
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Denise Raymond | -- | 2504 | 2023-07-12 15:20:52 | | | |
| 2 | Catherine Yang | Meta information modification | 2504 | 2023-07-13 04:01:24 | | |
Video Upload Options
Antiretrovirals (ARVs) reduce Human Immunodeficiency Virus (HIV) loads to undetectable levels in infected patients. However, HIV can persist throughout the body in cellular reservoirs partly due to the inability of some ARVs to cross anatomical barriers and the capacity of HIV-1 to establish latent infection in resting CD4+ T cells and monocytes/macrophages. A cure for HIV is not likely unless latency is addressed and delivery of ARVs to cellular reservoir sites is improved. Nanomedicine has been used in ARV formulations to improve delivery and efficacy. More specifically, researchers are exploring the benefit of using nanoparticles to improve ARVs and nanomedicine in HIV eradication strategies such as shock and kill, block and lock, and others.
1. Introduction
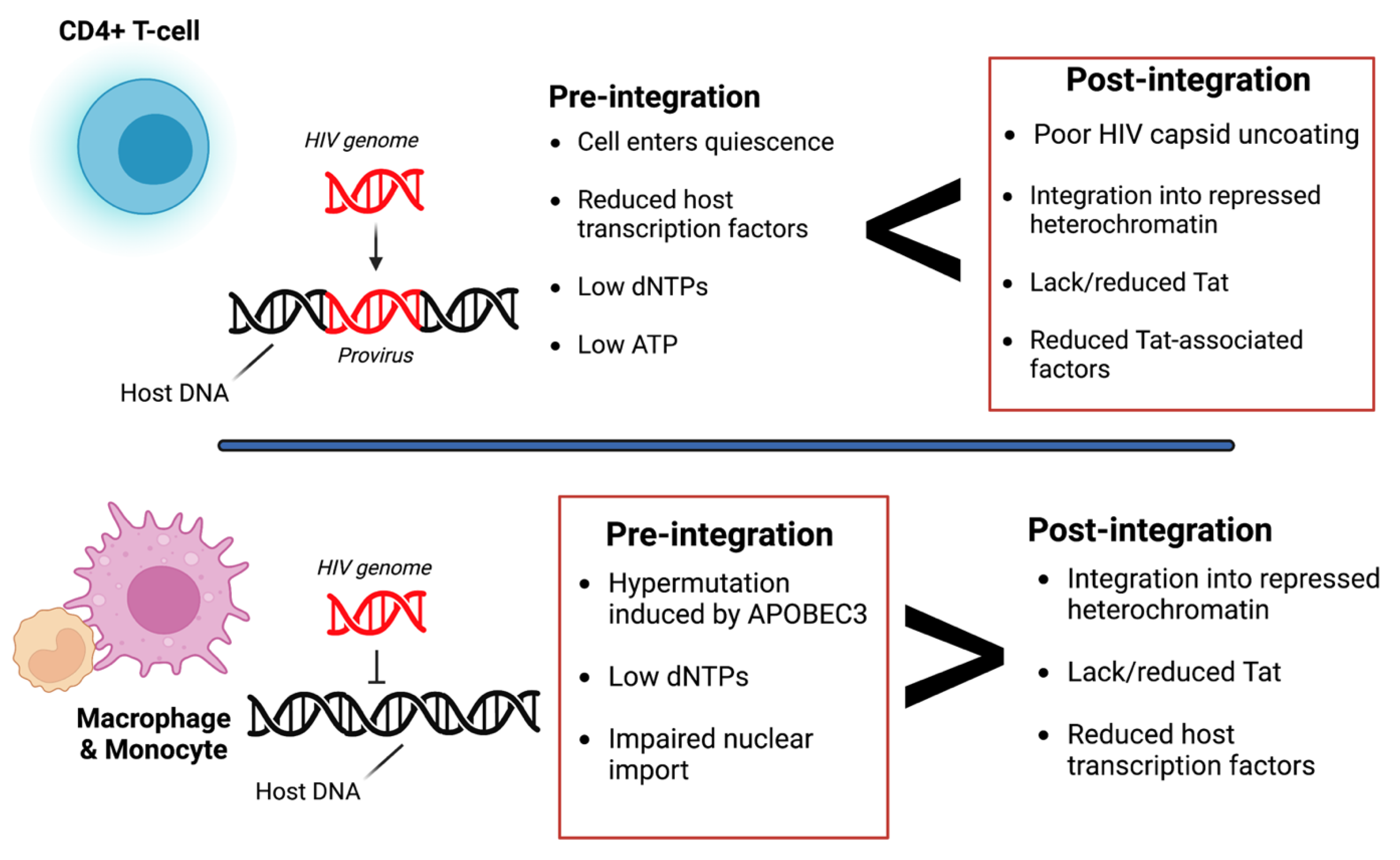
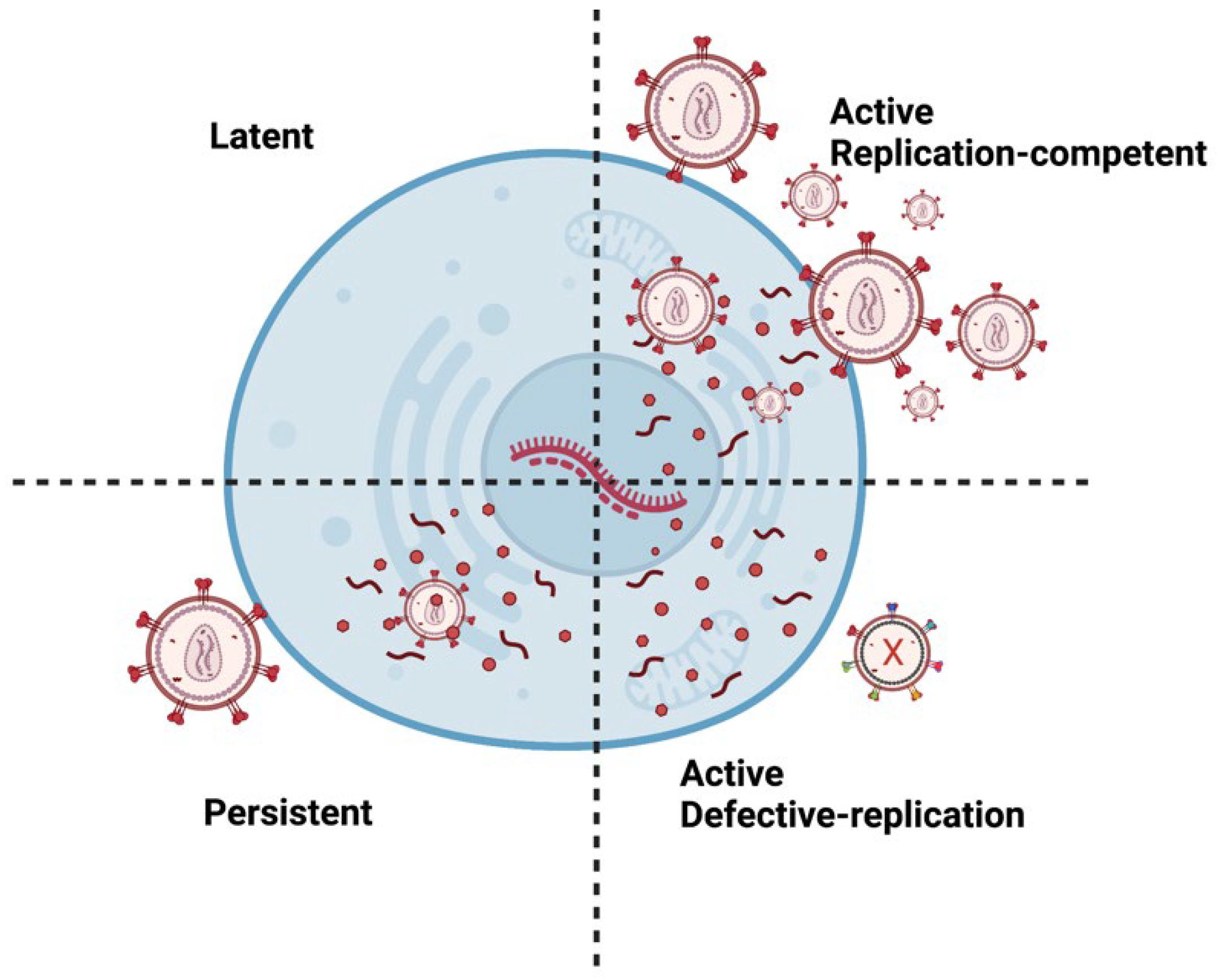
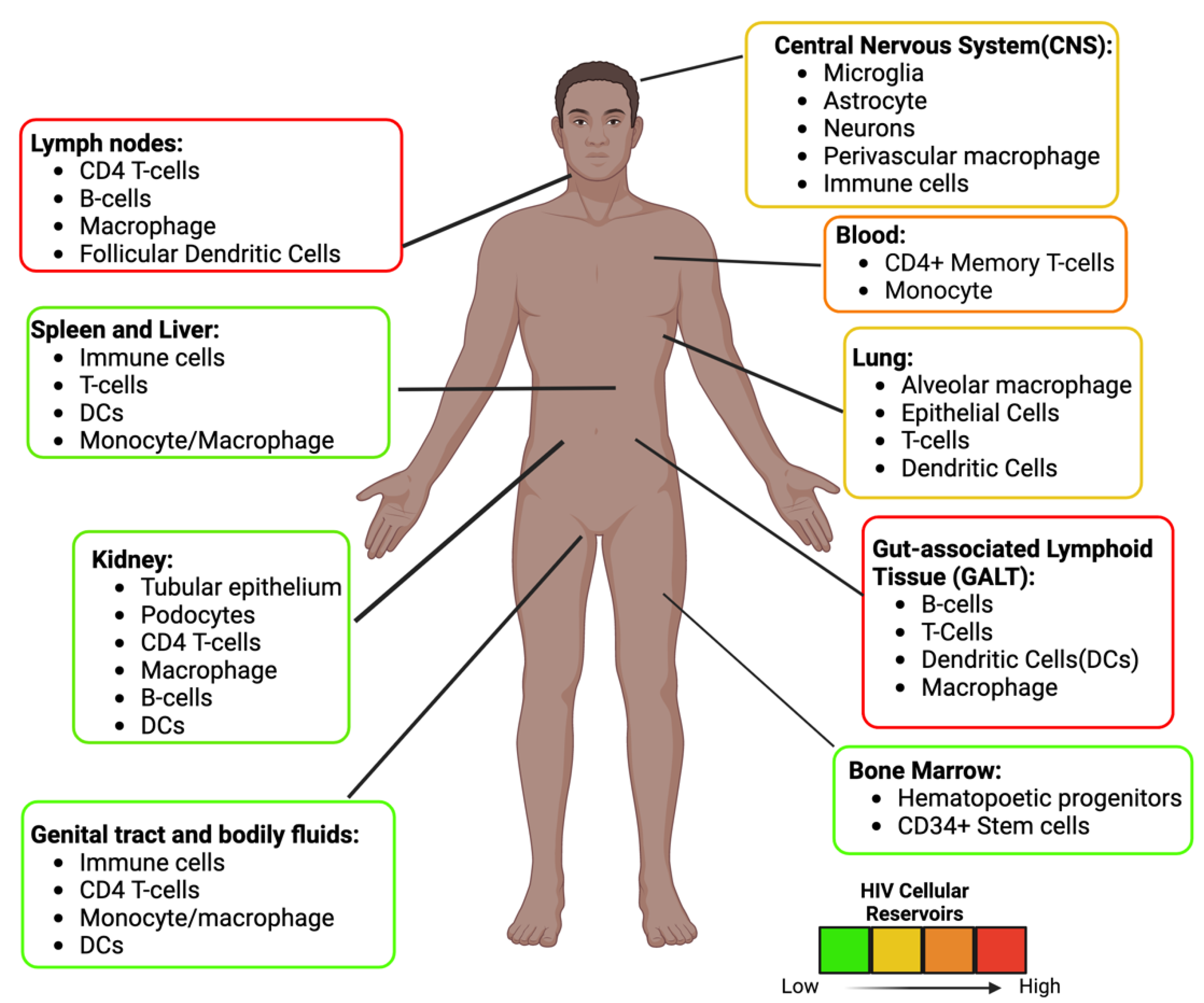
