| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vera Egorova | -- | 5424 | 2023-07-13 14:16:44 | | | |
| 2 | Peter Tang | -104 word(s) | 5320 | 2023-07-14 03:22:59 | | |
Video Upload Options
Cathepsin B is a lysosomal cysteine protease, contributing to vital cellular homeostatic processes including protein turnover, macroautophagy of damaged organelles, antigen presentation, and in the extracellular space, it takes part in tissue remodeling, prohormone processing, and activation. However, aberrant overexpression of cathepsin B and its enzymatic activity is associated with different pathological conditions, including cancer. Cathepsin B overexpression in tumor tissues makes this enzyme an important target for smart delivery systems, responsive to the activity of this enzyme. The generation of technologies which therapeutic effect is activated as a result of cathepsin B cleavage provides an opportunity for tumor-targeted therapy and controlled drug release.
1. Introduction
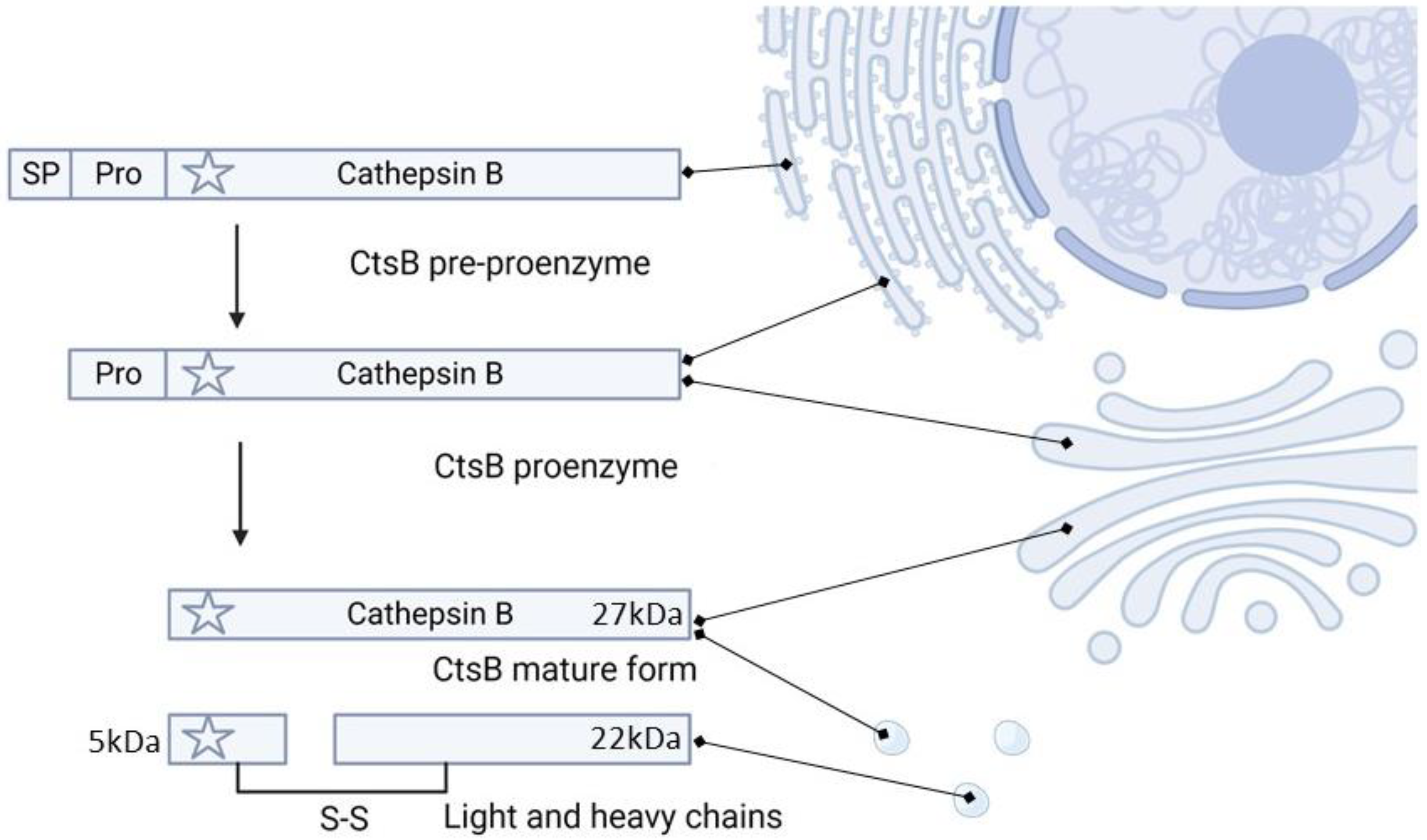
2. Cathepsin B Synthesis and Physiological Function
3. CtsB Activity and Targetability in Cancer
4. CtsB-Responsive Delivery Systems
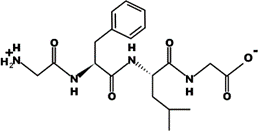
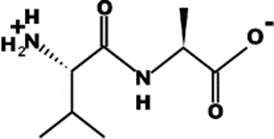
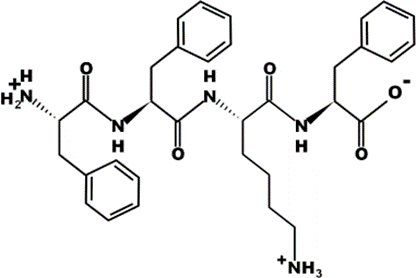
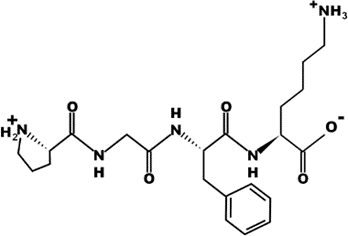
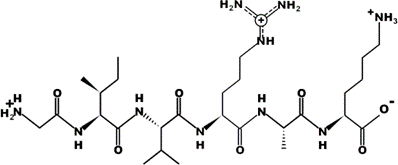
4.1. CtsB-Sensitive Conjugates Based on GFLG and Other Peptides
4.2. Antibody-Drug Conjugates
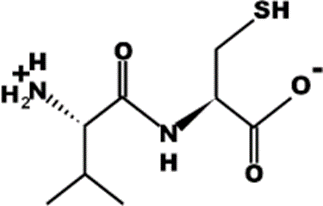
Different ADC were FDA-approved for cancer treatment and many of them use CtsB-sensitive linkers. Enhertu is an ADC composed of Transtuzumab (directed against tumor cells over-expressing Her-2) and was approved to treat breast [77], gastric [78] and non-small cell lung cancer [79]. The antibody is conjugated via the pentapeptide maleimide GGGPG (Gly-Gly-Gly-Pro-Gly) to the exatecan derivative Dxd. This linker is sensitive to CtsB and CtsL activity and is fundamental to control the release of Dxd where these proteases are overexpressed. Zynlonta comprises an antibody specific for CD19 conjugated via a VA linker to the DNA alkylating agent SG3199 and it was approved for treating B-cell non-Hodgkin lymphoma, over-expressing CD19. The ADC Adcetris was approved to treat Hodgkin lymphoma and systemic anaplastic large cell lymphoma and it is based on an antibody directed against CD30 (also known as the Reed–Sternberg cell-associated antigen). This antibody is conjugated to the drug monomethyl auristatin E (MMAE), through two chemical spacers (maleimidocaproyl and PABC groups), separated by the CtsB-sensitive VC dipeptide. The spacers have the primary function of physically conjugating the antibody, the linker and the MMAE, but also to favor the access of the enzyme to the peptide, that could be eventually affected by the drug [80]. This very similar technology was exploited to design the FDA-approved ADC Polivy and Padcev delivering MMAE. Polivy is used to treat diffuse large B-cell lymphoma by targeting CD79b while Padcev was approved to treat advanced or metastatic urothelial cancer and is composed of an antibody directed against Nectin-4 [81]. Tivdak comprised a human anti-TFIgG1κ antibody conjugated to MMAE via the CtsB-cleavable maleimidocaproyl-VC-PABC linker and was FDA-approved in 2021 to treat adult patients with recurrent or metastatic cervical cancer [82]. Disitamab vedotin, also known as RC48, with the same technology and CtsB sensitive linker, was approved by NMPA (China) to treat metastatic gastric cancer [83].
