Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Spyridon Kintzios | -- | 3456 | 2023-07-13 10:55:27 | | | |
| 2 | Sirius Huang | Meta information modification | 3456 | 2023-07-14 02:58:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Golfinopoulou, R.; Kintzios, S. Biosensors for Autoimmune Chronic Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/46745 (accessed on 04 February 2026).
Golfinopoulou R, Kintzios S. Biosensors for Autoimmune Chronic Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/46745. Accessed February 04, 2026.
Golfinopoulou, Rebecca, Spyridon Kintzios. "Biosensors for Autoimmune Chronic Disease" Encyclopedia, https://encyclopedia.pub/entry/46745 (accessed February 04, 2026).
Golfinopoulou, R., & Kintzios, S. (2023, July 13). Biosensors for Autoimmune Chronic Disease. In Encyclopedia. https://encyclopedia.pub/entry/46745
Golfinopoulou, Rebecca and Spyridon Kintzios. "Biosensors for Autoimmune Chronic Disease." Encyclopedia. Web. 13 July, 2023.
Copy Citation
Although relatively rare, affecting 10% of the general population, autoimmune disorders are causative linked with chronic diseases and morbidity. Control of the course of the disease is closely dependent on the ability to monitor its onset, as well as its response to treatment.
autoantibodies
autoimmune
biomarker
biosensor
chronic disease
cytokines
microRNAs
point-of-care
1. Introduction
Autoimmunity refers to long-term disorders that are characterized by the presence of autoantibodies that bind proteins of the human body, protein complexes or polypeptides, resulting in an autoimmune attack that is responsible for the pathogenesis of a disease [1]. Autoimmune diseases are classified into two categories: organ-specific, such as type I diabetes mellitus (DM), Graves’ disease and other thyroid disorders, myasthenia gravis (MG), etc., and systemic diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) or systemic sclerosis.
Detection of autoantibodies is mainly conducted by analytical techniques. Currently, enzyme-linked immunosorbent assay (ELISA), indirect immunofluorescence (IIF) and Western blotting are the most common traditional methods that have been developed for AAbs detection in autoimmune disorders [2]. All these techniques require specialized personnel and equipment, are time consuming and have a high cost and, in some cases, can only detect autoantibodies at high concentrations, co-occurring with permanent tissue damage, thus making their sensitivity as diagnostic methods less satisfactory [3]. Considering these limitations, it is important to develop new and reliable techniques for the detection of autoantibodies as a means to achieve early diagnosis in autoimmune diseases.
Biosensors are integrated receptor–transducer devices that combine a receptor (biochemical recognition system) and a detector (transducer), which transforms the biochemical (biological) response into a measurable output signal [4]. In 1962, Clark and Lyons introduced the first biosensor that meets this definition: an amperometric biosensor designed to monitor glucose that was called an “enzyme electrode” [5]. It was probably the first usable biosensor that led to a widespread use of electrochemical glucose sensors by patients at home and has led to a revolutionary care of patients with DM by allowing them to monitor their glucose levels at home instead of in a lab [6]. In this way, patients have more control over their disease and can achieve better management by having the option to measure glucose levels at their own convenience, more frequently and at a lower cost [7]. This is a good example of how modern glucose sensors helped achieve the current goals in the point of care (POC) with a simple measurement protocol, one that is foolproof and can be used by an untrained patient. In this case, a small portable device is used to draw a small quantity of blood sample, accompanied with test strips that are inserted into a meter that offers a readout on glucose level and provides the necessary information to the patient in a simple manner. The protocol can be more demanding in some cases, such as with immunosensors used for many types of biomolecules, that often require extra reagents and more steps and would need to be more automated and with a simple packaging suitable for POC use [8].
2. Biosensors
As mentioned, biosensors, as self-contained analytical devices, combine both a biological element and a signal transducer. The biological element is immobilized on a solid-state surface and enables a bio-specific interaction with the target analyte. The output signal caused by the binding of the analyte can be obtained by using labeled compounds or not, thus classifying the biosensors respectively as labeled and label-free types. One of the commonly used methods for labeling involves the use of enzymes, radionuclides, nanoparticles and fluorescent or electrochemiluminescent probes. There are various test formats available, including sandwich assays, competitive assays and indirect assays [9]. The primary benefit of labeled test formats is their ability to detect lower concentrations with greater accuracy, although higher operational costs are usually implied with longer assay times in comparison to most label-free test formats, which are performed without any labeled compound and are distinguished between direct and indirect assays [10].