2. Molecular Mechanisms of HIV Latency
3. Anti-HIV Therapy
3.1. Antiretroviral Therapy (ART)
3.2. A Hematopoietic Stem-Cell Transplantation
3.3. Shock and Kill/Kick and Kill
3.4. Block and Lock
3.5. Anti-HIV Vaccines
4. Nanomedicine in HIV Therapeutics

| Nanoparticle Type |
Drug/Agent | Target | Results | Reference |
|---|---|---|---|---|
| Chitosan | ||||
| Dolutegravir Tenofovir alafenamide |
HIV-infected cells | Dolutegravir became more soluble with the nanoparticle and had a higher concentration in multiple organs than drug alone. Extended release (56 %) of the drug for 16 days |
[44] [45] |
|
| Exosomes | ||||
| ARVs (Emtricitabine) Zinc Finger Protein (mRNA) |
TZM-bl cells HC69.5 |
Reduced HIV infection Repressor-loaded anti-HIV-1 exosomes suppress virus expression |
[46] [47] |
|
| Gold | ||||
| Tenofovir Cabotegravir Raltegravir |
TZM-bl cells HEK293 HeLa |
~15-fold higher anti-HIV-1 reverse transcriptase activity than drug alone and great biodistribution Less cytotoxicity than the drug alone Across the BBB and displayed antiretroviral activity and no toxicity |