Interestingly, a deeper investigation of this linker showed that CtsB expression inhibition through different biomolecular techniques did not affect the cytostatic properties of the system. This phenomenon was due to compensating mechanisms originating from the activity of other proteases (i.e., CtsS) mitigating the lack of CtsB. It is also worth highlighting that linking MMAE via a non-cleavable enantiomer did not completely suppress the cytostatic properties of the system because a toxic catabolic product of MMAE can generate in the lysosomal compartment. On the other hand, the cytotoxic dependence on CtsB activity was restored by substituting MMAE with another drug (pyrrolo [2,1-c][1,4] benzodiazepine dimer) that did not generate any toxic catabolite in the lysosomes. This work is important because it showed that functional ADC can be generated despite the linker sensitivity for a specific protease [84].
A different category of ADC agents has been developed utilizing PBD dimers, which are roughly 50–100 times more potent than the standard drugs used in the creation of ADCs (such as MMAE). Two examples of such agents are SGN-CD33A (Vadastuximab talirine) [85] and SGN-CD70A [86], which both include the same talirine cleavable linker, a maleimidocaproyl linker with a CtsB-sensitive VA-PAB moiety, PBD dimer (SG 1882) and anti-CD33 and anti-CD70 antibodies, respectively. Unfortunately, the clinical trials for these drugs had to be terminated due to severe side effects and increased patient mortality [87]. Furthermore, SGN-CD123A, ADC with a similar structure based on an anti-CD213 antibody, was developed to treat acute myeloid leukaemia [88].
Another ADC drugs containing CtsB-cleavable tesirine linker (tesirine linker in its turn comprises Val-Ala peptide) are evaluated in the ongoing clinical trials: Rovalpituzumab tesirine is an anti-DLL3 ADC developed to treat small cell lung cancer (phase III) and loncastuximab tesirine [89] and camidanlumab tesirine [90] are anti-CD19 and anti-CD25 ADCs (phase II), respectively, indicated to treat B cell acute lymphoblastic leukemia and Hodgkin lymphoma.
In a pre-clinical work, it was discovered that Carboxylesterase 1C can break down VC linkers in the plasma [91] affecting the complex stability in the blood. Nevertheless, modifying the linker by introducing an aminocaproyl or additional Glu residue upstream of the VC dipeptide has been shown to mitigate this issue [92][93], while maintaining or increasing sensitivity to CtsB activity.
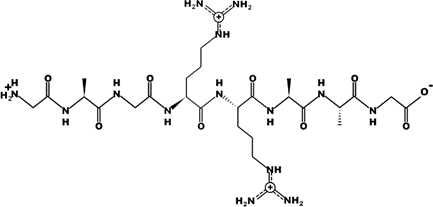
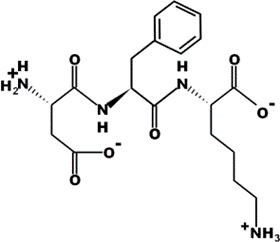
The most common CtsB-sensitive peptide linkers and their basic physicochemical properties are summarized in Table 1.
Table 1. Chemical structures and basic physicochemical properties of CtsB-cleavable linkers *.
|
Peptide Linker |
Chemical Structure |
Molecular Weight |
Net Charge |
Isoelectrical Point |
|
GFLG |
|
392.2 |
0 |
5.60 |
|
GAGRRAAG |
|
714.4 |
+2 |
12.49 |
|
VC |
|
220.1 |
0 |
4.95 |
|
DFK |
|
408.2 |
0 |
6.77 |
|
VA |
|
188.1 |
0 |
5.60 |
|
FFKF |
|
587.3 |
+1 |
9.93 |
|
PGFK |
|
447.2 |
+1 |
10.59 |
|
GIVRAK |
|
642.4 |
+2 |
11.56 |
* Based on data from www.pepdraw.com, accessed on 15 June 2023.
4.3. CtsB-Sensitive Nanoparticles
Ehrsam et al. [94] generated poly(dimethylsiloxane)-b-poly(methyloxazoline) (PDMS-PMOXA) NPs modified on their surface with the CtsB-responsive Fmoc-aminocaproic acid(Ahx)-GSGFLGSC peptide bearing PTX. A significant increase in OVCAR-3 and OVCAR-5 cell lines’ cytotoxicity was observed when the particle treatment was combined with purified CtsB, indicating that the enzyme could accelerate the release of the payload, while the addition of the CtsB inhibitor CA-074 decreased particle toxicity [103].
For cancer treatment, rare-earth doped upconversion nanocrystals (UCN) were embedded in a polymeric matrix bearing the photosensitizer Chlorin e6 (Ce6) and modified with the CtsB-sensitive peptide Ac-FKC(StBu)AC(SH)-CBT [104]. The peptide contained a protected reactive group comprising 2-cyanobenzothiazole that could covalently bind cysteine. CtsB cleavage of the peptide favored the covalent cross-linking between the exposed cysteine and 2-cyanobenzothiazole of adjacent particles, ultimately favoring their aggregation. UCN cross-linking enhanced their upconversion emission properties upon laser irradiation (wavelength of 808 nm) and the consequent generation of singlet oxygen by Ce6. The aggregation of UCN in an environment with a high content of CtsB was confirmed by electron microscopy and was accompanied by a shift in the UCN absorption peak. In vitro and in vivo fluorescence and photoacoustic imaging studies confirmed the success of this enzyme-induced cross-linking reaction.
Recently, self-assembling prodrug NPs have been proposed to generate a new concept of nanodelivery systems [105]. NPs assemble could occur by modifying the payload with hydrophobic or amphiphilic linkers inducing a particle self-assembly process. The prodrug molecules were modified with ester, thioether, thioketal and disulfide groups allowing for a selective activation of the system in the TME [106]. Proteolytic sensitivity against a particular enzyme could be imparted using sensitive peptides in the formula. It was known that Phe was the key amino acid residue for creating self-assembling nanostructures because of intermolecular hydrophobic and π-π interactions [107]. Peptides with the CtsB-cleavable sequence FFKF (Phe-Phe-Lys-Phe) demonstrated effective nanofiber formation and DOX loading and release efficacy [108]. The FRRL-DOX (Phe-Arg-Arg-Leu-DOX) self-assembled NPs (170 nm) showed a 16-times higher tumor targeting than free DOX [109].
Over the last year, only a few works have been published about CtsB-sensitive nanoparticles. One such work, by Huang et al. [110], involved the development of ferumoxytol nanoparticles that were linked to the MMAE drug by four polyethylene glycol linkers, a CtsB-cleavable VC dipeptide and a p-aminobenzylcarbamate spacer. These nanoparticles were tested in vitro using the U87-MG glioblastoma cell line and in vivo on nude mice that had been injected intracranially with these cells. The resulting nanoparticles were found to be effective in inducing the death of glioblastoma cells. The maximal anticancer effect was achieved through a combination of nanoparticle treatment and radiotherapy. Another work by Shi et al. [111] aimed to develop a complex of fluorocarbons linked with polyarginine and CtsB-sensitive GFLG peptides to anti-VEGF siRNA. Positively charged nanoparticles, about 90 nm in size, were prepared and the polyarginine and GFLG peptide sequences provided double cleavage mediated by GSH reduction and CtsB activity. The work resulted in efficient siRNA release and VEGF deregulation in HeLa cervical carcinoma cell line.
5. CtsB-Cleavable Surface Modifications
Gotov et al. [120] synthesized AuNPs modified with hyaluronic acid for targeted delivery of docetaxel (DTX). The hyaluronic acid was attached to the surface of the AuNPs using the CtsB-sensitive peptide GFLGC and allowed for increased circulation and targeting properties against the CD44 receptors overexpressed on cancer cells. The system showed greater cytotoxicity and higher tumor suppression efficacy in vivo than free DTX, providing a means to combine thermoablation and chemotherapy.