Direct assays are a simpler and inexpensive approach, as the signal response changes when analyte molecules bind to the transducer surface with no need for further steps or reagents in the procedure. In some cases, this simplicity could be a disadvantage when dealing with complex samples, as there could be binding of other non-analyte components onto the sensor surface and a false-positive result may arise. These kinds of unwanted effects can be resolved by ensuring that a significant change in the biosensor signal response is achieved only by the binding of the analyte to the corresponding element that is immobilized on the biosensor surface or using labeled test formats where the labeled compound usually determines the final biosensor signal response.
When considering the use of a biosensor for diagnostic applications, the core question of single use or multiuse arises. It is more than the biosensor itself that needs to be considered during the development stage to create a user-friendly and reliable device. The degree of specificity is determined by the biorecognition elements used in the biosensor, the majority of which bind with high affinity to the analyte molecules. The sensitivity is primarily influenced by the detector element that turns the biological response into a quantifiable output signal [11]. In the multiuse option, the sensing capability of the biosensor needs to be regained, and often, a regeneration step that leads to dissociating the binding between the biorecognition element and the analyte is necessary. This regeneration procedure may require additional expenses that exceed the savings that the use of multiuse biosensors provides. When considering the development of a POC-use sensor, single-use devices that are disposable are usually preferred [12], and similar considerations are applied to all system elements that come into contact with the sample provided.
One of the major challenges of creating a biosensor is to detect the disease biomarker selectively among a vast number of proteins and other elements in the sample that could potentially interfere with the analysis and the result. A patient’s prognosis can be benefited by the quantification of the appropriate biomarkers for that specific disease that can be detected in tissue or body fluids [13]. Selectively detecting these biomarkers requires meticulous consideration of the efficiency of the bioreceptor itself, its immobilization on the sensors surface and the way the signal is transduced, so that the output signal can be maximized, and the response to components bound nonspecifically is minimized. In Figure 1, the historical development of biosensors for selected chronic diseases is presented.

Figure 1. Biosensors development timeline.
2.1. Categories
2.1.1. Electrochemical
Due to their sensitivity, selectivity, quick response and cost effectiveness, electrochemical devices are the most common biosensors used for the early detection of disease-related biomarkers. Additionally, these biosensors have drawn a lot of interest as suitable POC tests for diagnostic purposes. Numerous nanobiosensors and electrochemical biosensors have been reported in the literature for the detection of different biomarkers in autoimmune diseases. The most frequently used techniques include amperometric, impedimetric and voltametric techniques [14][15][16]. Functionalized nanomaterials, such as metallic nanoparticles, conducting polymers and others, in association with electrochemical systems improved electron transfer, consequently improving the electrical signal transduction [17].
Amperometric biosensors use a fixed voltage to detect the amount of an analyte by measuring the produced current. Regarding autoimmune diseases and rheumatoid arthritis in particular, an amperometric nanobiosensor (NGP-NTiP-Thi) based on gold nanoparticles, titanium dioxide nanoparticles and thionine was designed by Li et al. in 2008 to detect macrophage migration inhibitory factor (MIF) in the serum of RA patients [18]. MIF was recognized by the IgM immunosensor in a linear relationship with the lower limit (S/N = 3) of 0.02 ng/mL. Villa et al. in 2011 [19] created an amperometric immunosensor for the measurement of anti-citrullinated peptide antibodies (ACPAs), a possible biomarker for the diagnosis of rheumatoid arthritis. A composite made of multiwalled carbon nanotubes and polystyrene (MWCNT-PS) was used to increase the platform’s sensitivity, achieving detection limits similar to those achieved with ELISA. Concerning systemic lupus erythematosus (SLE), in 2018, Fagúndez et al. [20] developed a sandwich-format electrochemical immunosensor for the anti-double-stranded DNA (anti-dsDNA) autoantibodies assay in serum samples from patients. The assay requires a total of only 30 min, and the sensor is capable of detecting 16 ng (8 μg mL−1) of α-dsDNA antibodies, according to the team [20].