[48] [49] [50] |
|
| Iron oxide | ||||
| LRA | Astrocytes | Across the BBB, and 33% reduction in p24 level and cell viability over 90% after five days |
[50] | |
| Liposomes | ||||
| ARVs (intracellular tenofovir, ritonavir, and lopinavir) HIV-1 Envelope |
CD4 T-cells Env-specific B cells |
50-fold higher intracellular ARV Efficiently activated Env-specific B cells |
[51] [52] |
|
| Magnetoelectric (MENPs) | ||||
| ARV drug | BBB model | Successfully crossed the BBB model and released the drug without producing heat | [53] | |
| Poly(lactic-co-glycolic) acid (PLGA) | ||||
| Protease inhibitor | Gut-homing T-cells |
Successfully penetrated the reservoirs in the GALT more effectively than the free drug | [54] | |
| Virus-Mimicking Polymer | ||||
| ARV drug | CD169+ Macrophages | Achieved inhibition of HIV-1 infection of primary human macrophages for up to 35 days. | [55] | |
References
- Abah, I.O.; Ncube, N.B.Q.; Bradley, H.A.; Agbaji, O.O.; Kanki, P. Antiretroviral Therapy-associated Adverse Drug Reactions and their Effects on Virologic Failure- A Retrospective Cohort Study in Nigeria. Curr. HIV Res. 2018, 16, 436–446.