In a recent study conducted by Li et al. [121], it was demonstrated that resveratrol encapsulated in mesoporous silica nanoparticles linked to transferrin molecules with CtsB-cleavable DEGFLGED peptide, exhibited high anticancer properties. In this case, transferrin acted as a capping and targeting agent. The resulting nanoparticles effectively reduced the viability of MCF7 cells and increased the apoptosis rate up to 80.8%. The authors have also planned to conduct further research using a mouse xenograft model.|
Peptide Linker |
Drug |
Delivery System |
Cancer |
Outcomes |
Ref. |
|
GFLG |
Doxorubicin |
Conjugate |
HepG2 cells |
The conjugate structure had an opposite effect on DOX release and tumor accumulation. The synergistic effect of these properties exhibited the highest antitumor efficacy |
[122] |
|
GFLG |
Paclitaxel and gemcitabine |
Conjugate with HPMA dendrimers |
A2780 human ovarian carcinoma cells |
The combination of PTX, GEM and diblock structures yielded the highest inhibition efficacy of tumor growth |
[123] |
|
GFLG |
Doxorubicin |
Conjugate with polymer |
Lung carcinoma, colorectal cancer and anthracycline-resistant breast cancer |
Antitumor activity in refractory cancers was demonstrated, and polymer-drug conjugation has been shown to reduce DOX dose-limiting toxicity |
[124] |
|
GFLG |
Doxorubicin |
Conjugate with polymer |
H22 mice tumor |
The conjugates were successfully internalized into the cell nuclei, resulting in an inhibition efficiency of ~90% for the tumor |
[125] |
|
GAGRRAAG |
Pheophorbide |
Conjugate |
Bone marrow cells |
The photodynamic effect was demonstrated to be greater than 60%, and the system could be used as a sensor for cathepsin activity |
[126] |
|
VC |
Doxorubicin |
Nanoparticle |
RKO colon carcinoma cells |
Conjugates can efficiently bind to and be internalized by EGFR-overexpressing cancer cells. This strategy could be used to reduce systemic toxicity |
[127] |
|
DFK |
SPION |
Nanoparticle |
MDA-MB-231 breast cancer cells |
The increased efficiency of NP internalization and spion release following exposure to CtsB were demonstrated |
[128] |
|
GSGFLGSC |
PTX |
Nanoparticle |
OVCAR-3 adenocarcinoma cells and OVCAR-5 ovarian cancer cells |
The time-dependent PTX release and a 25-fold reduction in IC50 compared to pure PTX were demonstrated |
[94] |
|
Ac-FKC(StBu)AC(SH)-CBT |
Chlorin e6 |
Nanoparticle |
H-29 human colorectal adenicarcinoma cells |
CtsB induced NPs self-assembly, resulting in an increased singlet oxygen generation and a significant enhancement of the photodynamic effect |
[104] |
|
FFKF |
Doxorubicin |
Self-assembled nanoparticle |
Tumor lysates |
A library of FFKF peptides with various N-terminal capping groups was studied, and their self-assembly and sensitivity to cathepsin B and L were analyzed. Cbz-FFKF-OH showed the highest potential and a release of 92% of DOX within 8 h |
[108] |
|
VA |
Duocarmycin and pyrrolobenzodiazepine |
ADC |
Hepatocellular carcinoma |
Using dipeptides, VC and VA, conjugating Duocarmycin SA and PBD dimers to antibodies targeting GPC3 on hepatocellular carcinoma cells advances in liver cancer therapy were achieved |
[129] |
|
VC |
(Pyrrolo [2,1-c][1,4]benzodiazepine dimer) |
ADC |
BT474 carcinoma cells, KPL-4 breast cancer cells and BJAM lymphoma cells |
The targeting agent used is of more importance for the effectiveness of ADC than the efficiency of linker cleavage |
[95] |
|
VC |
Auristatin-based |
ADC |
Expi293 cells |
Carboxylesterase 1C was identified as the enzyme responsible for the plasmatic hydrolysis of (VC-PABC)-based linkers |
[130] |
|
VC |
Monomethyl auristatin E |
Nanoparticle |
U87 glioblastoma cells |
The system provided efficient cellular uptake and high toxic effect on glioblastoma cells. The maximal anticancer effect was achieved using NPs and radiotherapy |
[110] |
|
GFLG |
Anti-VEGF siRNA |
Nanoparticle |
HeLa cells |
Efficient siRNA release and VEGF deregulation in HeLa cells were achieved |
[111] |
|
GFLG |
Doxorubicin |
Functionalized nanoparticle |
HeLa cells |
80% of DOX release was observed in 24 h in the presence of CtsB |
[131] |
|
GIVRAKEAEGIVRAK |
Safranin O or DOX |
Functionalized nanoparticle |
Hela cells |
A 5-fold increase in the release of Safarin O was observed in the presence of lysosomal extract, leading to a CtsB-dependent cytotoxic effect |
[132] |
|
PGFK |
Doxorubicin |
Functionalized nanoparticle |
A549 human non-small cell lung cancer cells, NIH-3T3 mouse fibroblast cells, A2780 human ovarian cancer cells |
At acidic pH, CtsB led to a four-fold increase in DOX release and consequent higher toxicity |
[133] |
|
GFLG |
Doxorubicin |
Functionalized nanoparticle |
MCF-7 human breast cancer cell |
The nanoparticles represented a promising system to overcome MDR phenomena |
[134] |
|
GFLGC |
Docetaxel |
Functionalized nanoparticle |
HeLa and MCF-7 breast cells |
The systems showed higher circulation properties, efficacy and safety |
[120] |
|
DEGFLGED |
Resveratrol |
Functionalized nanoparticle |
MCF-7 breast cells |
Anticancer activity exceeded 80% |
[121] |
|
VA |
SG3199 |
ADC |
B-cell non-Hodgkin Lymphoma |
Zynlonta® is FDA-approved ADC for the treatment of large B-cell lymphoma (USA) |
|
|
VC |
Monomethyl auristatin E |
ADC |
Hodgkin lymphoma |
Adcetris® was approved ADC for the treatment of Hodgkin lymphoma (USA) |
|
|
VC |
Monomethyl auristatin E |
ADC |
Large B-cell lymphoma |
Polivy® was approved for the treatment of large B-cell lymphoma (USA) |
[139] |
|
VC |
Monomethyl auristatin E |
ADC |
Metastatic urothelial cancer |
Padcev® was approved for the treatment of metastatic urothelial cancer (USA) |
[140] |
|
VC |
Monomethyl auristatin E |
ADC |
Metastatic cervical cancer |
Tivdak® was approved for the treatment of metastatic cervical cancer (USA) |
[82] |
|
VC |
Monomethyl auristatin E |
ADC |
HER-2 positive solid tumors |
RC-48® was approved for the treatment of metastatic cervical cancer (China) |
[83] |
|
VA |
SGD-1882 |
ADC |
Positive acute myeloid leukemia |
Clinical trials were stopped because of severe adverse events and increased patient mortality |
[85] |
|
VA |
SGD-1882 |
ADC |
Non-Hodgkin Lymphoma and Renal Cell Carcinoma |
Clinical trials were stopped because of severe adverse events and increased patient mortality |
[86] |
|
VA |
SG3199 |
ADC |
Large B-cell lymphoma |
Phase 2 of clinical trials of ADCT-402 (Loncastuximab Tesirine), NCT05296070 NCT05249959 |
[141] |
|
VA |
SG3199 |
ADC |
Hodgkin lymphoma |
Phase 2 of clinical trials of Camidanlumab tesirine NCT04052997 |
[90] |
6. Conclusions
Here, the researchers have highlighted the potential of developing CtsB-cleavable technologies for targeting cancer cells and tumor microenvironments. Although ADCs have shown superior results in terms of translational purposes, the use of nanosystems may expand the portfolio of possible therapies, such as photodynamic and photothermal therapy. However, although CtsB-cleavable conjugates, nanoparticles, ADCs and surface modifications are extensively studied in preclinical and even clinical settings, more work is necessary to define the specificity of these systems, since cathepsin proteases are numerous and have redundant activity. Additionally, the malignant properties of cancer cells must be identified as a function of CtsB overexpression, even though this enzyme is ubiquitous and expressed at varying levels in all cancer cells. Finally, for future clinical translation purposes, these systems need to be simplified in their synthesis as they often consist of various components. Fine control of the manufacturing process may be challenging, particularly for large-scale production.
References
- Parodi, A.; Kostyushev, D.; Brezgin, S.; Kostyusheva, A.; Borodina, T.; Akasov, R.; Frolova, A.; Chulanov, V.; Zamyatnin, A.A., Jr. Biomimetic approaches for targeting tumor inflammation. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022.
- Soltani, M.; Savvateeva, L.V.; Ganjalikhani-Hakemi, M.; Zamyatnin, A.A. Clinical Combinatorial Treatments Based on Cancer Vaccines: Combination with Checkpoint Inhibitors and Beyond. Curr. Drug Targets 2022, 23, 1072–1084.
- Soond, S.M.; Zamyatnin, A.A., Jr. Targeting G protein-coupled receptors in cancer therapy. Adv. Cancer Res. 2020, 145, 49–97.
- Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine cathepsins and their extracellular roles: Shaping the microenvironment. Cells 2019, 8, 264.
- Withana, N.P.; Blum, G.; Sameni, M.; Slaney, C.; Anbalagan, A.; Olive, M.B.; Bidwell, B.N.; Edgington, L.; Wang, L.; Moin, K. Cathepsin B Inhibition Limits Bone Metastasis in Breast Cancer Cathepsin B Inhibition Reduces Bone Metastasis. Cancer Res. 2012, 72, 1199–1209.
- Walker, E.; Mann, M.; Honda, K.; Vidimos, A.; Schluchter, M.D.; Straight, B.; Bogyo, M.; Popkin, D.; Basilion, J.P. Rapid visualization of nonmelanoma skin cancer. J. Am. Acad. Dermatol. 2017, 76, 209–216.e209.
- Rudzińska, M.; Parodi, A.; Soond, S.M.; Vinarov, A.Z.; Korolev, D.O.; Morozov, A.O.; Daglioglu, C.; Tutar, Y.; Zamyatnin, A.A., Jr. The role of cysteine cathepsins in cancer progression and drug resistance. Int. J. Mol. Sci. 2019, 20, 3602.
- Harbeck, N.; Alt, U.; Berger, U.; Krüger, A.; Thomssen, C.; Jänicke, F.; Höfler, H.; Kates, R.E.; Schmitt, M. Prognostic impact of proteolytic factors (urokinase-type plasminogen activator, plasminogen activator inhibitor 1, and cathepsins B, D, and L) in primary breast cancer reflects effects of adjuvant systemic therapy. Clin. Cancer Res. 2001, 7, 2757–2764.
- Kayser, K.; Richter, N.; Hufnagl, P.; Kayser, G.; Kos, J.; Werle, B. Expression, proliferation activity and clinical significance of cathepsin B and cathepsin L in operated lung cancer. Anticancer Res. 2003, 23, 2767–2772.
- Ma, K.; Chen, X.; Liu, W.; Chen, S.; Yang, C.; Yang, J. CTSB is a negative prognostic biomarker and therapeutic target associated with immune cells infiltration and immunosuppression in gliomas. Sci. Rep. 2022, 12, 4295.
- Olson, O.C.; Joyce, J.A. Cysteine cathepsin proteases: Regulators of cancer progression and therapeutic response. Nat. Rev. Cancer 2015, 15, 712–729.
- Sevenich, L.; Schurigt, U.; Sachse, K.; Gajda, M.; Werner, F.; Müller, S.; Vasiljeva, O.; Schwinde, A.; Klemm, N.; Deussing, J. Synergistic antitumor effects of combined cathepsin B and cathepsin Z deficiencies on breast cancer progression and metastasis in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 2497–2502.
- Li, Y.; Mei, T.; Han, S.; Han, T.; Sun, Y.; Zhang, H.; An, F. Cathepsin B-responsive nanodrug delivery systems for precise diagnosis and targeted therapy of malignant tumors. Chin. Chem. Lett. 2020, 31, 3027–3040.
- Zamyatnin, A.A., Jr.; Gregory, L.C.; Townsend, P.A.; Soond, S.M. Beyond Basic Research: The Contribution of Cathepsin B to Cancer Development, Diagnosis and Therapy. Expert Opin. Ther. Targets 2022, 26, 963–977.
- Xu, J.; Ding, Y.; Shi, C.; Yuan, F.; Sheng, X.; Liu, Y.; Xie, Y.; Lu, H.; Duan, C.; Hu, J. Identification of Cathepsin B as a Therapeutic Target for Ferroptosis of Macrophage after Spinal Cord Injury. Aging Dis. 2023.
- Tena Pérez, V.; Apaza Ticona, L.; Cabanillas, A.H.; Maderuelo Corral, S.; Rosero Valencia, D.F.; Martel Quintana, A.; Ortega Domenech, M.; Rumbero Sánchez, Á. Isolation of Nocuolin A and Synthesis of New Oxadiazine Derivatives. Design, Synthesis, Molecular Docking, Apoptotic Evaluation, and Cathepsin B Inhibition. Mar. Drugs 2023, 21, 284.
- Rudzińska, M.; Parodi, A.; Maslova, V.D.; Efremov, Y.M.; Gorokhovets, N.V.; Makarov, V.A.; Popkov, V.A.; Golovin, A.V.; Zernii, E.Y.; Zamyatnin, A.A., Jr. Cysteine cathepsins inhibition affects their expression and human renal cancer cell phenotype. Cancers 2020, 12, 1310.
- Park, S.-H.; Lee, J.-H.; Yang, S.-B.; Lee, D.-N.; Kang, T.-B.; Park, J. Development of a Peptide-Based Nano-Sized Cathepsin B Inhibitor for Anticancer Therapy. Pharmaceutics 2023, 15, 1131.
- Xie, Z.; Zhao, M.; Yan, C.; Kong, W.; Lan, F.; Narengaowa; Zhao, S.; Yang, Q.; Bai, Z.; Qing, H. Cathepsin B in programmed cell death machinery: Mechanisms of execution and regulatory pathways. Cell Death Dis. 2023, 14, 255.
- Megan Garland; Joshua J. Yim; Matthew Bogyo; A Bright Future for Precision Medicine: Advances in Fluorescent Chemical Probe Design and Their Clinical Application. Cell Chemical Biology 2016, 23, 122-136.
- Linke, M.; Herzog, V.; Brix, K. Trafficking of lysosomal cathepsin B—Green fluorescent protein to the surface of thyroid epithelial cells involves the endosomal/lysosomal compartment. J. Cell Sci. 2002, 115, 4877–4889.
- Mort, J.S.; Buttle, D.J. Cathepsin b. Int. J. Biochem. Cell Biol. 1997, 29, 715–720.
- Hook, G.; Reinheckel, T.; Ni, J.; Wu, Z.; Kindy, M.; Peters, C.; Hook, V. Cathepsin B Gene Knockout Improves Behavioral Deficits and Reduces Pathology in Models of Neurologic Disorders. Pharmacol. Rev. 2022, 74, 600–629.
- Brömme, D. Papain-like cysteine proteases. Curr. Protoc. Protein Sci. 2000, 21, 21.22.14–21.22.21.
- Yang, N.; Ye, Z.; Li, F.; Mahato, R.I. HPMA polymer-based site-specific delivery of oligonucleotides to hepatic stellate cells. Bioconjugate Chem. 2009, 20, 213–221.
- Krupa, J.C.; Hasnain, S.; Nägler, D.K.; Menard, R.; Mort, J.S. S′ 2 substrate specificity and the role of His110 and His111 in the exopeptidase activity of human cathepsin B. Biochem. J. 2002, 361, 613–619.
- Takahashi, T.; Dehdarani, A.H.; Yonezawa, S.; Tang, J. Porcine spleen cathepsin B is an exopeptidase. J. Biol. Chem. 1986, 261, 9375–9381.
- Almeida, P.C.; Nantes, I.L.; Chagas, J.R.; Rizzi, C.C.; Faljoni-Alario, A.; Carmona, E.; Juliano, L.; Nader, H.B.; Tersariol, I.L. Cathepsin B activity regulation: Heparin-like glycosaminoglycans protect human cathepsin B from alkaline pH-induced inactivation. J. Biol. Chem. 2001, 276, 944–951.
- Bohley, P.; Seglen, P. Proteases and proteolysis in the lysosome. Experientia 1992, 48, 151–157.
- Deussing, J.; Roth, W.; Saftig, P.; Peters, C.; Ploegh, H.L.; Villadangos, J.A. Cathepsins B and D are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc. Natl. Acad. Sci. USA 1998, 95, 4516–4521.
- Ni, J.; Lan, F.; Xu, Y.; Nakanishi, H.; Li, X. Extralysosomal cathepsin B in central nervous system: Mechanisms and therapeutic implications. Brain Pathol. 2022, 32, e13071.
- Wang, Y.; Niu, H.; Hu, Z.; Zhu, M.; Wang, L.; Han, L.; Qian, L.; Tian, K.; Yuan, H.; Lou, H. Targeting the lysosome by an aminomethylated Riccardin D triggers DNA damage through cathepsin B-mediated degradation of BRCA1. J. Cell. Mol. Med. 2019, 23, 1798–1812.
- Brix, K.; Linke, M.; Tepel, C.; Herzog, V. Cysteine proteinases mediate extracellular prohormone processing in the thyroid. Biol. Chem. 2001, 382, 717–726.
