Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Li Qi | -- | 1601 | 2023-07-13 09:37:24 | | | |
| 2 | Wendy Huang | Meta information modification | 1601 | 2023-07-14 06:34:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tang, K.; Zhang, S.; Liang, Z.; Wang, Y.; Ge, J.; Chen, W.; Qi, L. The Importance of Photoacoustic Tomography Image Post-Processing. Encyclopedia. Available online: https://encyclopedia.pub/entry/46734 (accessed on 09 March 2026).
Tang K, Zhang S, Liang Z, Wang Y, Ge J, Chen W, et al. The Importance of Photoacoustic Tomography Image Post-Processing. Encyclopedia. Available at: https://encyclopedia.pub/entry/46734. Accessed March 09, 2026.
Tang, Kaiyi, Shuangyang Zhang, Zhichao Liang, Yang Wang, Jia Ge, Wufan Chen, Li Qi. "The Importance of Photoacoustic Tomography Image Post-Processing" Encyclopedia, https://encyclopedia.pub/entry/46734 (accessed March 09, 2026).
Tang, K., Zhang, S., Liang, Z., Wang, Y., Ge, J., Chen, W., & Qi, L. (2023, July 13). The Importance of Photoacoustic Tomography Image Post-Processing. In Encyclopedia. https://encyclopedia.pub/entry/46734
Tang, Kaiyi, et al. "The Importance of Photoacoustic Tomography Image Post-Processing." Encyclopedia. Web. 13 July, 2023.
Copy Citation
Photoacoustic tomography (PAT) is a promising imaging technique that utilizes the detection of light-induced acoustic waves for both morphological and functional biomedical imaging. However, producing high-quality images using PAT is still challenging and requires further research. Besides improving image reconstruction, an alternative way to address this issue is through image post-processing, which enhances and optimizes the reconstructed PAT image. Image post-processing methods have rapidly emerged in PAT and are proven to be essential in improving image analysis performance.
image post-processing
photoacoustic tomography
image enhancement
image reconstruction
1. Introduction
Photoacoustic tomography (PAT) is a non-invasive, non-ionizing biomedical imaging technique that enables the reconstruction of the spatial distribution of photoacoustic pressure in the body. The photoacoustic effect [1], which underlies photoacoustic imaging, was first reported by Alexander Graham Bell in 1880. This effect occurs when a pulse, such as an optical or radio frequency wave, is absorbed by tissue. PAT offers high ultrasonic resolution and strong optical contrast in a single imaging modality. It is capable of providing high-resolution structural and molecular imaging in vivo by optically scattering biological media. By utilizing the photoacoustic effect, PAT overcomes the scattering of high-intensity light photons within biological tissues. As shown in Figure 1a, short pulses of laser-generated energy are directed to the tissue, generating thermal and acoustic pulse responses. The absorbed light is converted into heat, causing a rise in pressure due to the thermoelastic expansion of the irradiated tissues. The increased pressure is associated with localized energy injection and absorption from the light, along with other thermal and mechanical characteristics of the tissues, resulting in the generation of a photoacoustic signal. This signal is recorded using the tissue-facing side of an ultrasonic transducer, which is then amplified and digitized. A computational algorithm is used to process the PA signal, thus forming a PAT image.
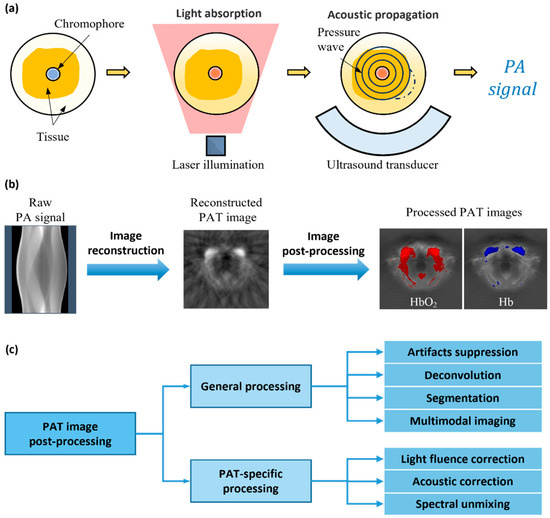
Figure 1. (a) PAT imaging principle. (b) PAT signal and image processing flow. (c) Categories of PAT image post-processing techniques.
