, E. cloacae IFO 12937, S. marcescens IFO 12648, Proteus vugaris (P. vugaris) IFO 3988, and P. aeruginosa IFO 3455, with MIC values of less than 3.13 µg/mL (Figure 4).
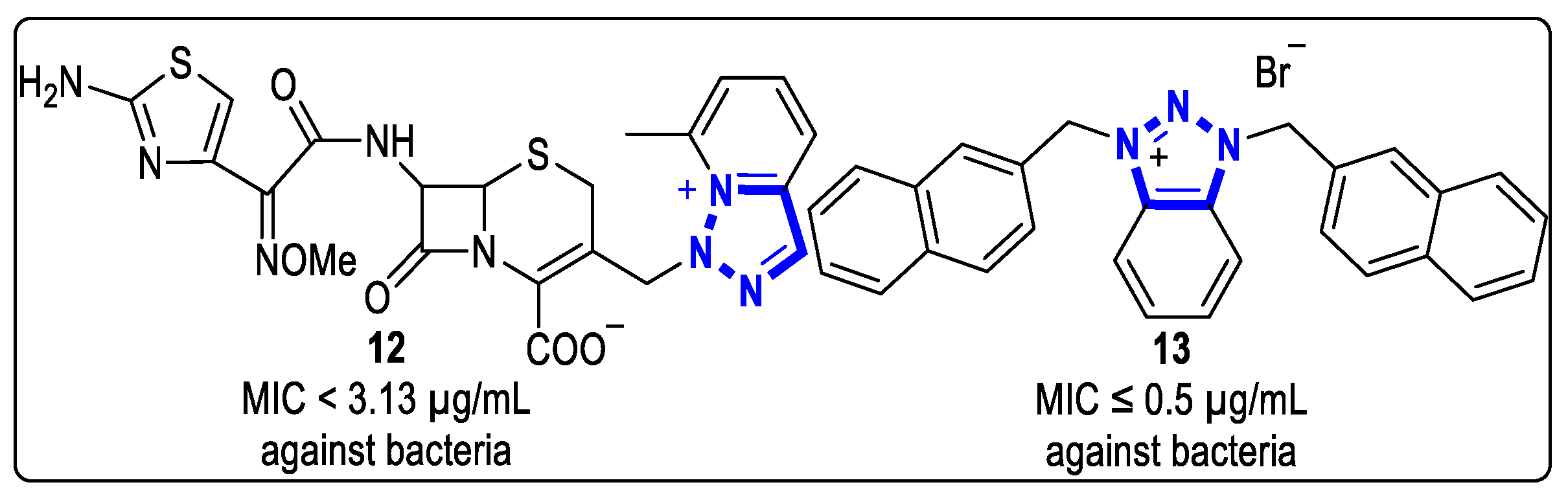
Figure 4. Structures of condensed-heterocyclic 1,2,3-triazolium salts with antibacterial activities.
Recently, Wilson et al. synthesized a series of N,N’-disubstituted triazolium salts, and tested their antibacterial activity against several bacteria, including Enterococcus faecium (E. faecium), S. aureus, Klebsiella pneumoniae (K. pneumoniae), Acinetobacter baumannii (A. baumannii), Pseudomonas aeruginosa (P. aeruginosa) and E. faecium, which are common pathogens of hospital-acquired infections [26]. Those compounds exhibited potent antibacterial properties against all bacterial strains. Specifically, compound 13 exhibited a MIC value of ≤0.5 µg/mL (Figure 4).
3.5. Antibacterial 1,2,3-Triazolium-Based Polymers
Polymers containing QACs are extensively studied as antibacterial and disinfectant agents [27][28]. The large-scale synthesis of the polymers with QACs can be cost-effective compared with antimicrobial peptides and antibiotics [27]. The antibacterial activity and blood toxicities of copolymers are significantly influenced by their molecular weight distribution, cationic property, density, and chemical composition [27][28][29][30][31].
In 2015, Tejero et al. prepared polys (methyl methacrylate) using alkynyl alcohols and thiazole azide derivatives as raw materials [27]. The N-alkylation of the azole ring allowed the preparation of unipolar and polar polyelectrolytes with different amphiphilic properties, resulting in their antibacterial activity against various microorganisms. Compound 14, for example, showed significant antibacterial activities, with MIC values less than 10 µg/mL against P. aeruginosa, S. aureus, S. epidermidis, and MRSA (Figure 5).