- Friedrichs, B.; Tepel, C.; Reinheckel, T.; Deussing, J.; von Figura, K.; Herzog, V.; Peters, C.; Saftig, P.; Brix, K. Thyroid functions of mouse cathepsins B, K, and L. J. Clin. Investig. 2003, 111, 1733–1745.
- Tran, A.P.; Sundar, S.; Yu, M.; Lang, B.T.; Silver, J. Modulation of receptor protein tyrosine phosphatase sigma increases chondroitin sulfate proteoglycan degradation through cathepsin B secretion to enhance axon outgrowth. J. Neurosci. 2018, 38, 5399–5414.
- Droga-Mazovec, G.; Bojic, L.; Petelin, A.; Ivanova, S.; Repnik, U.; Salvesen, G.S.; Stoka, V.; Turk, V.; Turk, B. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J. Biol. Chem. 2008, 283, 19140–19150.
- Ni, J.; Wu, Z.; Stoka, V.; Meng, J.; Hayashi, Y.; Peters, C.; Qing, H.; Turk, V.; Nakanishi, H. Increased expression and altered subcellular distribution of cathepsin B in microglia induce cognitive impairment through oxidative stress and inflammatory response in mice. Aging Cell 2019, 18, e12856.
- Zhang, H.; Zhong, C.; Shi, L.; Guo, Y.; Fan, Z. Granulysin induces cathepsin B release from lysosomes of target tumor cells to attack mitochondria through processing of bid leading to Necroptosis. J. Immunol. 2009, 182, 6993–7000.
- Meng, J.; Liu, Y.; Xie, Z.; Qing, H.; Lei, P.; Ni, J. Nucleus distribution of cathepsin B in senescent microglia promotes brain aging through degradation of sirtuins. Neurobiol. Aging 2020, 96, 255–266.
- Vasiljeva, O.; Korovin, M.; Gajda, M.; Brodoefel, H.; Bojic, L.; Krüger, A.; Schurigt, U.; Sevenich, L.; Turk, B.; Peters, C. Reduced tumour cell proliferation and delayed development of high-grade mammary carcinomas in cathepsin B-deficient mice. Oncogene 2008, 27, 4191–4199.
- Mijanović, O.; Branković, A.; Panin, A.N.; Savchuk, S.; Timashev, P.; Ulasov, I.; Lesniak, M.S. Cathepsin B: A sellsword of cancer progression. Cancer Lett. 2019, 449, 207–214.
- Kuester, D.; Lippert, H.; Roessner, A.; Krueger, S. The cathepsin family and their role in colorectal cancer. Pathol. Res. Pract. 2008, 204, 491–500.
- Kolwijck, E.; Massuger, L.F.; Thomas, C.M.; Span, P.N.; Krasovec, M.; Kos, J.; Sweep, F.C. Cathepsins B, L and cystatin C in cyst fluid of ovarian tumors. J. Cancer Res. Clin. Oncol. 2010, 136, 771–778.
- Ebert, M.P.; Krüger, S.; Fogeron, M.L.; Lamer, S.; Chen, J.; Pross, M.; Schulz, H.U.; Lage, H.; Heim, S.; Roessner, A. Overexpression of cathepsin B in gastric cancer identified by proteome analysis. Proteomics 2005, 5, 1693–1704.
- Rudzinska-Radecka, M.; Frolova, A.S.; Balakireva, A.V.; Gorokhovets, N.V.; Pokrovsky, V.S.; Sokolova, D.V.; Korolev, D.O.; Potoldykova, N.V.; Vinarov, A.Z.; Parodi, A. In Silico, In Vitro, and Clinical Investigations of Cathepsin B and Stefin A mRNA Expression and a Correlation Analysis in Kidney Cancer. Cells 2022, 11, 1455.
- Grabowska, M.M.; Day, M.L. Soluble E-cadherin: More than a symptom of disease. Front. Biosci. 2012, 17, 1948.
- Mitrović, A.; Fonović, U.P.; Kos, J. Cysteine cathepsins B and X promote epithelial-mesenchymal transition of tumor cells. Eur. J. Cell Biol. 2017, 96, 622–631.
- Nettesheim, A.; Shim, M.S.; Dixon, A.; Raychaudhuri, U.; Gong, H.; Liton, P.B. Cathepsin B localizes in the caveolae and participates in the proteolytic cascade in trabecular meshwork cells. Potential new drug target for the treatment of glaucoma. J. Clin. Med. 2020, 10, 78.
- Ruan, J.; Zheng, H.; Rong, X.; Rong, X.; Zhang, J.; Fang, W.; Zhao, P.; Luo, R. Over-expression of cathepsin B in hepatocellular carcinomas predicts poor prognosis of HCC patients. Mol. Cancer 2016, 15, 17.
- Nakao, S.; Zandi, S.; Sun, D.; Hafezi-Moghadam, A. Cathepsin B-mediated CD18 shedding regulates leukocyte recruitment from angiogenic vessels. FASEB J. 2018, 32, 143.
- Gondi, C.S.; Kandhukuri, N.; Kondraganti, S.; Gujrati, M.; Olivero, W.C.; Dinh, D.H.; Rao, J.S. RNA interference–mediated simultaneous down-regulation of urokinase-type plasminogen activator receptor and cathepsin B induces caspase-8–mediated apoptosis in SNB19 human glioma cells. Mol. Cancer Ther. 2006, 5, 3197–3208.
- Gondi, C.S.; Lakka, S.S.; Yanamandra, N.; Olivero, W.C.; Dinh, D.H.; Gujrati, M.; Tung, C.; Weissleder, R.; Rao, J.S. Adenovirus-mediated expression of antisense urokinase plasminogen activator receptor and antisense cathepsin B inhibits tumor growth, invasion, and angiogenesis in gliomas. Cancer Res. 2004, 64, 4069–4077.
- Malla, R.R.; Gopinath, S.; Gondi, C.S.; Alapati, K.; Dinh, D.H.; Gujrati, M.; Rao, J.S. Cathepsin B and uPAR knockdown inhibits tumor-induced angiogenesis by modulating VEGF expression in glioma. Cancer Gene Ther. 2011, 18, 419–434.
- Tummalapalli, P.; Spomar, D.; Gondi, C.S.; Olivero, W.C.; Gujrati, M.; Dinh, D.H.; Rao, J.S. RNAi-mediated abrogation of cathepsin B and MMP-9 gene expression in a malignant meningioma cell line leads to decreased tumor growth, invasion and angiogenesis. Int. J. Oncol. 2007, 31, 1039–1050.
- Nalla, A.K.; Gorantla, B.; Gondi, C.S.; Lakka, S.S.; Rao, J.S. Targeting MMP-9, uPAR, and cathepsin B inhibits invasion, migration and activates apoptosis in prostate cancer cells. Cancer Gene Ther. 2010, 17, 599–613.
- Bengsch, F.; Buck, A.; Günther, S.; Seiz, J.; Tacke, M.; Pfeifer, D.; Von Elverfeldt, D.; Sevenich, L.; Hillebrand, L.; Kern, U. Cell type-dependent pathogenic functions of overexpressed human cathepsin B in murine breast cancer progression. Oncogene 2014, 33, 4474–4484.
- Wei, B.; Gunzner-Toste, J.; Yao, H.; Wang, T.; Wang, J.; Xu, Z.; Chen, J.; Wai, J.; Nonomiya, J.; Tsai, S.P. Discovery of peptidomimetic antibody–drug conjugate linkers with enhanced protease specificity. J. Med. Chem. 2018, 61, 989–1000.
- Patel, D.K.; Menon, D.V.; Patel, D.H.; Dave, G. Linkers: A synergistic way for the synthesis of chimeric proteins. Protein Expr. Purif. 2022, 191, 106012.
- Schmitz, J.; Gilberg, E.; Löser, R.; Bajorath, J.; Bartz, U.; Gütschow, M. Cathepsin B: Active site mapping with peptidic substrates and inhibitors. Bioorganic Med. Chem. 2019, 27, 1–15.
- Loganzo, F.; Sung, M.; Gerber, H.-P. Mechanisms of resistance to antibody–drug conjugates. Mol. Cancer Ther. 2016, 15, 2825–2834.
- Yin, Q.; Shen, J.; Zhang, Z.; Yu, H.; Li, Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv. Drug Deliv. Rev. 2013, 65, 1699–1715.