A potent and nondestructive approach that has lately become more widely utilized as a diagnostic tool is the electrical impedance spectroscopy (EIS) biosensor. In multiple sclerosis (MS), two label-free EIS biosensors have been fabricated. The first is a cytokine immunosensor by Bhavsar et al. (2009), designed for MS diagnosis by detecting interleukin-12 (IL-12), and results indicate that this sensor can detect IL-12 at physiological levels <100 fM [21]. In a study conducted by Derkus et al. in 2013, a label-free electrochemical impedance immunosensor to determine anti-myelin basic protein (anti-MBP) autoantibodies in patients with MS was produced. In this biosensor, the use of titaniumdioxide (TiO2) nanoparticles resulted in a satisfactory detection range with the gelatin-MBP detection limit at 0.1528 ng mL−1 and gelatin-TiO2-MBP immunosensors detection limit of 0.1495 ng mL−1 [22].
Regarding voltametric biosensors, they utilize a variety of techniques, including square wave voltammetry (SWV), linear sweep voltammetry (LSV), differential pulse voltammetry (DPV) and cyclic voltammetry (CV). Their high sensitivity, selectivity and cost-effectiveness and their capacity for simultaneous quantification of their targets classify them among the most extensively used and available biosensor-based approaches for the detection of specific biomarkers in autoimmune disorders. In 2017, a new nanoimmunosensor based on graphene oxide (GO)/[poly(propyleneglycol)] (pPG) nanocomposite was published by Derkus et al. for simultaneously detecting MBP and Tau proteins in MS patients using serum and cerebrospinal fluid (CSF) as samples [23]. This team, in order to achieve the simultaneous quantification of MBP and Tau proteins in CSF and serum, modified the surface of screen-printed carbon electrodes (SPCE) using amine- and GO-functionalized first-generation trimethylolpropane tris[poly(propyleneglycol)] dendrimers (pPG). MBP and Tau antibodies were immobilized using a new carrier GO/pPG nanocomposite structure for the creation of a nanoimmunosensor. The next step was to apply to the matrix a secondary antibody conjugated with carboxyl-functionalized 3.5th-generation pPG/CdS and pPG/PbS probes and to obtain a sandwich complex. Following this phase, the nanoimmunosensor was characterized and optimized using electrochemical signals of Cd2+ and Pb2+ that were created by the ionization impact of nitric acid. This suggested approach aimed to quantify simultaneously with increased sensitivity both targets, and the developed nanoimmunosensor had detection limits of 0.30 nM for MBP and 0.15 nM for Tau proteins [23].
Another autoimmune disease for which voltametric biosensors have been studied is celiac disease (CeD), an inflammatory disorder mediated by T-cells in the upper small intestine caused by consuming gluten [24]. Examples of early detection of anti-tTG antibodies in patients include the nanobiosensor developed by Gupta et al. [25], a GQD/PAMAM nanohybrid-modified AuNP embedded in MWCNT (multiwalled carbon nanotube) with a lower limit of detection of anti-tissue transglutaminase antibody at 0.1 fg per 6 μL and another nanoimmunosensor based on cyclic voltammetry developed by Neves et al. [26]. With the use of cyclic voltammetry and square wave voltammetry, the team of Bellagha-Chenchah et al. developed an electrochemical biosensor in order to detect autoantibodies against CSG114(Glc), a synthetic glycopeptide [27] with the potential to detect antibodies in patients with MS [28]. The synthetic glycopeptide was modified in order to be suitable for bioelectrochemical detection, and a lower sample volume could be used because of a series of platinum microband electrodes employed on microfluidic channels, which offer other advantages, as well, toward the development of this type of system as a POC test device [28].
Another detection method that also has high sensitivity, fast response and low background signals is the photoelectrochemical (PEC) method [29]. This method has been studied for use in the early diagnosis of rheumatoid arthritis by the team of Pang et al., who developed a PEC biosensor based on ZnO nanorod (NR)/CH3NH3PbI3/nitrogen-doped carbon quantum dot (NCQD) nanocomposites for rapid determination of fibroblast-like synoviocyte (FLS) cells that showed a low detection limit of 2 cell/mL [30]. Overall, biosensors based on electrochemical methods are frequently used as a tool for the early detection of autoimmune diseases because of their several advantages, including their rapid detection capability, the fact that they can be reusable and the fact that they require a low sample volume. The quantitative analysis is feasible but false-positive results can occur originating from matrix electrolytes and lack of adequate control of the response of the working electrode at higher currents [31].