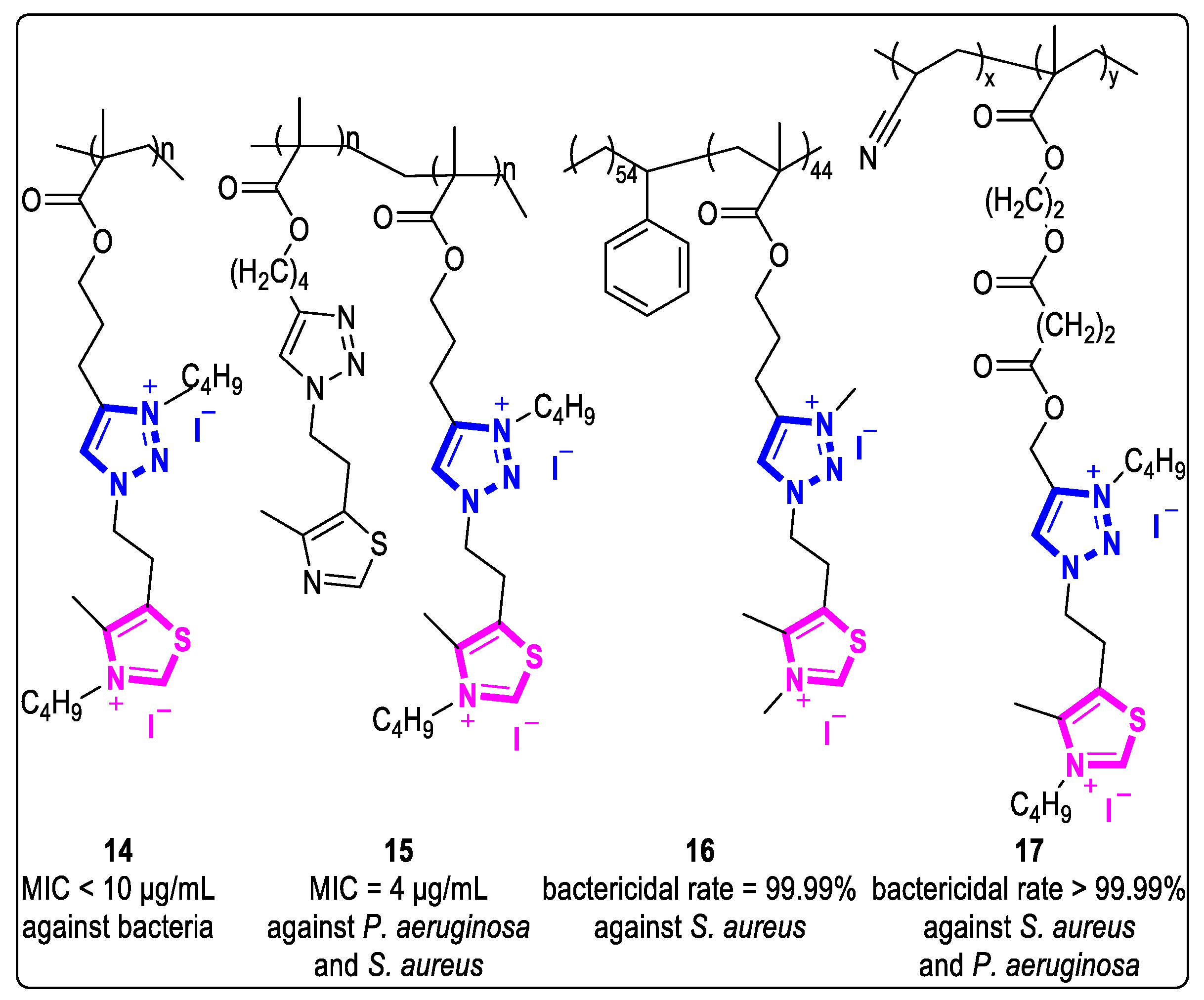
Figure 5. Structures of 1,2,3-triazolium-based polymers with antibacterial activities.
In the same year, they also prepared a series of compounds with amphiphilic quaternary ammonium salts through controlled quaternization of triazolium side chains by polymethacrylates [28]. The MIC values of compound 15 against P. aeruginosa and S. aureus were 4 µg/mL at 100% quaternization. Meanwhile, compound 15 with 50% quaternization can also kill 100% of bacteria within 5 min at 2 × MIC. Interestingly, these polymers with a low degree of quaternization still exhibited strong and rapid bactericidal behavior, possibly due to the synergistic effect between the unquaternized heterocyclic and the quaternized heterocyclic (Figure 5).