- Shim, M.K.; Moon, Y.; Yang, S.; Kim, J.; Cho, H.; Lim, S.; Yoon, H.Y.; Seong, J.-K.; Kim, K. Cancer-specific drug-drug nanoparticles of pro-apoptotic and cathepsin B-cleavable peptide-conjugated doxorubicin for drug-resistant cancer therapy. Biomaterials 2020, 261, 120347.
- Rejmanová, P.; Kopeček, J.; Pohl, J.; Baudyš, M.; Kostka, V. Polymers containing enzymatically degradable bonds, 8. Degradation of oligopeptide sequences in N-(2-hydroxypropyl) methacrylamide copolymers by bovine spleen cathepsin B. Die Makromol. Chem. 1983, 184, 2009–2020.
- Omelyanenko, V.; Gentry, C.; Kopečková, P.; Kopeček, J. HPMA copolymer–anticancer drug–OV-TL16 antibody conjugates. II. Processing in epithelial ovarian carcinoma cells in vitro. Int. J. Cancer 1998, 75, 600–608.
- Chen, Z.; Zhang, P.; Cheetham, A.G.; Moon, J.H.; Moxley, J.W., Jr.; Lin, Y.-a.; Cui, H. Controlled release of free doxorubicin from peptide–drug conjugates by drug loading. J. Control. Release 2014, 191, 123–130.
- Dubikovskaya, E.A.; Thorne, S.H.; Pillow, T.H.; Contag, C.H.; Wender, P.A. Overcoming multidrug resistance of small-molecule therapeutics through conjugation with releasable octaarginine transporters. Proc. Natl. Acad. Sci. USA 2008, 105, 12128–12133.
- Zhang, P.; Lock, L.L.; Cheetham, A.G.; Cui, H. Enhanced cellular entry and efficacy of tat conjugates by rational design of the auxiliary segment. Mol. Pharm. 2014, 11, 964–973.
- Zhang, H.; Sun, Z.; Wang, K.; Li, N.; Chen, H.; Tan, X.; Li, L.; He, Z.; Sun, J. Multifunctional tumor-targeting cathepsin B-sensitive gemcitabine prodrug covalently targets albumin in situ and improves cancer therapy. Bioconjugate Chem. 2018, 29, 1852–1858.
- Ford, C.; Newman, C.; Johnson, J.; Woodhouse, C.; Reeder, T.; Rowland, G.; Simmonds, R. Localisation and toxicity study of a vindesine-anti-CEA conjugate in patients with advanced cancer. Br. J. Cancer 1983, 47, 35–42.
- Ponziani, S.; Di Vittorio, G.; Pitari, G.; Cimini, A.M.; Ardini, M.; Gentile, R.; Iacobelli, S.; Sala, G.; Capone, E.; Flavell, D.J. Antibody-drug conjugates: The new frontier of chemotherapy. Int. J. Mol. Sci. 2020, 21, 5510.
- Jain, N.; Smith, S.W.; Ghone, S.; Tomczuk, B. Current ADC linker chemistry. Pharm. Res. 2015, 32, 3526–3540.
- Tong, J.T.; Harris, P.W.; Brimble, M.A.; Kavianinia, I. An insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules 2021, 26, 5847.
- Bryden, F.; Martin, C.; Letast, S.; Lles, E.; Viéitez-Villemin, I.; Rousseau, A.; Colas, C.; Brachet-Botineau, M.; Allard-Vannier, E.; Larbouret, C. Impact of cathepsin B-sensitive triggers and hydrophilic linkers on in vitro efficacy of novel site-specific antibody–drug conjugates. Org. Biomol. Chem. 2018, 16, 1882–1889.
- Dubowchik, G.M.; Mosure, K.; Knipe, J.O.; Firestone, R.A. Cathepsin B-sensitive dipeptide prodrugs. 2. Models of anticancer drugs paclitaxel (Taxol®), mitomycin C and doxorubicin. Bioorganic Med. Chem. Lett. 1998, 8, 3347–3352.
- Fu, Y.; Urban, D.J.; Nani, R.R.; Zhang, Y.F.; Li, N.; Fu, H.; Shah, H.; Gorka, A.P.; Guha, R.; Chen, L. Glypican-3-specific antibody drug conjugates targeting hepatocellular carcinoma. Hepatology 2019, 70, 563–576.
- Bargh, J.D.; Isidro-Llobet, A.; Parker, J.S.; Spring, D.R. Cleavable linkers in antibody–drug conjugates. Chem. Soc. Rev. 2019, 48, 4361–4374.
- Shanu Modi; Cristina Saura; Toshinari Yamashita; Yeon Hee Park; Sung-Bae Kim; Kenji Tamura; Fabrice Andre; Hiroji Iwata; Yoshinori Ito; Junji Tsurutani; et al.Joohyuk SohnNeelima DenduluriChristophe PerrinKenjiro AogiEriko TokunagaSeock-Ah ImKeun Seok LeeSara A. HurvitzJavier CortesCaleb LeeShuquan ChenLin ZhangJavad ShahidiAntoine YverIan Krop Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. The New England Journal of Medicine 2020, 382, 610-621.
- Kohei Shitara; Yung-Jue Bang; Satoru Iwasa; Naotoshi Sugimoto; Min-Hee Ryu; Daisuke Sakai; Hyun-Cheol Chung; Hisato Kawakami; Hiroshi Yabusaki; Jeeyun Lee; et al.Kaku SaitoYoshinori KawaguchiTakahiro KamioAkihito KojimaMasahiro SugiharaKensei Yamaguchi Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. The New England Journal of Medicine 2020, 382, 2419-2430.
- ENHERTU® Approved in the US as the First HER2 Directed Therapy for Patients with Previously Treated HER2 Mutant Metastatic Non-Small Cell Lung Cancer. . FDA. Retrieved 2023-7-11
- Tong, J.T.; Harris, P.W.; Brimble, M.A.; Kavianinia, I. An insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules 2021, 26, 5847.
- Thomas Powles; Jonathan E. Rosenberg; Guru P. Sonpavde; Yohann Loriot; Ignacio Durán; Jae-Lyun Lee; Nobuaki Matsubara; Christof Vulsteke; Daniel Castellano; Chunzhang Wu; et al.Mary CampbellMaria MatsangouDaniel P. Petrylak Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. The New England Journal of Medicine 2021, 384, 1125-1135.
- Anthony Markham; Tisotumab Vedotin: First Approval. Drugs 2021, 81, 2141-2147.
- Fan Shi; Yanli Liu; Xuexiao Zhou; Pei Shen; Ran Xue; Min Zhang; Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Delivery 2022, 29, 1335-1344.
- Zhi, X.; Jiang, Y.; Xie, L.; Li, Y.; Fang, C.-J. Gold Nanorods Functionalized with Cathepsin B Targeting Peptide and Doxorubicin for Combinatorial Therapy against Multidrug Resistance. ACS Appl. Bio Mater. 2019, 2, 5697–5706.
- May S. Kung Sutherland; Roland B. Walter; Scott C. Jeffrey; Patrick J. Burke; Changpu Yu; Heather Kostner; Ivan Stone; Maureen C. Ryan; Django Sussman; Robert P. Lyon; et al.Weiping ZengKimberly H. HarringtonKerry KlussmanLori WestendorfDavid MeyerIrwin D. BernsteinPeter D. SenterDennis R. BenjaminJonathan G. DrachmanJulie A. McEarchern SGN-CD33A: a novel CD33-targeting antibody–drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 2013, 122, 1455-1463.
- Sumanta K. Pal; Andres Forero‐Torres; John A. Thompson; John C. Morris; Saurabh Chhabra; Christopher J. Hoimes; Nicholas J. Vogelzang; Thomas Boyd; Paulo G. Bergerot; Jacob J. Adashek; et al.Hong LiXuesong YuElaina M. GartnerAnne‐Sophie CarretDavid C. Smith A phase 1 trial of SGN‐CD70A in patients with CD70‐positive, metastatic renal cell carcinoma. Cancer 2019, 125, 1124-1132.
- Nicolas Joubert; Alain Beck; Charles Dumontet; Caroline Denevault-Sabourin; Antibody–Drug Conjugates: The Last Decade. Pharmaceuticals 2020, 13, 245.