2.1.2. Optical Biosensors
A compact analytical device that contains a biorecognition sensing element that is integrated with an optical transducer system is characterized as an optical biosensor, and its main objective is to produce a proportionate signal to the concentration of the analyte (the measured substance). The exploitation of the interaction of the optical field with a biorecognition element provides optical detection. As a category, they can be divided into label based and label free. The first category, label-based biosensors, involve the use of a specific label and a method (fluorescent, luminescent or colorimetric) to generate the optical signal. On the other hand, the label-free biosensors generate the signal directly by the interaction of the transducer with the sample [32]. The advantages of label-free detection can be offered by optical biosensors as well, and in the field of autoimmune diseases, the screening of biomarkers for diagnostic purposes has recently evolved. A number of studies for the detection of specific biomarkers have been conducted regarding optical biosensors.
The use of electrochemiluminescence (ECL) has advantages including high sensitivity, a wide detection range, simple controllability and a rapid response, and thus, ECL biosensors have a wide use to detect biomarkers [33]. Rheumatoid arthritis has been one of the autoimmune diseases targeted with an attempt to have early diagnosis by sensitive quantification of the anti-CCP (anti-cyclic citrullinated peptide) antibody by the team of Zhao et al. [34]. Their team developed a label-free ECL sensor that is based on asymmetric heterogeneous polyaniline–gold (PANI-Au) nanomaterial, which was incorporated with graphite-like carbon nitride (g-C3N4) in order to improve stability with a lower detection limit of 0.2 pg mL−1 obtained for the determination of an anti-CCP antibody. Another common and widely used optical method is fluorescence. In the case of MS, the team of Mansourian et al. [35] developed a biosensor for determining microRNA-145 as a biomarker in patients’ plasma, based on fluorescent DNA-hosted silver nanoclusters (AgNCs) and hybridization chain reaction (HCR) amplification. The use of silver nanoclusters is considered to have many advantages, including simple synthesis, low toxicity, high stability and biocompatibility [36].
Fluorescence enhancement, along with surface-enhanced Raman scattering (SERS), has been applied to develop ultrasensitive bioassays. Fluorescence is enhanced in the vicinity of metals that might interact with both the stimulating laser beam and the fluorophores that are radiating. For example, a paper-based nanoplatform was created by Campu et al. for LSPR, SER and metal-enhanced fluorescence (MEF)-based multimodal biodetection [37][38]. Gold nanobipyramids (Au-BPs) were used to deposit the modulated platform with variable LSPR responses onto the cellulose fiber using a commercial pen and a plasmonic calligraphy technique. Target proteins were detected using three different sensing approaches. In order to provide portable point-of-care diagnostics, the researchers combined several LSPR, SERS and MEF nanosensors with multiplex capabilities into a single flexible and portable plasmonic nanoplatform [37].
SERS has developed into a high-throughput detection technique with a wide range of substrates [39] that can be fabricated, and clinical diagnosis [40][41] is among the possible applications of this technique [42]. As a technique with huge potential when combined with the technological progress achieved in the development of related instrumentation, vast research is being conducted around SERS, and a broad spectrum of analytical, physical and chemical applications has been proposed. The practical accomplishment of nanostructures with outstanding quality and customized morphologies, in some cases having smaller and more precisely engineered 2D and 3D nanogaps, has been one of the main driving forces behind recent advancements in SERS. These manufacturing methodologies and methods have also made it possible to produce substrates and nanotags on a wide scale with a reliable SERS response for both general and goal-oriented sensing applications.
Surface plasmon resonance (SPR) and surface plasmon resonance imaging (SPRi) are also techniques used in optical biosensors for detecting specific biomarkers in patients with autoimmune diseases. The SPR phenomenon occurs when the surface of metal is illuminated by polarized light at a specific angle (resonant angle) and electrons get excited (plasmon) when they absorb light energy. The intensity of the reflected laser beam decreases with the resonance angle, and the number of absorbed molecules on the metal surface strongly influences resonance, thus allowing for a calibration curve for a specific analyte by a resonance angle change [43][44].