- Dufour, C.; Gantner, P.; Fromentin, R.; Chomont, N. The multifaceted nature of HIV latency. J. Clin. Investig. 2020, 130, 3381–3390.
- Fujinaga, K.; Cary, D.C. Experimental Systems for Measuring HIV Latency and Reactivation. Viruses 2020, 12, 1279.
- Chen, B. Molecular Mechanism of HIV-1 Entry. Trends Microbiol. 2019, 27, 878–891.
- Gallo, S.A.; Finnegan, C.M.; Viard, M.; Raviv, Y.; Dimitrov, A.; Rawat, S.S.; Puri, A.; Durell, S.; Blumenthal, R. The HIV Env-mediated fusion reaction. Biochim. Biophys. Acta 2003, 1614, 36–50.
- Miyauchi, K.; Kim, Y.; Latinovic, O.; Morozov, V.; Melikyan, G.B. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 2009, 137, 433–444.
- Chauhan, A.; Mehla, R.; Vijayakumar, T.S.; Handy, I. Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes. Virology 2014, 456–457, 1–19.
- Gray, L.R.; Turville, S.G.; Hitchen, T.L.; Cheng, W.J.; Ellett, A.M.; Salimi, H.; Roche, M.J.; Wesselingh, S.L.; Gorry, P.R.; Churchill, M.J. HIV-1 entry and trans-infection of astrocytes involves CD81 vesicles. PLoS ONE 2014, 9, e90620.
