Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ramón Román-Doval | -- | 1221 | 2023-07-14 02:53:21 | | | |
| 2 | Wendy Huang | Meta information modification | 1221 | 2023-07-14 06:36:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. General Characteristics of Chitosan. Encyclopedia. Available online: https://encyclopedia.pub/entry/46793 (accessed on 08 February 2026).
Román-Doval R, Torres-Arellanes SP, Tenorio-Barajas AY, Gómez-Sánchez A, Valencia-Lazcano AA. General Characteristics of Chitosan. Encyclopedia. Available at: https://encyclopedia.pub/entry/46793. Accessed February 08, 2026.
Román-Doval, Ramón, Sandra P. Torres-Arellanes, Aldo Y. Tenorio-Barajas, Alejandro Gómez-Sánchez, Anai A. Valencia-Lazcano. "General Characteristics of Chitosan" Encyclopedia, https://encyclopedia.pub/entry/46793 (accessed February 08, 2026).
Román-Doval, R., Torres-Arellanes, S.P., Tenorio-Barajas, A.Y., Gómez-Sánchez, A., & Valencia-Lazcano, A.A. (2023, July 14). General Characteristics of Chitosan. In Encyclopedia. https://encyclopedia.pub/entry/46793
Román-Doval, Ramón, et al. "General Characteristics of Chitosan." Encyclopedia. Web. 14 July, 2023.
Copy Citation
Chitosan is a naturally occurring compound that can be obtained from deacetylated chitin, which is obtained from various sources such as fungi, crustaceans, and insects. Commercially, chitosan is produced from crustaceans. Based on the range of its molecular weight, chitosan can be classified into three different types, namely, high molecular weight chitosan (HMWC, >700 kDa), medium molecular weight chitosan (MMWC, 150–700 kDa), and low molecular weight chitosan (LMWC, less than 150 kDa).
chitosan
crustaceans
functional groups
particle size
molecular weight
crystalline structure
level of deacetylation
surface area
1. The Structure and Natural Origins of Chitosan
Chitosan is a linear polymer composed of two subunits, D-glucosamine and N-acetyl-D-glucosamine, that are joined by 1,4-glycosidic bonds. The three rings of the chitosan molecule make up its overall structure (Figure 1). It has three functional groups including amino groups, primary and secondary hydroxyl groups, which make its chemical modification easy. Additionally, these functional groups influence chitosan’s solubility and mechanical properties. Furthermore, chitosan possesses -1,4 glycosidic linkages. In acidic aqueous environments, chitosan is more soluble than chitin. The primary reason for chitosan’s solubility is the protonation of –NH2 at the C–1 position of the D-glucosamine repeat unit, which transforms the polysaccharide into a polyelectrolyte under acidic conditions. Due to its solubility properties, nearly all areas of human life and health (agriculture, medicine, process engineering, and industries) can benefit from using chitosan. Chitosan can be directly extracted from a variety of fungus; it can also be produced by extracting chitin and then passing it through a deacetylation process. In the fungal kingdom, chitin is a polymer that is made more frequently than chitosan, being produced by zygomycetes, ascomycetes, basidomycetes, deuteromycetes and phycomycetes. In contrast, chitosan is exclusively found in the cell walls of a few taxa of fungi, particularly, zygomycetes. Extraction of chitosan from fungal biomass is greatly advantageous as it can be done at any time and is not prone to seasonal changes. A process similar to that used to extract chitosan from crustaceans is used to extract chitin from fungi whose cell walls are the sole source of it. Therefore, using only fungi that currently produce the desired product is more cost effective [1]. Another very important and abundant source of chitin comes from the exoskeletons of crustaceans; mainly from a variety of marine crustaceans such as shrimp, crabs, and lobsters. Currently, the main source of chitin comes from waste from the shrimp industry, where exoskeletons are obtained to obtain chitin and calcium. Chitosan is obtained through a chemical process of N-deacetylation, where the cationic nature of chitosan is owed to the free amino group left by the partial removal of the acetyl group of chitin [2]. The diverse sources of chitosan and chitin are shown in Table 1.