In general, optical biosensors, by enabling the direct, real-time and label-free detection of many different biomarkers, provide advantages over analytical techniques used for detecting autoimmunity. Currently, they are a widespread technology, and scientists focus on improving the sensitivity and resolution of a conventional SPR, with modifications including surface plasmon resonance imaging (SPRi), long-range surface plasmon (LRSP) and localized surface plasmon resonance (LSPR) in the area of biosensing with a direction to make the technology compatible with miniaturization for portable devices [45]. A new analytical tool may emerge through their application for autoantibody detection in several autoimmune diseases, common or rarer, such as myasthenia gravis, in which patients present autoantibodies against AChR (acetylcholine receptor) and other proteins of the neuromuscular junction [46]. They may be of great diagnostic interest, as they can allow for an analysis of the serum level of the autoantibody in question and, furthermore, of its binding characteristics, making them applicable to a wide spectrum of autoimmune disorders and their serological evaluation [3].
2.1.3. Mechanical Biosensors
In interactions between biomolecules such as biorecognition, mechanical transducers may detect changes in mechanical parameters such as mass, surface stress and viscoelasticity [47]. Their complexity makes them less common and popular than optical or electrochemical biosensors. This type of biosensor can be categorized into four broad categories depending on the chemical interactions of the sensor and the analyte: (1) affinity-based assays that achieve highly selective target identification by applying high specificity between the device surface and the target, (2) separation-based assays, where spatiotemporal separation of analytes is permitted because of chemical affinities between immobilized molecules and flowing analytes, (3) fingerprint assays, where the target is identified through specific binding affinities to sensors and (4) spectrometric assays, where the target’s identification is enabled through deducing its optical properties or its mass [48].
Quartz crystal microbalance (QCM) sensors are the most established ones, which are based on quartz crystal resonators [49]. They are centimeter-scale mechanical resonators that measure the mass of analytes on their surface in fluid, gas or vacuum. When the crystal is deformed with the use of the piezoelectric technique, a mass change occurs when the analyte binds to the biorecognition element that is immobilized on the crystal surface. There have been attempts to develop RA-specific peptide-coated single-walled carbon nanotube (SWCNT)-based QCM biosensors [50]. By binding an RA-specific peptide (containing citric citrulline) to a functionalized SWCNT and depositing it on a QCM sensing crystal, it was possible to identify the related autoantibodies in patients’ serum. The finding demonstrated that the QCM sensor detected 34.4% more RA patients with anti-citrullinated peptide antibodies than those detected by the classic analytical method of ELISA and 37.5% more patients than microarray analysis [50].
Biology is fundamentally based on mechanical interactions. On a cellular level, mechanical forces of chemical origin control adhesion and motility, and on a molecular level, they control transport and affinity. Unique opportunities to detect forces, displacements and changes in mass from cellular and subcellular activities are provided by biological sensing in the mechanical domain [51]. Mechanical biosensors often benefit from properties that scale advantageously as physical size is decreased. Exquisite mass resolution is provided by nanoscale mechanical sensors. Nanoelectromechanical systems (NEMS) have achieved nanogram resolution in a fluid environment and zeptogramscale mass resolution when operating in a vacuum. The ability of a device’s mechanical compliance to be displaced or disformed is significantly increased by uniform reduction in its dimensions [52]. One of the biggest challenges for all NEMS devices has been the development of transduction and actuation methods that are efficient, but significant advances have been made over these past years [53].
Mechanical biosensors, such as QCM, have the benefit of being very sensitive, having a large dynamic range of detection ranging from nanomolar to femtomolar ranges. Another benefit is that they are flexible enough to utilize almost any surface coating for tests [52]. The main disadvantage of mechanical biosensors is that handling samples is often cumbersome and susceptible to measurement artifacts [54]. There are still many challenges, from integrating arrays of advanced nanosensors with conventional techniques to developing better capture agents, but developing tools capable of high-throughput studies at the level of a single cell for understanding biological systems remains the goal for developing tools that will bring advances in the field.
A schematical overview of the main biosensing approaches for the diagnosis and monitoring of autoimmune diseases is presented in Figure 2.
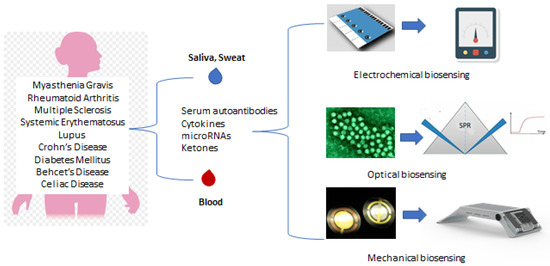
Figure 2. Overview of the main biosensing approaches for the diagnosis and monitoring of chronic and autoimmune diseases.