- Hao, H.N.; Lyman, W.D. HIV infection of fetal human astrocytes: The potential role of a receptor-mediated endocytic pathway. Brain Res. 1999, 823, 24–32.
- Letendre, S.; Marquie-Beck, J.; Capparelli, E.; Best, B.; Clifford, D.; Collier, A.C.; Gelman, B.B.; McArthur, J.C.; McCutchan, J.A.; Morgello, S.; et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch. Neurol. 2008, 65, 65–70.
- Blanco-Rodriguez, G.; Gazi, A.; Monel, B.; Frabetti, S.; Scoca, V.; Mueller, F.; Schwartz, O.; Krijnse-Locker, J.; Charneau, P.; Di Nunzio, F. Remodeling of the Core Leads HIV-1 Preintegration Complex into the Nucleus of Human Lymphocytes. J. Virol. 2020, 94, e00135-20.
- Zamborlini, A.; Lehmann-Che, J.; Clave, E.; Giron, M.-L.; Tobaly-Tapiero, J.; Roingeard, P.; Emiliani, S.; Toubert, A.; de Thé, H.; Saïb, A. Centrosomal pre-integration latency of HIV-1 in quiescent cells. Retrovirology 2007, 4, 63.
- Williams, S.A.F.; Greene, W.C. Host factors regulating post-integration latency of HIV. Trends Microbiol. 2005, 13, 137–139.
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front. Cell. Infect. Microbiol. 2019, 9, 362.
- Li, G.-H.; Henderson, L.; Nath, A. Astrocytes as an HIV Reservoir: Mechanism of HIV Infection. Curr. HIV Res. 2016, 14, 373–381.
- Vincendeau, M.; Kramer, S.; Hadian, K.; Rothenaigner, I.; Bell, J.; Hauck, S.M.; Bickel, C.; Nagel, D.; Kremmer, E.; Werner, T.; et al. Control of HIV replication in astrocytes by a family of highly conserved host proteins with a common Rev-interacting domain (Risp). AIDS 2010, 24, 2433–2442.
- Narasipura, S.D.; Kim, S.; Al-Harthi, L. Epigenetic regulation of HIV-1 latency in astrocytes. J. Virol. 2014, 88, 3031–3038.
- Henderson, L.J.; Reoma, L.B.; Kovacs, J.A.; Nath, A. Advances toward Curing HIV-1 Infection in Tissue Reservoirs. J. Virol. 2020, 94, e00375-19.
- Falcinelli, S.D.; Ceriani, C.; Margolis, D.M.; Archin, N.M. New Frontiers in Measuring and Characterizing the HIV Reservoir. Front. Microbiol. 2019, 10, 2878.
- Ho, Y.C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551.
- Lorenzo-Redondo, R.; Fryer, H.R.; Bedford, T.; Kim, E.-Y.; Archer, J.; Pond, S.L.K.; Chung, Y.-S.; Penugonda, S.; Chipman, J.; Fletcher, C.V.; et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016, 530, 51–56.
- Liu, R.; Simonetti, F.R.; Ho, Y.-C. The forces driving clonal expansion of the HIV-1 latent reservoir. Virol. J. 2020, 17, 4.
- Bui, J.K.; Sobolewski, M.D.; Keele, B.F.; Spindler, J.; Musick, A.; Wiegand, A.; Luke, B.T.; Shao, W.; Hughes, S.H.; Coffin, J.M.; et al. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog. 2017, 13, e1006283.
- Hosmane, N.N.; Kwon, K.J.; Bruner, K.M.; Capoferri, A.A.; Beg, S.; Rosenbloom, D.I.; Keele, B.F.; Ho, Y.C.; Siliciano, J.D.; Siliciano, R.F. Proliferation of latently infected CD4(+) T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J. Exp. Med. 2017, 214, 959–972.
- Thompson, C.G.; Gay, C.L.; Kashuba, A.D.M. HIV Persistence in Gut-Associated Lymphoid Tissues: Pharmacological Challenges and Opportunities. AIDS Res. Hum. Retrovir. 2017, 33, 513–523.