- Fu Li; May Kung Sutherland; Changpu Yu; Roland B. Walter; Lori Westendorf; John Valliere-Douglass; Lucy Pan; Ashley Cronkite; Django Sussman; Kerry Klussman; et al.Michelle UlrichMartha E. AndersonIvan J. StoneWeiping ZengMechthild JonasTimothy S. LewisMaitrayee GoswamiSa A. WangPeter D. SenterChe-Leung LawEric J. FeldmanDennis R. Benjamin Characterization of SGN-CD123A, A Potent CD123-Directed Antibody–Drug Conjugate for Acute Myeloid Leukemia. Molecular Cancer Therapeutics 2018, 17, 554-564.
- Kahl, B.S.; Hamadani, M.; Radford, J.; Carlo-Stella, C.; Caimi, P.; Reid, E.; Feingold, J.M.; Ardeshna, K.M.; Solh, M.; Heffner, L.T.; et al. A Phase I Study of ADCT-402 (Loncastuximab Tesirine), a Novel Pyrrolobenzodiazepine-Based Antibody–Drug Conjugate, in Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma Phase I Study of ADCT-402 in Relapsed/Refractory B-Cell NHL. . Clin. Cancer Res. 2019, 25, 6986–6994.
- Hamadani, M.; Collins, G.P.; Caimi, P.F.; Samaniego, F.; Spira, A.; Davies, A.; Radford, J.; Menne, T.; Karnad, A.; Zain, J.M.; et al. Camidanlumab tesirine in patients with relapsed or refractory lymphoma: A phase 1, open-label, multicentre, dose-escalation, dose-expansion study.. Lancet Haematol. 2021, 8, e433–e445.
- Dorywalska, M.; Dushin, R.; Moine, L.; Farias, S.E.; Zhou, D.; Navaratnam, T.; Lui, V.; Hasa-Moreno, A.; Casas, M.G.; Tran, T.-T.; et al. Molecular Basis of Valine-Citrulline-PABC Linker Instability in Site-Specific ADCs and Its Mitigation by Linker Design Molecular Basis of VC-PABC Linker Instability. . Mol. Cancer Ther. 2016, 15, 958–970.
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C. Enfortumab vedotin in previously treated advanced urothelial carcinoma. New Engl. J. Med. 2021, 384, 1125–1135.
- Anami, Y.; Yamazaki, C.M.; Xiong, W.; Gui, X.; Zhang, N.; An, Z.; Tsuchikama, K. Glutamic acid–valine–citrulline linkers ensure stability and efficacy of antibody–drug conjugates in mice. Nat. Commun. 2018, 9, 2512.
- Daniel Ehrsam; Fabiola Porta; Janine Hussner; Isabell Seibert; Henriette E Meyer Zu Schwabedissen; PDMS-PMOXA-Nanoparticles Featuring a Cathepsin B-Triggered Release Mechanism. Materials 2019, 12, 2836.
- Niña G. Caculitan; Josefa Dela Cruz Chuh; Yong Ma; Donglu Zhang; Katherine R. Kozak; Yichin Liu; Thomas H. Pillow; Jack Sadowsky; Tommy K. Cheung; Qui Phung; et al.Benjamin HaleyByoung-Chul LeeRobert W. AkitaMark X. SliwkowskiAndrew G. Polson Cathepsin B Is Dispensable for Cellular Processing of Cathepsin B-Cleavable Antibody–Drug Conjugates. Cancer Research 2017, 77, 7027-7037.
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291.
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17, 849–865.
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Nanomater. Neoplasms 2021, 53, 31–142.
- Ye, Z.; Zhang, Y.; Liu, Y.; Liu, Y.; Tu, J.; Shen, Y. EGFR targeted cetuximab-valine-citrulline (vc)-doxorubicin immunoconjugates-loaded bovine serum albumin (BSA) nanoparticles for colorectal tumor therapy. Int. J. Nanomed. 2021, 16, 2443.
- Kolesova, E.P.; Egorova, V.S.; Syrocheva, A.O.; Frolova, A.S.; Kostyushev, D.; Kostyusheva, A.; Brezgin, S.; Trushina, D.B.; Fatkhutdinova, L.; Zyuzin, M. Proteolytic Resistance Determines Albumin Nanoparticle Drug Delivery Properties and Increases Cathepsin B, D, and G Expression. Int. J. Mol. Sci. 2023, 24, 10245.
- Satsangi, A.; Roy, S.S.; Satsangi, R.K.; Tolcher, A.W.; Vadlamudi, R.K.; Goins, B.; Ong, J.L. Synthesis of a novel, sequentially active-targeted drug delivery nanoplatform for breast cancer therapy. Biomaterials 2015, 59, 88–101.
- Satsangi, A.; Roy, S.S.; Satsangi, R.K.; Vadlamudi, R.K.; Ong, J.L. Design of a paclitaxel prodrug conjugate for active targeting of an enzyme upregulated in breast cancer cells. Mol. Pharm. 2014, 11, 1906–1918.
- M. Montaser; G. Lalmanach; L. Mach; CA-074, But Not Its Methyl Ester CA-074Me, Is a Selective Inhibitor of Cathepsin B within Living Cells. Biological Chemistry 2002, 383, 1305-8.
- Xiangzhao Ai; Chris Jun Hui Ho; Junxin Aw; Amalina Binte Ebrahim Attia; Jing Mu; Yu Wang; Xiaoyong Wang; Yong Wang; Xiaogang Liu; Huabing Chen; et al.Mingyuan GaoXiaoyuan ChenEdwin K.L. YeowGang LiuMalini OlivoBengang Xing In vivo covalent cross-linking of photon-converted rare-earth nanostructures for tumour localization and theranostics. Nature Communications 2016, 7, 10432-10432.
- Anne Nguyen; Roland Böttger; Shyh-Dar Li; Recent trends in bioresponsive linker technologies of Prodrug-Based Self-Assembling nanomaterials. Biomaterials 2021, 275, 120955.
- Xiao Dong; Rajeev K. Brahma; Chao Fang; Shao Q. Yao; Stimulus-responsive self-assembled prodrugs in cancer therapy. Chemical Science 2022, 13, 4239-4269.
- Helen W. German; Sahin Uyaver; Ulrich H. E. Hansmann; Self-Assembly of Phenylalanine-Based Molecules. The Journal of Physical Chemistry A 2014, 119, 1609-1615.
- Yael Ben-Nun; Galit Fichman; Lihi Adler-Abramovich; Boris Turk; Ehud Gazit; Galia Blum; Cathepsin nanofiber substrates as potential agents for targeted drug delivery. Journal of Controlled Release 2016, 257, 60-67.
- Nayeon Shim; Seong Ik Jeon; Suah Yang; Jung Yeon Park; Mihee Jo; Jinseong Kim; Jiwoong Choi; Wan Su Yun; Jeongrae Kim; Youngjoo Lee; et al.Man Kyu ShimYongju KimKwangmeyung Kim Comparative study of cathepsin B-cleavable linkers for the optimal design of cathepsin B-specific doxorubicin prodrug nanoparticles for targeted cancer therapy. Biomaterials 2022, 289, 121806.
- Ching-Hsin Huang; Edwin Chang; Li Zheng; Joe Gerald Jesu Raj; Wei Wu; Laura J. Pisani; Heike E. Daldrup-Link; Tumor protease-activated theranostic nanoparticles for MRI-guided glioblastoma therapy. Theranostics 2023, 13, 1745-1758.
- Zhen Shi; Yuhan Yang; Ziyang Guo; Shun Feng; Yu Wan; A cathepsin B/GSH dual-responsive fluorinated peptide for effective siRNA delivery to cancer cells. Bioorganic Chemistry 2023, 135, 106485.
- Cha, H.; Yoon, J.H.; Yoon, S. Probing quantum plasmon coupling using gold nanoparticle dimers with tunable interparticle distances down to the subnanometer range. ACS Nano 2014, 8, 8554–8563.
- Zhang, N.; Wu, H.; Liang, Y.; Ye, J.; Zhang, H.; Miao, Y.; Luo, Y.; Fan, H.; Yue, T. Design and Preparation of “corn-like” DFK-SBP-M13 Assembly for Improvement of Effective Internalization. Int. J. Nanomed. 2021, 16, 7091.
- He, X.; Alves, C.S.; Oliveira, N.; Rodrigues, J.; Zhu, J.; Bányai, I.; Tomás, H.; Shi, X. RGD peptide-modified multifunctional dendrimer platform for drug encapsulation and targeted inhibition of cancer cells. Colloids Surf. B Biointerfaces 2015, 125, 82–89.
- Agostini, A.; Mondragón, L.; Coll, C.; Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Pérez-Payá, E.; Amorós, P. Dual enzyme-triggered controlled release on capped nanometric silica mesoporous supports. ChemistryOpen 2012, 1, 17–20.