In 2017, Muñoz-Bonilla et al. prepared a cationic copolymer containing thiazole and triazole groups [29]. They blended it with commercial polystyrene by a simple spin coating method to obtain a series of contact-type active antibacterial films. These blended films exhibited significant microbicidal activity against both G+ and G- bacteria, as well as fungi. At 30% and 50% PS/copolymer content, the killing efficiency of the blended film 16 exceeded 99.99%. In 2018, the researchers reported that even a small amount (3~9 wt %) of PS/copolymer breath figure films prepared using the breath figures approach still had considerable antibacterial effects against G+ bacteria, such as S. aureus and C. parapsilonosis [30]. The breath figure film 16 was able to kill over 90% of the cells from both bacteria through surface contact (Figure 5).
In 2018, Fernández-García and coworkers synthesized a series of copolymers with quaternary ammonium salt groups that exhibited contact active antibacterial properties [31]. These copolymers were made using quaternary ammonium salts and polyacrylonitrile as raw materials. Compound 17 has extremely strong bactericidal activities against S. aureus and P. aeruginosa, with cell-killing rates of over 99.99% (Figure 5).
3.6. Antibacterial 1,2,3-Triazolium-Based Complex
In 2022, Muñoz-Bonilla and coworkers synthesized compound 18 via free radical polymerization and click reaction with a hydantoin moiety (Figure 6). It underwent further N-alkylation and chlorination reactions to form triazolium salt polylactide-based fibers with antibacterial activity. Polylactide-based fiber 18 containing cationic triazolium and N-haloamine groups showed good antibacterial activities against both G+ and G- bacteria [32].
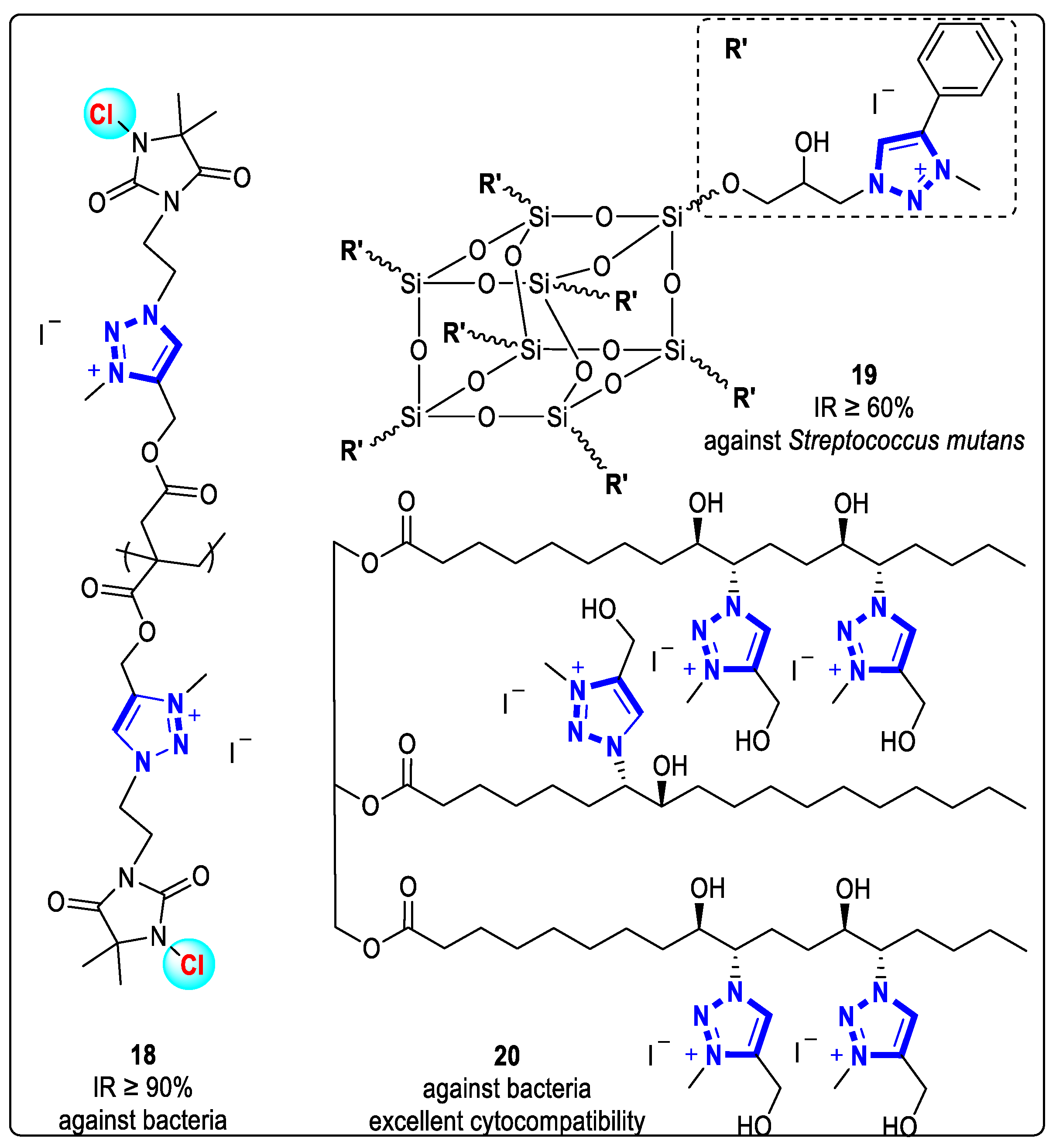
Figure 6. Structures of 1,2,3-triazolium-based complex with antibacterial activities.