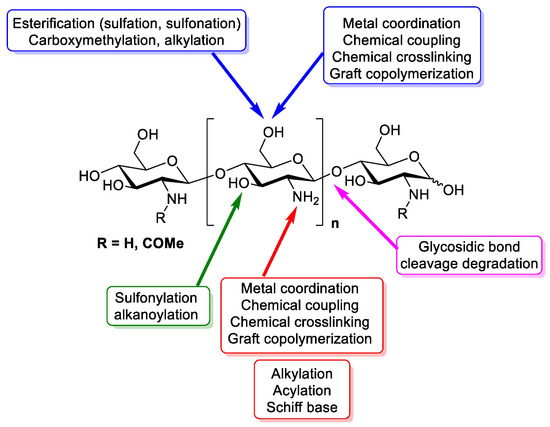
Figure 1. Design of chitin and chitosan structure, chemistry and functional groups that are able to be modified [3].
Table 1. Main origins of chitosan or chitin in both terrestrial and marine organisms [4].
| Marine |
|---|
| Sellfish |
| Crab |
| Chionoecetes opilio [5] |
| Podophthalmus vigil [6] |
| Paralithodes camtschaticus [7] |
| Carcinus mediterraneus [8] |
| Water lobster |
| Crawfish [9] |
| Shrimp |
| Aristens antennatus [10] |
| Krill |
| Daphnia longispina [11] |
| Anax imperator [12] |
| Hyrophilus piceus [12] |
| Notonecta glauca [12] |
| Agabus bipustulatus [12] |
| Asellus aquaticus [12] |
| Molluscs |
| Squid pens |
| Loligo sp. [13] |
| Todarodes pacificus [14] |
| Terrestrial |
| Arthropods |
| Spyders |
| Geolycosa vultuosa [15] |
| Nephila edulis [15] |
| Scorpions |
| Mesobuthus gibbosus [16] |
| Beetles |
| Bombyx mori [17] |
| Holotrichia parallela [18] |
| Leptinotarsa decemlineata [19] |
| Cockroaches [20] |
| Brachiopods |
| Lingula seta [21] |
| Microorganisms |
| Fungus (cell walls) |
| Ascomydes |
| Mucor rouxii [22] |
| Blastomycota |
| Blastocladiaceae [23] |
| Chytridiomycota |
| Chytridiaceae |
| Protista |
| Brown algae [24] |
| Plantae |
| Green algae [24] |
2. The Primary Properties That Determine the Efficacy and Scope of Applications for Chitosan
The source and method of extraction have a direct impact on the properties of chitosan. The primary properties that determine the efficacy and scope of applications for chitosan include particle size, molecular weight, crystalline structure, level of deacetylation, and surface area.
The molecular weight (MW) of chitosan depends on how many monomeric units are in the biopolymer. Viscosity and solubility are two properties that are impacted by MW, thus their control, assessment, and modification are crucial. The MW of chitosan typically varies from 20 to 1200 kDa [25]. It can be determined using light scattering and high-performance liquid chromatography, but the most popular and straightforward method is the viscosimetric method.
The deacetylation degree (DD) is another important factor that influences the characteristics and applications of chitosan. The relation between units of 2-acetamido-2-deoxy-D-glucopyranose is known as DD. When the biopolymer only contains monomeric forms of 2-amino-2-deoxy-D-glucopyranose, the degree of deacetylation is 100 percent, and the biopolymer is totally deacetylated. When the percentage of 2-amino-2-deoxy-D-glucopyranose units reaches 50%, the polymer is typically known as chitosan and turns soluble in aqueous acidic environment. The properties and uses of chitosan are influenced by the deacetylation degree, much like MW [26].
Crystallinity, a property of chitosan, reflects the proportion of the biopolymer’s crystalline and its amorphous fractions. This property is measured by the crystallinity index (CI). Chitosan is a polymorphic biopolymer and is semi-crystalline in its solid form. It has an orthorhombic unit cell with two antiparallel chains without water molecules. The source and preparation methods influence the crystallinity of chitosan. The highest value of crystallinity is shared by chitin (0% deacetylated) and 100% fully deacetylated chitosan. Quantifying CI is essential since it influences how chitosan swells as well as its porosity, water absorption and moisture retention. The relation between X-ray diffraction characteristic peaks can be used to determine CI [27].