The quality of PAT images is limited by the imaging hardware, signal acquisition and processing operations, and image reconstruction algorithm. The size and bandwidth of transducer elements are typically limited, and the geometry of the constituent arrays has a significant impact on the imaging results. The inverse model in the reconstruction algorithm is often an approximate and simplified description of the transducer array and medium properties. Imperfect illumination conditions and discrete data acquisition also affect the quality of the collected PA signal, ultimately impacting the fidelity in the resulting images. As a result of these factors, PAT images can be noisy and blurred or even distorted. Numerous methods were previously suggested to deal with the above challenges, including improved image reconstruction [2][3][4][5] and image post-processing methods. Specifically, as shown in Figure 1b, image reconstruction involves turning the raw signal into a desired image, while image post-processing entails enhancing and optimizing the reconstructed images. While a majority of previous research in PAT imaging was dedicated to image reconstruction [6], there has been a recent surge in studies focused on PAT image post-processing methods. As researchers delve deeper into the potential of the image post-processing technique, it is becoming increasingly evident that it plays a crucial role in enhancing the accuracy and reliability of PAT.
In an ideal PAT imaging scenario, a stationary object is illuminated by a high-power light source in an acoustically homogeneous medium. A transducer array with a wide bandwidth and detection angle is used to detect the PA signal. The image reconstruction algorithm must be robust, taking into account tissue properties while performing real-time operation. However, real-world conditions do not always satisfy these criteria. The cost and technical level of the imaging hardware reduces the accuracy of the acquisition process. Also, the biological properties of the tissue are obscured in the reconstruction algorithm by the crude estimation of the simplified imaging model. This issue leads to a deterioration in the reconstructed images’ quality. Therefore, to compensate for these problems, image enhancement using post-processing methods is required. Table 1 underscores the importance of image post-processing techniques for improving the final result.
Table 1. Main limiting factors of PAT imaging quality. Bulleted symbol (√) indicates correspondence between limiting factors (table rows) and manifestations (table columns).
| Structural Distortion | Spatial Aliasing | Negative Value | Reflection Artifacts |
Clutter | Noise | ||
|---|---|---|---|---|---|---|---|
| Limitations in hardware | Poor illumination | √ | |||||
| Limited view | √ | √ | |||||
| Limited bandwidth |
√ | √ | √ | ||||
| Sparse sampling | √ | √ | |||||
| Motion | √ | ||||||
| Limitations in tissue | Optical attenuation |
√ | |||||
| Out-of-plane absorption |
√ | ||||||
| Acoustic attenuation |
√ | √ | √ | ||||
| Acoustic heterogeneity |
√ | √ | √ | √ | |||
| Limitations in algorithms | Inappropriate algorithms |
√ | √ | √ |
2. Hardware
Researchers provide an overview of the hardware limitations in PAT systems, which generally include three essential components: illumination source, ultrasound transducer, and signal acquisition unit.
In PAT imaging, the intensity of illumination is restricted by safety guidelines, necessitating that the imaged tissue be irradiated within a standard range to meet the non-invasive imaging requirements. Excessive optical irradiation can cause tissue damage due to the absorption of excessive heat, whereas inadequate illumination can result in weak signals being obscured by electronic or thermal noise [7].
Although PAT imaging allows the illumination of tissues at a certain depth, the absorbing molecules at that depth have different characteristics from those at the skin surface. As a result of the light fluence, tissues at depth receive less light, resulting in weaker pressure.
The photoacoustic wave produced by the excited tissue is broadband, and the bandwidths of the actual transducer probes used for detection are limited. The emitted signals from large-volume targets tend to be at lower frequencies, while those from small-volume targets tend to be at higher frequencies. Given that the area of interest in PAT imaging often contains targets of varying sizes, the limited receiving bandwidth of the transducers is clearly inadequate to capture the entire photoacoustic signal [8]. This issue results in information being missing from the image [9], which may be seen as the hollowing out of the middle part or the loss of finer structures at image boundaries.
The shape of the transducer element and the geometry of the array can significantly affect the received signal. The surface of the transducer element, as well as its geometry, determines its sensitivity angle, which limits its ability to respond to signals from different directions [10]. Even if arranged in a circular or spherical array to achieve a complete imaging field of view, there may still be blind spots in the near-edge field section. Furthermore, a closely spaced array can be costly, which is another important limitation to be considered.