References
- Tozzoli, R. The diagnostic role of autoantibodies in the prediction of organ-specific autoimmune diseases. Clin. Chem. Lab. Med. 2008, 46, 577–587.
- Conrad, N.; Misra, S.; Verbakel, J.Y.; Verbeke, G.; Molenberghs, G.; Taylor, P.N.; Mason, J.; Sattar, N.; McMurray, J.J.V.; McInnes, I.B.; et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: A population-based cohort study of 22 million individuals in the UK. Lancet 2023, 401, 1878–1890.
- Zhang, X.; Zambrano, A.; Lin, Z.-T.; Xing, Y.; Rippy, J.; Wu, T. Immunosensors for Biomarker Detection in Autoimmune Diseases. Arch. Immunol. Ther. Exp. 2017, 65, 111–121.
- Conrad, K.; Roggenbuck, D.; Reinhold, D.; Sack, U. Autoantibody diagnostics in clinical practice. Autoimmun. Rev. 2012, 11, 207–211.
- Campuzano, S.; Pedrero, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical biosensors for autoantibodies in autoimmune and cancer diseases. Anal. Methods 2019, 11, 871–887.
- Thaler, M.; Buhl, A.; Welter, H.; Schreiegg, A.; Kehrel, M.; Alber, B.; Metzger, J.; Luppa, P.B. Biosensor analyses of serum autoantibodies: Application to antiphospholipid syndrome and systemic lupus erythematosus. Anal. Bioanal. Chem. 2009, 393, 1417–1429.
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131.
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45.
- Forster, J.R.; Cumba, R.L. Optimizing Glucose Sensing for Diabetes Monitoring. In Electronic and Optical Materials, Bioelectronics and Medical Devices; Pal, K., Kraatz, H.-B., Khasnobish, A., Bag, S., Banerjee, I., Kuruganti, U., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 765–778.
- Jones, A.; Dhanapala, L.; Kankanamage, R.N.T.; Kumar, C.V.; Rusling, J.F. Multiplexed Immunosensors and Immunoarrays. Anal. Chem. 2020, 92, 345–362.
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95.
- Jiang, X.; Li, D.; Xu, X.; Ying, Y.; Li, Y.; Ye, Z.; Wang, J. Immunosensors for detection of pesticide residues. Biosens. Bioelectron. 2008, 23, 1577–1587.
- Gauglitz, G. Direct optical sensors: Principles and selected applications. Anal. Bioanal. Chem. 2005, 381, 141–155.
- Leca-Bouvier, B.; Blum, L.J. Biosensors for Protein Detection: A Review. Anal. Lett. 2005, 38, 1491–1517.
- Mascini, M.; Tombelli, S. Biosensors for biomarkers in medical diagnostics. Biomarkers 2008, 13, 637–657.
- Spain, E.; Keyes, T.E.; Forster, R.J. DNA sensor based on vapour polymerised pedot films functionalised with gold nanoparticles. Biosens. Bioelectron. 2013, 41, 65–70.
- Huang, Y.; Xu, J.; Liu, J.; Wang, X.; Chen, B. Disease-Related Detection with Electrochemical Biosensors: A Review. Sensors 2017, 17, 2375.
- Li, S.; Zhang, R.; Li, P.; Yi, W.; Zhang, Z.; Chen, S.; Su, S.; Zhao, L.; Hu, C. Development of a novel method to measure macrophage migration inhibitory factor (MIF) in sera of patients with rheumatoid arthritis by combined electrochemical immunosensor. Int. Immunopharmacol. 2008, 8, 859–865.
- de Gracia Villa, M.; Jiménez-Jorquera, C.; Haro, I.; Gomara, M.J.; Sanmartí, R.; Fernández-Sánchez, C.; Mendoza, E. Carbon nanotube composite peptide-based biosensors as putative diagnostic tools for rheumatoid arthritis. Biosens. Bioelectron. 2011, 27, 113–118.
- Fagúndez, P.; Brañas, G.; Cairoli, E.; Laíz, J.; Tosar, J.P. An electrochemical biosensor for rapid detection of anti-dsDNA antibodies in absolute scale. Analyst 2018, 143, 3874–3882.