To minimize the decreasing effect of ionic bactericidal compounds on the mechanical strength of dental composites, Yeganeh et al. (2017) prepared bactericidal dental nanocomposites containing 1,2,3-triazolium-based functionalized polyhedral oligomeric silsesquioxane (POSS) additives through thiol-one click polymerization. The addition of the triazolium cation resulted in increased bactericidal activity of complex 19 (Figure 6) compared to similar compositions containing dimethyl aminoethyl methacrylate monomers (DMAEMA-BC). The inhibition rate (IR) of complex 19 against Streptococcus pyogenes (S. pyogenes) was above 60% [33].
Compound 20, an antimicrobial polyurethane wound dressing film, was prepared through an N-alkylation reaction between 1,2,3-triazolium functional soybean oil (TSBO) and methyl iodide (Figure 6). The introduction of 1,2,3-triazolium cation groups into the dressing skeleton increased their antibacterial activity against a range of bacteria. Appropriate concentrations of these cationic groups still maintained the membrane’s good cytocompatibility with dermal fibroblast [34].
3.7. Structure-Activity Relationship Analysis of Antibacterial 1,2,3-Triazolium
The cationic properties of triazolium salts are necessary for their antibacterial efficacy. Compounds 1 and 2 have good antibacterial activity, whereas their parent 1,2,3-triazole analogs have no significant antibacterial activity with a MIC value of ≥250 µM [13][14]. The phenolic ketone group in compound 3 is a nonpolar component, which can reduce the hydrophilicity of the triazolium group. There is a balance between the lipophilicity and hydrophilicity of compound 3, which supports good inhibitory activity against G+ bacteria [15]. However, compound 3 has poor activity against G- bacteria. It is caused by the presence of an outer layer of lipopolysaccharide that hinders the penetration of hydrophobic compounds [15]. Connecting a hydrophobic group at the N-3 and C-4 positions of 1,2,3-triazolium salts is beneficial to the resistance of G+ bacteria. However, excessive increases in hydrophobicity (lipophilicity) will make it more difficult for compounds to pass through bacterial cell membranes, resulting in decreased antibacterial activity (Figure 7).
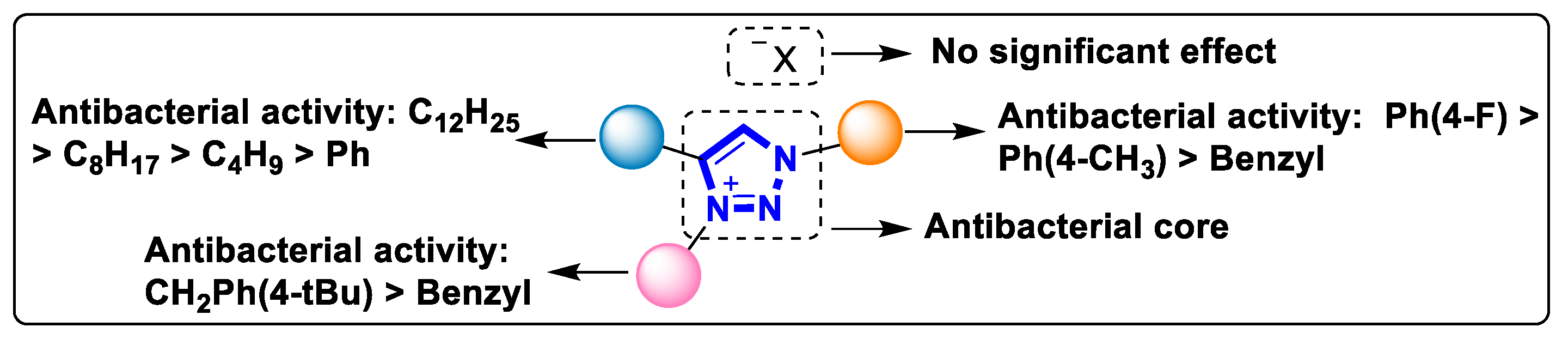
Figure 7. The summarized structure–activity relationship of antibacterial 1,2,3-triazolium.