- Labarthe, L.; Gelé, T.; Gouget, H.; Benzemrane, M.S.; Le Calvez, P.; Legrand, N.; Lambotte, O.; Le Grand, R.; Bourgeois, C.; Barrail-Tran, A. Pharmacokinetics and tissue distribution of tenofovir, emtricitabine and dolutegravir in mice. J. Antimicrob. Chemother. 2022, 77, 1094–1101.
- Ferrara, M.; Bumpus, N.N.; Ma, Q.; Ellis, R.J.; Soontornniyomkij, V.; Fields, J.A.; Bharti, A.; Achim, C.L.; Moore, D.J.; Letendre, S.L. Antiretroviral drug concentrations in brain tissue of adult decedents. Aids 2020, 34, 1907–1914.
- Rosen, E.P.; Deleage, C.; White, N.; Sykes, C.; Brands, C.; Adamson, L.; Luciw, P.; Estes, J.D.; Kashuba, A.D.M. Antiretroviral drug exposure in lymph nodes is heterogeneous and drug dependent. J. Int. AIDS Soc. 2022, 25, e25895.
- Doohan, P.T.; Oldfield, L.D.; Arnold, J.C.; Anderson, L.L. Cannabinoid Interactions with Cytochrome P450 Drug Metabolism: A Full-Spectrum Characterization. AAPS J. 2021, 23, 91.
- Nasrin, S.; Watson, C.J.W.; Perez-Paramo, Y.X.; Lazarus, P. Cannabinoid Metabolites as Inhibitors of Major Hepatic CYP450 Enzymes, with Implications for Cannabis-Drug Interactions. Drug Metab. Dispos. 2021, 49, 1070–1080.
- Qian, Y.; Gurley, B.J.; Markowitz, J.S. The Potential for Pharmacokinetic Interactions Between Cannabis Products and Conventional Medications. J. Clin. Psychopharmacol. 2019, 39, 462–471.
- Brown, T.R. I Am the Berlin Patient: A Personal Reflection. AIDS Res. Hum. Retrovir. 2015, 31, 2–3.
- Yukl, S.A.; Boritz, E.; Busch, M.; Bentsen, C.; Chun, T.-W.; Douek, D.; Eisele, E.; Haase, A.; Ho, Y.-C.; Hütter, G.; et al. Challenges in detecting HIV persistence during potentially curative interventions: A study of the Berlin patient. PLoS Pathog. 2013, 9, e1003347.
- Hill, A.L.; Rosenbloom, D.I.S.; Goldstein, E.; Hanhauser, E.; Kuritzkes, D.R.; Siliciano, R.F.; Henrich, T.J. Real-Time Predictions of Reservoir Size and Rebound Time during Antiretroviral Therapy Interruption Trials for HIV. PLOS Pathog. 2016, 12, e1005535.
- Zerbato, J.M.; Purves, H.V.; Lewin, S.R.; Rasmussen, T.A. Between a shock and a hard place: Challenges and developments in HIV latency reversal. Curr. Opin. Virol. 2019, 38, 1–9.
- McBrien, J.B.; Mavigner, M.; Franchitti, L.; Smith, S.A.; White, E.; Tharp, G.K.; Walum, H.; Busman-Sahay, K.; Aguilera-Sandoval, C.R.; Thayer, W.O.; et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8+ cells. Nature 2020, 578, 154–159.
- Fidler, S.; Stöhr, W.; Pace, M.; Dorrell, L.; Lever, A.; Pett, S.; Kinloch-de Loes, S.; Fox, J.; Clarke, A.; Nelson, M.; et al. Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): A phase 2, randomised trial. Lancet 2020, 395, 888–898.
- Lewin, S.R.; Rasmussen, T.A. Kick and kill for HIV latency. Lancet 2020, 395, 844–846.
- Vansant, G.; Bruggemans, A.; Janssens, J.; Debyser, Z. Block-And-Lock Strategies to Cure HIV Infection. Viruses 2020, 12, 84.
- Méndez, C.; Ledger, S.; Petoumenos, K.; Ahlenstiel, C.; Kelleher, A.D. RNA-induced epigenetic silencing inhibits HIV-1 reactivation from latency. Retrovirology 2018, 15, 67.
- Gray, G.E.; Corey, L. The path to find an HIV vaccine. J. Int. AIDS Soc. 2021, 24, e25749.