- Bhavsar, K.; Fairchild, A.; Alonas, E.; Bishop, D.K.; La Belle, J.T.; Sweeney, J.; Alford, T.; Joshi, L. A cytokine immunosensor for Multiple Sclerosis detection based upon label-free electrochemical impedance spectroscopy using electroplated printed circuit board electrodes. Biosens. Bioelectron. 2009, 25, 506–509.
- Derkus, B.; Emregul, E.; Yucesan, C.; Emregul, K.C. Myelin basic protein immunosensor for multiple sclerosis detection based upon label-free electrochemical impedance spectroscopy. Biosens. Bioelectron. 2013, 46, 53–60.
- Derkus, B.; Bozkurt, P.A.; Tulu, M.; Emregul, K.C.; Yucesan, C.; Emregul, E. Simultaneous quantification of Myelin Basic Protein and Tau proteins in cerebrospinal fluid and serum of Multiple Sclerosis patients using nanoimmunosensor. Biosens. Bioelectron. 2017, 89, 781–788.
- Pasinszki, T.; Krebsz, M. Chapter One—Advances in celiac disease testing. Adv. Clin. Chem. 2019, 91, 1–29.
- Gupta, S.; Kaushal, A.; Kumar, A.; Kumar, D. Ultrasensitive transglutaminase based nanosensor for early detection of celiac disease in human. Int. J. Biol. Macromol. 2017, 105, 905–911.
- Neves, M.M.; González-García, M.B.; Nouws, H.P.; Costa-García, A. Celiac disease detection using a transglutaminase electrochemical immunosensor fabricated on nanohybrid screen-printed carbon electrodes. Biosens. Bioelectron. 2012, 31, 95–100.
- Lolli, F.; Mazzanti, B.; Pazzagli, M.; Peroni, E.; Alcaro, M.C.; Sabatino, G.; Lanzillo, R.; Morra, V.B.; Santoro, L.; Gasperini, C.; et al. The glycopeptide CSF114(Glc) detects serum antibodies in multiple sclerosis. J. Neuroimmunol. 2005, 167, 131–137.
- Bellagha-Chenchah, W.; Sella, C.; Fernandez, F.R.; Peroni, E.; Lolli, F.; Amatore, C.; Thouin, L.; Papini, A. Interactions between Human Antibodies and Synthetic Conformational Peptide Epitopes: Innovative Approach for Electrochemical Detection of Biomarkers of Multiple Sclerosis at Platinum Electrodes. Electrochim. Acta 2015, 176, 1239–1247.
- Abolhasan, R.; Mehdizadeh, A.; Rashidi, M.R.; Aghebati-Maleki, L.; Yousefi, M. Application of hairpin DNA-based biosensors with various signal amplification strategies in clinical diagnosis. Biosens. Bioelectron. 2019, 129, 164–174.
- Pang, X.; Zhang, Y.; Pan, J.; Zhao, Y.; Chen, Y.; Ren, X.; Ma, H.; Wei, Q.; Du, B. A photoelectrochemical biosensor for fibroblast-like synoviocyte cell using visible light-activated NCQDs sensitized-ZnO/CH3NH3PbI3 heterojunction. Biosens. Bioelectron. 2016, 77, 330–338.
- Ghorbani, F.; Abbaszadeh, H.; Dolatabadi, J.E.N.; Aghebati-Maleki, L.; Yousefi, M. Application of various optical and electrochemical aptasensors for detection of human prostate specific antigen: A review. Biosens. Bioelectron. 2019, 142, 111484.
- Damborsky, P.; Svitel, J.; Katrlik, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100.
- Liu, S.; Tong, Z.; Mu, X.; Liu, B.; Du, B.; Liu, Z.; Gao, C. Detection of Abrin by Electrochemiluminescence Biosensor Based on Screen Printed Electrode. Sensors 2018, 18, 357.
- Zhao, Y.; Liu, Y.; Li, X.; Wang, H.; Zhang, Y.; Ma, H.; Wei, Q. Label-free ECL immunosensor for the early diagnosis of rheumatoid arthritis based on asymmetric heterogeneous polyaniline-gold nanomaterial. Sens. Actuators B Chem. 2018, 257, 354–361.