The surface area and size of particles are two of chitosan’s most crucial characteristics. The porosity of chitosan is related to the pore size distribution and volume of its pores, which in turn depend on the source and extraction process. Due to its nonporous nature, chitosan flake or powder has a surface area less than 10 m2/g. In most applications, particles lower than 1 mm is often used. Chitosan applications like adsorption and enzyme immobilization require several accessible sites and a porous structure; therefore, surface area and particle size are crucial factors. The nonporous nature of chitosan necessitates several modifications to increase its surface area. In fact, precise measurement of the size of the particles and its surface area is crucial. Nitrogen adsorption–desorption isotherms using the BET method are typically used to determine surface area. Particle size can be determined using a particle analyzer, sieving tests, or scanning electron microscopy [28].
Due to the diversity associated with its chemical structure, chitosan is a very attractive compound. Two methods to express this diversity are the molecular weight, which ranges from oligo-chitosan to high, medium, or low molecular weight, and DD, which ranges from acetylated chitosan to partially deacetylated chitin [29]. Three basic types of chitosan may be categorized according to their molecular weight ranges: low molecular, weight chitosan (LMWC, less than 150 kDa), medium molecular weight chitosan (MMWC, 150–700 kDa), and high molecular weight chitosan (HMWC, >700 kDa) [30]. The preparation process and raw material sources influence chitosan’s molecular weight. Native chitin typically has a molecular weight of over 1,000,000 Da, whereas industrial chitosan products typically range from 100,000 to 1,200,000 Da. Generally, shear stress, dissolved oxygen, and high temperatures may all cause chitosan to deteriorate. For instance, chitosan undergoes heat degradation at temperatures higher than 280 °C, which causes polymer chains to break down quickly and reduce the molecular weight. Additionally, when EDTA is used, maximum depolymerization brought on by the use of strong or high-temperature acids such sulfuric acid, acetic acid, and hydrochloric acid leads to molecular weight alterations with little degradation. Chromatography, light scattering, and viscosimetry are some of the techniques that can measure chitosan’s molecular weight [31].
References
- Sharif, R.; Mujtaba, M.; Ur Rahman, M.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The Multifunctional Role of Chitosan in Horticultural Crops; A review. Molecules 2018, 23, 872.
- Dima, J.B.; Sequeiros, C.; Zaritzky, N. Chitosan from marine crustaceans: Production, characterization and applications. In Biological Activities and Application of Marine Polysaccharides; IntechOpen: London, UK, 2017; pp. 39–56.
- Aranaz, I.; Alcántara, A.; Civera, M.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256.
- Crespo, M.; Martínez, M.; Hernández, J.; Lage Yusty, M. High-performance liquid chromatographic determination of chitin in the snow crab, Chionoecetes opilio. J. Chromatogr. 2006, 1116, 189–192.
- Sunita, D. Extraction of Chitin from Trash Crabs (Podophthalmus vigil) by an Eccentric Method. Curr. Res. J. Biol. Sci. 2010, 2, 72–75.
- Sperstad, S.; Haug, T.; Paulsen, V.; Rode, T.; Strandskog, G.; Solem, S.; Styrvold, B.; Stensvåg, K. Characterization of crustins from the hemocytes of the spider crab, Hyas araneus, and the red king crab, Paralithodes camtschaticus. Dev. Comp. Immunol. 2009, 33, 583–591.
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014, 65, 298–306.
- Abdou, E.; Nagy, K.; Elsabee, M. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367.
- Mahlous, M.; Tahtat, D.; Benamer, S.; Khodja, A. Gamma irradiation-aided chitin/chitosan extraction from prawn shells. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 265, 414–417.