- Singh, N.; Karambelkar, A.; Gu, L.; Lin, K.; Miller, J.S.; Chen, C.S.; Sailor, M.J.; Bhatia, S.N. Bioresponsive mesoporous silica nanoparticles for triggered drug release. J. Am. Chem. Soc. 2011, 133, 19582–19585.
- Stephen, S.; Gorain, B.; Choudhury, H.; Chatterjee, B. Exploring the role of mesoporous silica nanoparticle in the development of novel drug delivery systems. Drug Deliv. Transl. Res. 2021, 12, 105–123.
- Cheng, Y.-J.; Luo, G.-F.; Zhu, J.-Y.; Xu, X.-D.; Zeng, X.; Cheng, D.-B.; Li, Y.-M.; Wu, Y.; Zhang, X.-Z.; Zhuo, R.-X. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 9078–9087.
- De la Torre, C.; Mondragón, L.; Coll, C.; Sancenón, F.; Marcos, M.D.; Martínez-Máñez, R.; Amorós, P.; Pérez-Payá, E.; Orzáez, M. Cathepsin-B induced controlled release from peptide-capped mesoporous silica nanoparticles. Chem. A Eur. J. 2014, 20, 15309–15314.
- Oyuntuya Gotov; Gantumur Battogtokh; Young Tag Ko; Docetaxel-Loaded Hyaluronic Acid–Cathepsin B-Cleavable-Peptide–Gold Nanoparticles for the Treatment of Cancer. Molecular Pharmaceutics 2018, 15, 4668-4676.
- Dongning Li; Chengzhu Song; Jie Zhang; Xiaoyan Zhao; Targeted delivery and apoptosis induction activity of peptide-transferrin targeted mesoporous silica encapsulated resveratrol in MCF-7 cells. Journal of Pharmacy and Pharmacology 2022, 75, 49-56.
- Zhipeng Chen; Pengcheng Zhang; Andrew G. Cheetham; Jae Hyon Moon; James W. Moxley; Yi-An Lin; Honggang Cui; Controlled release of free doxorubicin from peptide–drug conjugates by drug loading. Journal of Controlled Release 2014, 191, 123-130.
- Jiyuan Yang; Rui Zhang; Huaizhong Pan; Yuling Li; Yixin Fang; Libin Zhang; Jindřich Kopeček; Backbone Degradable N-(2-Hydroxypropyl)methacrylamide Copolymer Conjugates with Gemcitabine and Paclitaxel: Impact of Molecular Weight on Activity toward Human Ovarian Carcinoma Xenografts. Molecular Pharmaceutics 2017, 14, 1384-1394.
- P A Vasey; S B Kaye; R Morrison; C Twelves; P Wilson; R Duncan; A H Thomson; L S Murray; T E Hilditch; T Murray; et al.S BurtlesD FraierE FrigerioJ Cassidy Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee.. Clinical Cancer Research 1999, 5, 83–94.
- Zi-Yan Wu; Jian-Min Shen; Hao Lang; Ting Yue; Chan Sun; pH/enzyme dual sensitive and nucleus-targeting dendrimer nanoparticles to enhance the antitumour activity of doxorubicin. Pharmaceutical Development and Technology 2022, 27, 357-371.
- Manish Jain; Jordan Bouilloux; Ines Borrego; Stéphane Cook; Hubert Van Den Bergh; Norbert Lange; Georges Wagnieres; Marie-Noelle Giraud; Cathepsin B-Cleavable Polymeric Photosensitizer Prodrug for Selective Photodynamic Therapy: In Vitro Studies. Pharmaceuticals 2022, 15, 564.
- Zixuan Ye; Yue Zhang; Yuanfen Liu; Yanyan Liu; Jiasheng Tu; Yan Shen; EGFR Targeted Cetuximab-Valine-Citrulline (vc)-Doxorubicin Immunoconjugates- Loaded Bovine Serum Albumin (BSA) Nanoparticles for Colorectal Tumor Therapy. International Journal of Nanomedicine 2021, ume 16, 2443-2459.
- Na Zhang; /sup> Hui Wu; /sup> Yingzhi Liang; /sup> Jianming Ye; Huan Zhang; Yuqing Miao; Yane Luo; Haiming Fan; Tianli Yue; Design and Preparation of “corn-like” SPIONs@DFK-SBP-M13 Assembly for Improvement of Effective Internalization. International Journal of Nanomedicine 2021, ume 16, 7091-7102.
- Ying Fu; Daniel J. Urban; Roger R. Nani; Yi‐Fan Zhang; Nan Li; Haiying Fu; Hamzah Shah; Alexander P. Gorka; Rajarshi Guha; Lu Chen; et al.Matthew D. HallMartin J. SchnermannMitchell Ho Glypican‐3‐Specific Antibody Drug Conjugates Targeting Hepatocellular Carcinoma. Hepatology 2019, 70, 563-576.
- Dorywalska, M.; Dushin, R.; Moine, L.; Farias, S.E.; Zhou, D.; Navaratnam, T.; Lui, V.; Hasa-Moreno, A.; Casas, M.G.; Tran, T.-T.; et al. Molecular Basis of Valine-Citrulline-PABC Linker Instability in Site-Specific ADCs and Its Mitigation by Linker DesignMo-lecular Basis of VC-PABC Linker Instability.. Mol. Cancer Ther. 2016, 15, 958–970.
- Yin-Jia Cheng; Guo-Feng Luo; Jing-Yi Zhu; Xiao-Ding Xu; Xuan Zeng; Dong-Bing Cheng; You-Mei Li; Yan Wu; Xian-Zheng Zhang; Ren-Xi Zhuo; et al.Feng He Enzyme-Induced and Tumor-Targeted Drug Delivery System Based on Multifunctional Mesoporous Silica Nanoparticles. ACS Applied Materials & Interfaces 2015, 7, 9078-9087.
- Cristina De La Torre; Laura Mondragón; Carmen Coll; Félix Sancenón; María D. Marcos; Laura Mondragon Martinez; Pedro Amorós; Enrique Pérez-Payá; Mar Orzáez; Cathepsin-B Induced Controlled Release from Peptide-Capped Mesoporous Silica Nanoparticles. Chemistry – A European Journal 2014, 20, 15309-15314.
- Jinming Li; Fang Liu; Qing Shao; Yuanzeng Min; Marianne Costa; Edwin K. L. Yeow; Bengang Xing; Enzyme-Responsive Cell-Penetrating Peptide Conjugated Mesoporous Silica Quantum Dot Nanocarriers for Controlled Release of Nucleus-Targeted Drug Molecules and Real-Time Intracellular Fluorescence Imaging of Tumor Cells. Advanced Healthcare Materials 2014, 3, 1230-1239.
- Xiaomin Zhi; Yuqian Jiang; Linlin Xie; Yanbo Li; Chen-Jie Fang; Gold Nanorods Functionalized with Cathepsin B Targeting Peptide and Doxorubicin for Combinatorial Therapy against Multidrug Resistance. ACS Applied Bio Materials 2019, 2, 5697-5706.
- Arnold Lee; Loncastuximab Tesirine: First Approval. Drugs 2021, 81, 1229-1233.
- Kendall C. Shultes; Loncastuximab Tesirine-lpyl (Zynlonta®). Oncology Times 2022, 44, 14-14.
- A. Copeland; A. Younes; Brentuximab vedotin. Drugs of the Future 2010, 35, 797.
- Peter D Senter; Eric L Sievers; The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nature Biotechnology 2012, 30, 631-637.
- Emma D. Deeks; Polatuzumab Vedotin: First Global Approval. Drugs 2019, 79, 1467-1475.
- Padcev (Enfortumab Vedotin-ejfv) FDA Approved for the Treatment of Metastatic Urothelial Carcinoma. . FDA. Retrieved 2023-7-13
- Kahl, B.S.; Hamadani, M.; Radford, J.; Carlo-Stella, C.; Caimi, P.; Reid, E.; Feingold, J.M.; Ardeshna, K.M.; Solh, M.; Heffner, L.T.; et al. A Phase I Study of ADCT-402 (Loncastuximab Tesirine), a Novel Pyrrolobenzodiazepine-Based Antibody–Drug Conjugate, in Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma Phase I Study of ADCT-402 in Relapsed/Refractory B-Cell NHL.. Clin. Cancer Res. 2019, 25, 6986–6994..