- Mansourian, N.; Rahaie, M.; Hosseini, M. A Nanobiosensor Based on Fluorescent DNA-Hosted Silver Nanocluster and HCR Amplification for Detection of MicroRNA Involved in Progression of Multiple Sclerosis. J. Fluoresc. 2017, 27, 1679–1685.
- Dong, H.; Hao, K.; Tian, Y.; Jin, S.; Lu, H.; Zhou, S.-F.; Zhang, X. Label-free and ultrasensitive microRNA detection based on novel molecular beacon binding readout and target recycling amplification. Biosens. Bioelectron. 2014, 53, 377–383.
- Campu, A.; Susu, L.; Orzan, F.; Maniu, D.; Craciun, A.M.; Vulpoi, A.; Roiban, L.; Focsan, M.; Astilean, S. Multimodal Biosensing on Paper-Based Platform Fabricated by Plasmonic Calligraphy Using Gold Nanobypiramids Ink. Front. Chem. 2019, 7, 55.
- Susu, L.; Campu, A.; Astilean, S.; Focsan, M. Calligraphed Selective Plasmonic Arrays on Paper Platforms for Complementary Dual Optical “ON/OFF Switch” Sensing. Nanomaterials 2020, 10, 1025.
- Itoh, T.; Procházka, M.; Dong, Z.-C.; Ji, W.; Yamamoto, Y.S.; Zhang, Y.; Ozaki, Y. Toward a New Era of SERS and TERS at the Nanometer Scale: From Fundamentals to Innovative Applications. Chem. Rev. 2023, 123, 1552–1634.
- Debreczeni, M.L.; Szekacs, I.; Kovacs, B.; Saftics, A.; Kurunczi, S.; Gál, P.; Dobó, J.; Cervenak, L.; Horvath, R. Human primary endothelial label-free biochip assay reveals unpredicted functions of plasma serine proteases. Sci. Rep. 2020, 10, 3303.
- Avram, L.; Iancu, S.D.; Stefancu, A.; Moisoiu, V.; Colnita, A.; Marconi, D.; Donca, V.; Buzdugan, E.; Craciun, R.; Leopold, N.; et al. SERS-Based Liquid Biopsy of Gastrointestinal Tumors Using a Portable Raman Device Operating in a Clinical Environment. J. Clin. Med. 2020, 9, 212.
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117.
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, 15, 10481–10510.
- Shrivastav, A.M.; Cvelbar, U.; Abdulhalim, I. A comprehensive review on plasmonic-based biosensors used in viral diagnostics. Commun. Biol. 2021, 4, 70.
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628.
- Golfinopoulou, R.; Papageorgiou, L.; Efthimiadou, A.; Bacopoulou, F.; Chrousos, G.P.; Eliopoulos, E.; Vlachakis, D. Clinical Genomic, phenotype and epigenetic insights into the pathology, autoimmunity and weight management of patients with Myasthenia Gravis (Review). Mol. Med. Rep. 2021, 24, 512.
- Tamayo, J.; Kosaka, P.M.; Ruz, J.J.; Paulo, Á.S.; Calleja, M. Biosensors based on nanomechanical systems. Chem. Soc. Rev. 2013, 42, 1287–1311.
- Arlett, J.; Myers, E.; Roukes, M. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011, 6, 203–215.
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519.
- Drouvalakis, K.A.; Bangsaruntip, S.; Hueber, W.; Kozar, L.G.; Utz, P.J.; Dai, H. Peptide-coated nanotube-based biosensor for the detection of disease-specific autoantibodies in human serum. Biosens. Bioelectron. 2008, 23, 1413–1421.
- Ekinci, K.L. Electromechanical Transducers at the Nanoscale: Actuation and Sensing of Motion in Nanoelectromechanical Systems (NEMS). Small 2005, 1, 786–797.
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63.
- Florea, A.; Melinte, G.; Simon, I.; Cristea, C. Electrochemical Biosensors as Potential Diagnostic Devices for Autoimmune Diseases. Biosensors 2019, 9, 38.
- Anstey, N.M.; Bastian, I.; Dunckley, H.; Currie, B.J. Systemic lupus erythematosus in Australian Aborigines: High prevalence, morbidity and mortality. Aust. N. Z. J. Med. 1993, 23, 646–651.
More
Information
Subjects:
Pathology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
14 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