- Kaya, M.; Cakmak, Y.; Baran, T.; Asan-Ozusaglam, M.; Menteş, A.; Tozak, K. New chitin, chitosan, and O-carboxymethyl chitosan sources from resting eggs of Daphnia longispina (Crustacea); with physicochemical characterization, and antimicrobial and antioxidant activities. Biotechnol. Bioprocess Eng. 2014, 19, 58–69.
- Kaya, M.; Baran, T.; Menteş, A.; Asaroglu, M.; Sezen, G.; Tozak, K. Extraction and Characterization of α-Chitin and Chitosan from Six Different Aquatic Invertebrates. Food Biophys. 2014, 9, 145–157.
- Chaussard, G.; Domard, A. New Aspects of the Extraction of Chitin from Squid Pens. Biomacromolecules 2014, 5, 559–564.
- Fan, Y.; Saito, T.; Isogai, A. Preparation of Chitin Nanofibers from Squid Pen β-Chitin by Simple Mechanical Treatment under Acid Conditions. Biomacromolecules 2008, 9, 1919–1923.
- Davies, G.; Knight, D.; Vollrath, F. Chitin in the Silk Gland Ducts of the Spider Nephila edulis and the Silkworm Bombyx mori. PLoS ONE 2013, 8, e73225.
- John, C.C. The chemistry and Chemical Ecology of Octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 1992, 92, 613–631.
- Kaya, M.; Asan-Ozusaglam, M.; Erdogan, S. Comparison of antimicrobial activities of newly obtained low molecular weight scorpion chitosan and medium molecular weight commercial chitosan. J. Biosci. Bioeng. 2016, 121, 678–684.
- Zhang, H.; Neau, S. In vitro degradation of chitosan by a commercial enzyme preparation: Effect of molecular weight and degree of deacetylation. Biomaterials 2001, 22, 1653–1658.
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and Characterization of Chitin from the Beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611.
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105.
- Kaya, M.; Baran, T. Description of a new surface morphology for chitin extracted from wings of cockroach (Periplaneta americana). Int. J. Biol. Macromol. 2015, 75, 7–12.
- Tanaka, K.; Katsura, N.; Saku, T.; Kasuga, S. Composite Texture of Chitin and Keratin in an Animal Organ, Lingula seta. Polym. J. 2015, 20, 119–123.
- Synowiecki, J.; Al-Khateeb, N. Mycelia of Mucor rouxii as a source of chitin and chitosan. Food Chem. 1997, 60, 605–610.
- Mathur, N.; Narang, C. Chitin and chitosan, versatile polysaccharides from marine animals. J. Chem. Educ. 1990, 67, 938.
- Chobot, V.; Kremenák, J.; Opletal, L. Phytotherapeutic aspects of diseases of the circulatory system. 4. Chitin and chitosan. Ceska Slov. Farm. 1995, 44, 190–195.
- Kulikov, S.N.; Chirkov, S.N.; Il’Ina, A.V.; Lopatin, S.A.; Varlamov, V.P. Effect of the molecular weight of chitosan on its antiviral activity in plants. Appl. Biochem. Microbiol. 2006, 42, 200–203.
- De Alvarenga, E.S.; de Oliveira, C.P.; Bellato, C.R. An approach to understanding the deacetylation degree of chitosan. Carbohydr. Polym. 2010, 80, 1155–1160.
- Jaworska, M.; Sakurai, K.; Gaudon, P.; Guibal, E. Influence of chitosan characteristics on polymer properties. I: Crystallographic properties. Polym. Int. 2003, 52, 198–205.
- Dotto, G.; Campana-Filho, S.; de Almeida Pinto, L.A. Frontiers in Biomaterials; Bentham Science Publishers: Bussum, The Netherlands, 2017.
- Verlee, A.; Mincke, S.; Stevens, C. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283.
- Minh, N.; van Hoa, N.; Trung, T. Preparation, properties, and application of low-molecular-weight chitosan. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 453–471.
- Niazi, S. Production, Classification, Properties and Application of Chitosan. Int. J. Res. Agric. Sci. 2016, 3, 2358–3997.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
14 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