Therefore, the maximum effectiveness of 1,2,3-triazolium salts against G- bacteria requires the connection of a sufficient but not excessive hydrophobic group. Furthermore, a decrease in hydrophobicity at one substituent position can be compensated by an increase in hydrophobicity at other substituent positions. For example, connecting alkyl chains with different carbon chain lengths at different sites in compounds 5 and 6 maintains their antibacterial effectiveness by maintaining a balance in the overall hydrophobicity of the compounds [17][18].
References
- Xie, M.; Tang, H.; Zhang, Y.; Guo, Z.; Guo, H.; Qu, G. A new method for the synthesis of alkyl-substituted 1,2,3-triazole compounds. Chin. J. Org. Chem. 2015, 35, 2589–2594.
- Weiss, Z.F.; Little, J.; Hammond, S. Evolution of antifungals for invasive mold infections in immunocompromised hosts, then and now. Expert Rev. Anti-Infect. Ther. 2023, 21, 535–549.
- Aizpurua, J.M.; Fratila, R.M.; Monasterio, Z.; Pérez-Esnaola, N.; Andreieff, E.; Irastorzaa, A.; Sagartzazu-Aizpuruaa, M. Triazolium cations: From the “click” pool to multipurpose applications. New J. Chem. 2014, 38, 474–480.
- Yacob, Z.; Liebscher, J. 1,2,3-Triazolium salts as a versatile new class of ionic liquids. In Ionic Liquids; Handy, S.T., Ed.; IntechOpen: Rijeka, Germany, 2011; Volume 1, pp. 3–23.
- Li, Q.; Qiu, L.; Tan, W.; Gu, G.; Guo, Z. Novel 1,2,3-triazolium-functionalized inulin derivatives: Synthesis, free radical-scavenging activity, and antifungal activity. RSC Adv. 2017, 7, 42225–42232.
- Tan, W.; Li, Q.; Dong, F.; Qiu, S.; Zhang, J.; Guo, Z. Novel 1,2,3-triazolium-functionalized starch derivatives: Synthesis, characterization, and evaluation of antifungal property. Carbohydr. Polym. 2017, 160, 163–171.
- Tan, W.; Li, Q.; Gao, Z.; Qiu, S.; Dong, F.; Guo, Z. Design, synthesis of novel starch derivative bearing 1,2,3-triazolium and pyridinium and evaluation of its antifungal activity. Carbohydr. Polym. 2017, 157, 236–243.
- Tan, W.; Zhang, J.; Luan, F.; Wei, L.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and antifungal evaluation of novel 1,2,3-triazolium-functionalized starch derivative. Int. J. Biol. Macromol. 2017, 101, 845–851.
- Tan, W.; Li, Q.; Dong, F.; Zhang, J.; Luan, F.; Wei, L.; Chen, Y.; Guo, Z. Novel cationic chitosan derivative bearing 1,2,3-triazolium and pyridinium: Synthesis, characterization, and antifungal property. Carbohydr. Polym. 2018, 182, 180–187.
- Steiner, I.; Stojanovic, N.; Bolje, A.; Brozovic, A.; Polancec, D.; Ambriovic-Ristov, A.; Stojkovic, M.R.; Piantanida, I.; Eljuga, D.; Kosmrlj, J.; et al. Discovery of ‘click’ 1,2,3-triazolium salts as potential anticancer drugs. Radiol. Oncol. 2016, 50, 280–288.
- Rokitskaya, T.I.; Khailova, L.S.; Makarenkov, A.V.; Shunaev, A.V.; Tatarskiy, V.V.; Shtil, A.A.; Ol’shevskaya, V.A.; Antonenko, Y.N. Carborane derivatives of 1,2,3-triazole depolarize mitochondria by transferring protons through the lipid part of membranes. Biochim. Biophys. Acta Biomembr. 2019, 1861, 573–583.
- Almeida, A.C.; Meinel, R.S.; Leal, Y.L.; Silva, T.P.; Glanzmann, N.; Mendonca, D.V.C.; Perin, L.; Cunha-Júnior, E.F.; Coelho, E.A.F.; Melo, R.C.N.; et al. Functionalized 1,2,3-triazolium salts as potential agents against visceral leishmaniasis. Parasitol. Res. 2022, 121, 1389–1406.