The accuracy of raw data in PAT imaging is also significantly affected by the signal acquisition process. Due to hardware cost constraints, the arrangement of transducer elements is usually sparse, causing insufficient spatial sampling [11]. This issue can lead to information being missing and affect the overall accuracy of the final image [12]. Furthermore, temporal resolution of the signal acquisition hardware also limits PAT imaging performance. Unexpected movements during acquisition, such as breath or cardiac pulsatile response, can cause relative displacements, resulting in misalignment and blurred image features [13]. This problem is due to insufficient temporal resolution to observe continuous small movements.
3. Tissue Heterogeneity
Except for the limitations in imaging hardware, the imaged object also affects the quality of the PAT image. One of the key challenges in PAT imaging is imaging depth, which is primarily limited by light attenuation [14] in tissue caused by absorption and multiple scattering. The laser illuminated at the object surface must penetrate biological tissue to reach the desired imaging depth. However, in this process, the light energy is gradually absorbed by the tissue and is not uniformly absorbed, resulting in a non-uniform and unknown distribution of light fluence [15].
In addition, the resolution of PAT imaging is affected by the return of the acoustic signal after optical–acoustic conversion. The acoustic signal is subject to attenuation and scattering during propagation, resulting in lower amplitude and a wider waveform of the high-frequency component. Consequently, the imaging resolution is reduced [16], as the high-frequency information of fine structures is lost and cannot be displayed in the image. Moreover, biological tissues are acoustically heterogeneous due to variations in the types and concentrations of constituent molecules, leading to differences in SOS (speed of sound) as a medium. At locations where there are boundaries with high acoustic contrast, incident sound waves generate acoustic reflections. These reflections overlap with the original image features to form acoustic reflection artifacts and can be mistaken for a real existing structure [17], which can greatly impact the accuracy of the reconstructed image.
In addition, when there are molecules with strong optical absorption outside the imaging plane of interest, the pressure wave signals they generate cannot be ignored. These spurious signals can even obscure the weak signal from deep tissue, resulting in spatial blending or clutter artifacts formed by the same transducer acceptance [18].
4. Image Reconstruction Algorithms
The recorded PA signal needs to undergo a mathematical transformation to form the final PAT image, which is referred to as image reconstruction. Ideally, the reconstruction algorithm should account for the PA wave generation and propagation physics. However, due to the uncertainty and complexity of the process, the reconstruction algorithm is often simplified and approximated, leading to a reconstruction error and resulting in low-quality reconstructed images.
Currently, common PAT image reconstruction algorithms include delay and sum (DAS) [6][19], back projection (BP) [20], and model-based (MB) [21][22] methods. Under ideal conditions, no additional processing steps are required for image reconstruction. However, in real-world PAT-imaging scenarios, algorithms possess distinct characteristics and may produce images with different levels of degradation [9][23][24]. Direct reconstruction algorithms, such as BP and DAS, can suffer from artifacts [12] and noise, resulting in low contrast and resolution of reconstructed images. Model-based algorithms can address some of these limitations by incorporating prior knowledge of the tissue and improving the physical acoustic wave propagation model; however, they are sensitive to errors in the model matrix and require higher computational costs.
References
- Das, D.; Sharma, A.; Rajendran, P.; Pramanik, M. Another decade of photoacoustic imaging. Phys. Med. Biol. 2021, 66, 05TR01.
- Li, X.; Qi, L.; Zhang, S.; Huang, S.; Wu, J.; Lu, L.; Feng, Y.; Feng, Q.; Chen, W. Model-based optoacoustic tomography image reconstruction with non-local and sparsity regularizations. IEEE Access 2019, 7, 102136–102148.
- Qi, L.; Huang, S.; Li, X.; Zhang, S.; Lu, L.; Feng, Q.; Chen, W. Cross-sectional photoacoustic tomography image reconstruction with a multi-curve integration model. Comput. Methods Programs Biomed. 2020, 197, 105731.
- Jeon, S.; Choi, W.; Park, B.; Kim, C. A Deep Learning-Based Model That Reduces Speed of Sound Aberrations for Improved In Vivo Photoacoustic Imaging. IEEE Trans Image Process. 2021, 30, 8773–8784.
- Wang, K.; Schoonover, R.W.; Su, R.; Oraevsky, A.; Anastasio, M.A. Discrete imaging models for three-dimensional optoacoustic tomography using radially symmetric expansion functions. IEEE Trans. Med. Imaging 2014, 33, 1180–1193.