- Borducchi, E.N.; Cabral, C.; Stephenson, K.E.; Liu, J.; Abbink, P.; Ng’ang’a, D.; Nkolola, J.P.; Brinkman, A.L.; Peter, L.; Lee, B.C.; et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 2016, 540, 284–287.
- Priya Dharshini, K.; Ramya Devi, D.; Banudevi, S.; Narayanan, V.H.B. In-vivo pharmacokinetic studies of Dolutegravir loaded spray dried Chitosan nanoparticles as milk admixture for paediatrics infected with HIV. Sci. Rep. 2022, 12, 13907.
- Narayanan, V.H.B.; Lewandowski, A.; Durai, R.; Gonciarz, W.; Wawrzyniak, P.; Brzezinski, M. Spray-dried tenofovir alafenamide-chitosan nanoparticles loaded oleogels as a long-acting injectable depot system of anti-HIV drug. Int. J. Biol. Macromol. 2022, 222, 473–486.
- Welch, J.L.; Kaddour, H.; Winchester, L.; Fletcher, C.V.; Stapleton, J.T.; Okeoma, C.M. Semen Extracellular Vesicles From HIV-1-Infected Individuals Inhibit HIV-1 Replication In Vitro, and Extracellular Vesicles Carry Antiretroviral Drugs In Vivo. J. Acquir. Immune Defic. Syndr. 2020, 83, 90–98.
- Shrivastava, S.; Ray, R.M.; Holguin, L.; Echavarria, L.; Grepo, N.; Scott, T.A.; Burnett, J.; Morris, K.V. Exosome-mediated stable epigenetic repression of HIV-1. Nat. Commun. 2021, 12, 5541.
- Garrido, C.; Simpson, C.A.; Dahl, N.P.; Bresee, J.; Whitehead, D.C.; Lindsey, E.A.; Harris, T.L.; Smith, C.A.; Carter, C.J.; Feldheim, D.L.; et al. Gold nanoparticles to improve HIV drug delivery. Future Med. Chem. 2015, 7, 1097–1107.
- Rawat, P.; Imam, S.S.; Gupta, S. Formulation of Cabotegravir Loaded Gold Nanoparticles: Optimization, Characterization to In-Vitro Cytotoxicity Study. J. Cluster Sci. 2022, 1–13.
- Nair, M.; Jayant, R.D.; Kaushik, A.; Sagar, V. Getting into the brain: Potential of nanotechnology in the management of NeuroAIDS. Adv. Drug Deliv. Rev. 2016, 103, 202–217.
- Freeling, J.P.; Koehn, J.; Shu, C.; Sun, J.; Ho, R.J.Y. Long-acting three-drug combination anti-HIV nanoparticles enhance drug exposure in primate plasma and cells within lymph nodes and blood. AIDS 2014, 28, 2625–2627.
- Damm, D.; Suleiman, E.; Theobald, H.; Wagner, J.T.; Batzoni, M.; Ahlfeld Née Kohlhauser, B.; Walkenfort, B.; Albrecht, J.C.; Ingale, J.; Yang, L.; et al. Design and Functional Characterization of HIV-1 Envelope Protein-Coupled T Helper Liposomes. Pharmaceutics 2022, 14, 1385.
- Nair, M.; Guduru, R.; Liang, P.; Hong, J.; Sagar, V.; Khizroev, S. Externally controlled on-demand release of anti-HIV drug using magneto-electric nanoparticles as carriers. Nat. Commun. 2013, 4, 1707.
- Cao, S.; Jiang, Y.; Zhang, H.; Kondza, N.; Woodrow, K.A. Core-shell nanoparticles for targeted and combination antiretroviral activity in gut-homing T cells. Nanomedicine 2018, 14, 2143–2153.
- Eshaghi, B.; Fofana, J.; Nodder, S.B.; Gummuluru, S.; Reinhard, B.M. Virus-Mimicking Polymer Nanoparticles Targeting CD169(+) Macrophages as Long-Acting Nanocarriers for Combination Antiretrovirals. ACS Appl. Mater. Interfaces 2022, 14, 2488–2500.
- What’s New in the Guidelines?|NIH. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new-guidelines (accessed on 3 January 2023).