- Fletcher, J.T.; Sobczyk, J.M.; Gwazdacz, S.C.; Blanck, A.J. Antimicrobial 1,3,4-trisubstituted-1,2,3-triazolium salts. Bioorg. Med. Chem. Lett. 2018, 28, 3320–3323.
- Godard, J.; Gibbons, D.; Leroy-Lhez, S.; Williams, R.M.; Villandier, N.; Ouk, T.S.; Brégier, F.; Sol, V. Development of phenalenone-triazolium salt derivatives for aPDT: Synthesis and antibacterial screening. Antibiotics 2021, 10, 626.
- Lejcher, C.A.; Villa, E.M.; Fletcher, J.T. Merging antimicrobial and visible emission properties within 1,3,4-trisubstituted-1,2,3-triazolium salts. Med. Chem. Res. 2022, 31, 474–484.
- Chan, K.Y.; Zhang, J.; Chang, C.W. Mode of action investigation for the antibacterial cationic anthraquinone analogs. Bioorg. Med. Chem. Lett. 2011, 21, 6353–6356.
- Fosso, M.Y.; Chan, K.Y.; Gregory, R.; Chang, C.W. Library synthesis and antibacterial investigation of cationic anthraquinone analogs. ACS Comb. Sci. 2012, 14, 231–235.
- Zhang, J.; Redman, N.; Litke, A.P.; Zeng, J.; Zhan, J.; Chan, K.Y.; Chang, C.W.T. Synthesis and antibacterial activity study of a novel class of cationic anthraquinone analogs. Bioorg. Med. Chem. 2011, 19, 498–503.
- Shrestha, J.P.; Baker, C.; Kawasaki, Y.; Subedi, Y.P.; Vincent de Paul, N.N.; Takemoto, J.Y.; Chang, C.T. Synthesis and bioactivity investigation of quinone-based dimeric cationic triazolium amphiphiles selective against resistant fungal and bacterial pathogens. Eur. J. Med. Chem. 2017, 126, 696–704.
- Subedi, Y.P.; Alfindee, M.N.; Shrestha, J.P.; Chang, C.T. Tuning the biological activity of cationic anthraquinone analogues specifically toward Staphylococcus aureus. Eur. J. Med. Chem. 2018, 157, 683–690.
- Subedi, Y.P.; Chang, C.T. Cationic anthraquinone analogs as selective antimicrobials. Microbiol. Insights 2019, 12, 1178636119847809.
- Nziko, V.P.N.; Fosso, M.Y.; Chang, C.-W.T. Quantitative structure–activity relationship analysis of antibacterial cationic anthraquinone analogs using Hansch and Fujita models. Med. Chem. Res. 2014, 23, 5058–5062.
- Shyam, R.; Charbonnel, N.; Job, A.; Blavignac, C.; Forestier, C.; Taillefumier, C.; Faure, S. 1,2,3-Triazolium-based cationic amphipathic peptoid oligomers mimicking antimicrobial helical peptides. ChemMedChem 2018, 13, 1513–1516.
- Shyam, R.; Forestier, C.; Charbonnel, N.; Roy, O.; Taillefumier, C.; Faure, S. Solution-phase synthesis of backbone-constrained cationic peptoid hexamers with antibacterial and anti-biofilm activities. Eur. J. Org. Chem. 2021, 2021, 5813–5822.
- Yoshimura, Y.; Tomimatsu, K.; Nishimura, T.; Miyake, A.; Hashimoto, N. Studies on condensed-heterocyclic azolium cephalosporins. V. Synthesis and antibacterial activity of 3-(condensed-triazolo-pyridinium, -pyrimidinium, and -pyridazinium)-methyl cephalosporins. J. Antibiot. 1992, 45, 721–734.
- Wilson, J.A.; Lin, Z.J.; Rodriguez, I.; Ta, T.; Martz, L.; Fico, D.; Johnson, S.S.; Gorden, J.D.; Shelton, K.L.; King, L.B. Synthesis, characterization, and antimicrobial activity of lipophilic N,N’-bis-substituted triazolium salts. J. Heterocycl. Chem. 2021, 59, 577–587.
- Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. Antimicrobial polymethacrylates based on quaternized 1,3-thiazole and 1,2,3-triazole side-chain groups. Polym. Chem. 2015, 6, 3449–3459.
- Tejero, R.; Lopez, D.; Lopez-Fabal, F.; Gomez-Garces, J.L.; Fernandez-Garcia, M. High efficiency antimicrobial thiazolium and triazolium side-chain polymethacrylates obtained by controlled alkylation of the corresponding azole derivatives. Biomacromolecules 2015, 16, 1844–1854.