- Ruiz-Veloz, M.; Gutiérrez-Juárez, G.; Polo-Parada, L.; Cortalezzi, F.; Kline, D.D.; Dantzler, H.A.; Cruz-Alvarez, L.; Castro-Beltrán, R.; Hidalgo-Valadez, C. Image reconstruction algorithm for laser-induced ultrasonic imaging: The single sensor scanning synthetic aperture focusing technique. J. Acoust. Soc. Am. 2023, 153, 560–572.
- Winkler, A.M.; Maslov, K.; Wang, L.V. Noise-equivalent sensitivity of photoacoustics. J. Biomed. Opt. 2013, 18, 097003.
- Ku, G.; Wang, X.; Stoica, G.; Wang, L. Multiple-bandwidth photoacoustic tomography. Phys. Med. Biol. 2004, 49, 1329–1338.
- Choi, W.; Oh, D.; Kim, C. Practical photoacoustic tomography: Realistic limitations and technical solutions. J. Appl. Phys. 2020, 127, 230903.
- Shen, K.; Liu, S.; Feng, T.; Yuan, J.; Zhu, B.; Tian, C. Negativity artifacts in back-projection based photoacoustic tomography. J. Phys. D-Appl. Phys. 2021, 54, 074001.
- Cox, B.T.; Arridge, S.R.; Beard, P.C. Photoacoustic tomography with a limited-aperture planar sensor and a reverberant cavity. Inverse Probl. 2007, 23, S95.
- Tian, C.; Zhang, C.; Zhang, H.; Xie, D.; Jin, Y. Spatial resolution in photoacoustic computed tomography. Rep. Prog. Phys. 2021, 84, 036701.
- Bise, R.; Zheng, Y.; Sato, I.; Toi, M. Vascular Registration in Photoacoustic Imaging by Low-Rank Alignment via Foreground, Background and Complement Decomposition. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2016; Springer: Cham, Switzerland, 2016; pp. 326–334.
- Ammari, H.; Bretin, E.; Jugnon, V.; Wahab, A. Photoacoustic imaging for attenuating acoustic media. Math. Model. Biomed. Imaging II 2012, 2035, 53–80.
- Bu, S.; Liu, Z.; Shiina, T.; Kondo, K.; Yamakawa, M.; Fukutani, K.; Someda, Y.; Asao, Y. Model-Based Reconstruction Integrated With Fluence Compensation for Photoacoustic Tomography. IEEE Trans. Biomed. Eng. 2012, 59, 1354–1363.
- Huang, C.; Nie, L.; Schoonover, R.W.; Wang, L.V.; Anastasio, M.A. Photoacoustic computed tomography correcting for heterogeneity and attenuation. J. Biomed. Opt. 2012, 17, 0612111–0612115.
- Nguyen, H.N.Y.; Hussain, A.; Steenbergen, W. Reflection artifact identification in photoacoustic imaging using multi-wavelength excitation. Biomed. Opt. Express 2018, 9, 4613–4630.
- Preisser, S.; Held, G.; Akarcay, H.G.; Jaeger, M.; Frenz, M. Study of clutter origin in in-vivo epi-optoacoustic imaging of human forearms. J. Opt. 2016, 18, 094003.
- Thomenius, K.E. Evolution of ultrasound beamformers. In Proceedings of the 1996 IEEE Ultrasonics Symposium, San Antonio, TX, USA, 3–4 November 1996; pp. 1615–1622.
- Xu, M.; Wang, L.V. Universal back-projection algorithm for photoacoustic computed tomography. Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 2005, 71, 016706.
- Dean-Ben, X.L.; Buehler, A.; Ntziachristos, V.; Razansky, D. Accurate model-based reconstruction algorithm for three-dimensional optoacoustic tomography. IEEE Trans. Med. Imaging 2012, 31, 1922–1928.
- Jiang, H.; Iftimia, N.V.; Xu, Y.; Eggert, J.A.; Fajardo, L.L.; Klove, K.L. Near-infrared optical imaging of the breast with model-based reconstruction. Acad. Radiol. 2002, 9, 186–194.
- Allman, D.; Reiter, A.; Bell, M.A.L. Photoacoustic Source Detection and Reflection Artifact Removal Enabled by Deep Learning. IEEE Trans. Med. Imaging 2018, 37, 1464–1477.
- Muhammad, M.; Prakash, J.; Liapis, E.; Ntziachristos, V.; Jüstel, D. Weighted model-based optoacoustic reconstruction for partial-view geometries. J. Biophotonics 2022, 15, e202100334.
More
Information
Subjects:
Engineering, Biomedical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
849
Revisions:
2 times
(View History)
Update Date:
14 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