- Cuervo-Rodriguez, R.; Lopez-Fabal, F.; Gomez-Garces, J.L.; Munoz-Bonilla, A.; Fernandez-Garcia, M. Contact active antimicrobial coatings prepared by polymer blending. Macromol. Biosci. 2017, 17, 1700258.
- Munoz-Bonilla, A.; Cuervo-Rodriguez, R.; Lopez-Fabal, F.; Gomez-Garces, J.L.; Fernandez-Garcia, M. Antimicrobial porous surfaces prepared by breath figures approach. Materials 2018, 11, 1266.
- Tejero, R.; Gutierrez, B.; Lopez, D.; Lopez-Fabal, F.; Gomez-Garces, J.L.; Munoz-Bonilla, A.; Fernandez-Garcia, M. Tailoring macromolecular structure of cationic polymers towards efficient contact active antimicrobial surfaces. Polymers 2018, 10, 241.
- Chiloeches, A.; Cuervo-Rodriguez, R.; Gil-Romero, Y.; Fernandez-Garcia, M.; Echeverria, C.; Munoz-Bonilla, A. Electrospun polylactic acid-Based fibers loaded with multifunctional antibacterial biobased polymers. ACS Appl. Polym. Mater. 2022, 4, 6543–6552.
- Burujeny, S.B.; Yeganeh, H.; Atai, M.; Gholami, H.; Sorayya, M. Bactericidal dental nanocomposites containing 1,2,3-triazolium-functionalized POSS additive prepared through thiol-ene click polymerization. Dent. Mater. 2017, 33, 119–131.
- Gholami, H.; Yeganeh, H.; Burujeny, S.B.; Sorayya, M.; Shams, E. Vegetable oil based polyurethane containing 1,2,3-triazolium functional groups as antimicrobial wound dressing. J. Polym. Environ. 2017, 26, 462–473.
- Khazhieva, I.S.; Demkin, P.M.; Eltsov, O.S.; Nein, Y.I.; Kalinina, T.A.; Fan, Z.-J.; Chen, L.; Zhao, B.; Glukhareva, T.V.; Morzherin, Y.Y. Synthesis and fungicidal activity of monocyclic and fused 1,2,3-triazolium-5-olates. Chem. Heterocycl. Compd. 2018, 54, 956–963.
- Souza-Fagundes, E.M.; Delp, J.; Prazeres, P.D.M.; Marques, L.B.; Carmo, A.M.L.; Stroppa, P.H.F.; Glanzmann, N.; Kisitu, J.; Szamosvari, D.; Böttcher, T.; et al. Correlation of structural features of novel 1,2,3-triazoles with their neurotoxic and tumoricidal properties. Chem. Biol. Interact. 2018, 291, 253–263.
- Ramos, J.P.; Abdel-Salam, M.A.L.; Nobre, D.A.B.; Glanzmann, N.; de Souza, C.P.; Leite, E.A.; de Abreu Teles, P.P.; Barbosa, A.S.; Barcelos, L.S.; Dos Reis, D.C.; et al. Acute toxicity and antitumor potential of 1,3,4-trisubstituted-1,2,3-triazole dhmtAc-loaded liposomes on a triple-negative breast cancer model. Arch. Pharm. 2022, 355, e2200004.
- Shrestha, J.P.; Chang, C.W. Safe and easy route for the synthesis of 1,3-dimethyl-1,2,3-triazolium salt and investigation of its anticancer activities. Bioorg. Med. Chem. Lett. 2013, 23, 5909–5911.
- Shrestha, J.P.; Subedi, Y.P.; Chen, L.; Chang, C.W.T. A mode of action study of cationic anthraquinone analogs: A new class of highly potent anticancer agents. MedChemComm 2015, 6, 2012–2022.
- Wang, R.; Li, Y.; Dehaen, W. Antiproliferative effect of mitochondria-targeting allobetulin 1,2,3-triazolium salt derivatives and their mechanism of inducing apoptosis of cancer cells. Eur. J. Med. Chem. 2020, 207, 112737.
- Riela, S.; Massaro, M.; Colletti, C.G.; Bommarito, A.; Giordano, C.; Milioto, S.; Noto, R.; Poma, P.; Lazzara, G. Development and characterization of co-loaded curcumin/triazole-halloysite systems and evaluation of their potential anticancer activity. Int. J. Pharm. 2014, 475, 613–623.
- Massaro, M.; Colletti, C.G.; Noto, R.; Riela, S.; Poma, P.; Guernelli, S.; Parisi, F.; Milioto, S.; Lazzara, G. Pharmaceutical properties of supramolecular assembly of co-loaded cardanol/triazole-halloysite systems. Int. J. Pharm. 2015, 478, 476–485.
- Stroppa, P.H.F.; Antinarelli, L.M.R.; Carmo, A.M.L.; Gameiro, J.; Coimbra, E.S.; da Silva, A.D. Effect of 1,2,3-triazole salts, non-classical bioisosteres of miltefosine, on Leishmania amazonensis. Bioorg. Med. Chem. 2017, 25, 3034–3045.
- Meinel, R.S.; Almeida, A.D.C.; Stroppa, P.H.F.; Glanzmann, N.; Coimbra, E.S.; da Silva, A.D. Novel functionalized 1,2,3-triazole derivatives exhibit antileishmanial activity, increase in total and mitochondrial-ROS and depolarization of mitochondrial membrane potential of Leishmania amazonensis. Chem. Biol. Interact. 2020, 315, 108850.
- Lucio, H.; Revuelto, A.; Carriles, A.A.; de Castro, S.; Garcia-Gonzalez, S.; Garcia-Soriano, J.C.; Alcon-Calderon, M.; Sanchez-Murcia, P.A.; Hermoso, J.A.; Gago, F.; et al. Identification of 1,2,3-triazolium salt-based inhibitors of Leishmania infantum trypanothione disulfide reductase with enhanced antileishmanial potency in cellulo and increased selectivity. Eur. J. Med. Chem. 2022, 244, 114878.
- Martín-Montes, Á.; Ballesteros-Garrido, R.; Martín-Escolano, R.; Marín, C.; Guitiérrez-Sánchez, R.; Abarca, B.; Ballesteros, R.; Sanchez-Moreno, M. Synthesis and in vitro leishmanicidal activity of novel triazolopyridine salts. RSC Adv. 2017, 7, 15715–15726.
- Peiteado, Z.M. Synthesis, Applications and Reactivity of 1,2,3-Triazolium Salts. Ph.D. Thesis, University of the Basque Country-UPV/EHU, Bilbao, Spain, 2015.
- Yacob, Z.; Liebscher, J. Chemistry of 1,2,3-triazolium salts. In Chemistry of 1,2,3-Triazoles; Dehaen, W., Bakulev, V.A., Eds.; Springer International Publishing: Cham, Germany, 2015; Volume 40, pp. 167–210.
- Huang, D.; Zhao, P.; Astruc, D. Catalysis by 1,2,3-triazole- and related transition-metal complexes. Coord. Chem. Rev. 2014, 272, 145–165.
- Mishra, R.; Mishra, J.S.; Chaubey, S.A. Recent advances on ttriazolium iionic liquids: Synthesis and applications. Curr. Org. Chem. 2019, 23, 1239–1255.
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021.
- Maity, R.; Sarkar, B. Chemistry of compounds based on 1,2,3-triazolylidene-type mesoionic carbenes. J. Am. Chem. Soc. 2022, 2, 22–57.
- Monasterio, Z.; Irastorza, A.; Miranda, J.I.; Aizpurua, J.M. Site-selective N-dealkylation of 1,2,3-triazolium salts: A metal-free route to 1,5-substituted 1,2,3-triazoles and related bistriazoles. Org. Lett. 2016, 18, 2511–2514.
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. 1963, 2, 565–598.
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 14.
- Purnell, L.G.; Shepherd, J.C.; Hodgson, D.J. Interaction of metal ions with 8-azapurines. synthesis and structure of tetrachlorobis-2triazole]copper(II) monohydrate. J. Am. Chem. Soc. 1974, 97, 9–13.
- Ma, J.; Ding, S. Transition metal-catalyzed cycloaddition of azides with internal alkynes. Asian J. Org. Chem. 2020, 9, 1872–1888.
- Boren, B.C.; Narayan, S.; Rasmussen, L.K.; Zhang, L.; Zhao, H.; Lin, Z.; Jia, G.; Fokin, V.V. Ruthenium-catalyzed azide-alkyne cycloaddition: Scope and mechanism. J. Am. Chem. Soc. 2008, 130, 8923–8930.
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary ammonium compounds (QACs) and ionic liquids (ILs) as biocides: From simple antiseptics to tunable antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793.
- Sakarya, F.K.; Haznedaroglu, B.Z.; Tezel, U. Biological removal of benzalkonium chlorides from wastewater by immobilized cells of Pseudomonas sp. BIOMIG1 in an up-flow packed bed reactor. J. Hazard. Mater. 2021, 418, 126210.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
874
Revisions:
3 times
(View History)
Update Date:
17 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




