2. Molecular Mechanisms of HIV Latency
At the beginning of HIV cellular infection, HIV glycoprotein (gp120) binds to the host CD4 receptor. HIV can then enter the cell in two common pathways. In the primary pathway, gp120 undergoes a conformational change and exposes the gp120’s V3 domain. The V3 domain binds to the host chemokine coreceptors, such as CCR5 or CXCR4, depending on the viral tropism caused by a point mutation
[4][11]. A post-conformational change dissociates gp120 from gp41, which facilitates fusion with the host membrane
[5][12]. The second pathway involves endocytosis. Upon gp120 binding to the CD4+ receptor, the virus enters the cell via an endocytic vesicle
[6][13]. Chauhal et al. demonstrated that HIV-1 enters astrocytes via the endocytosis pathway in which gp41 facilitates fusion with the luminal membrane of the endosome. The endocytosis pathway has been reported to produce more defective viruses
[7][8][9][14,15,16]. Once fusion occurs in both pathways, the capsid is released into the cytosol.
Cells latently infected with HIV establish reservoirs at specific anatomical sites such as the brain
[10][25]. At these reservoirs, two types of latency exist, pre-integration and post-integration (
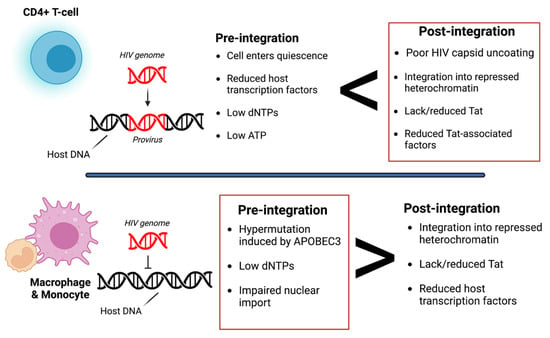
Figure 1). Pre-integration latency primarily occurs when the host cells transition to a quiescent state before HIV viral DNA is integrated. The mechanisms of pre-integration latency include poor nuclear transportation of the PIC, defective viral proteins, and the state of the cell life cycle
[11][22]. In addition, the viral PIC can remain stable for several weeks on centrosomes and possibly integrate into the host genome when the host cell becomes reactivated
[12][26]. Pre-integration latency is less stable than post-integration latency. Post-integration latency occurs after the viral genome has integrated into the host genome and remains transcriptionally silenced
[13][27]. Memory CD4+ T-cells transitioning from an active to a quiescent state also exhibit post-integration latency. Several factors can contribute to this state, such as low transcriptional factors, DNA modification, transcriptional suppression, and low Tat and P-TEFb (
Figure 1).
Figure 1. Overview of pre- and post-integration HIV latency. Pre-integration latency is more common in monocytes/macrophages due to poor nuclear transport of the pre-integration complex (PIC), change in cell cycle phase, and defective reverse transcriptase. Post-integration latency in memory T-cells during ARV treatment is partly due to transcriptional interference, low cellular transcription factors, DNA methylation, chromatin organization, and reduced p-TEFb and Tat protein. Red boxes indicate which type of latency is more common. It is also important to note that pre-integration is more common in the T-cells of untreated individuals. Image created with biorender.com and Adobe Illustrator/Photoshop.
Post-integration latency can depend on the expression threshold of the HIV Tat protein, which forms a positive feedback loop that upregulates HIV transcription. When Tat expression is above the threshold, transcription can occur. However, when Tat expression is below the threshold, the provirus is considered dormant/latent. As a result, HIV LTR transcription is significantly reduced/inhibited. Several factors can contribute to the low expression of Tat, such as low levels of host transcriptional factors, the presence of host transcriptional repressors, and the integration of the HIV genome into a heterochromatin region
[14][28]. For example, in microglia, Sp3 (a host transcriptional repressor) is regulated and induces latency by blocking HIV transcription. However, latency in astrocytes is induced by low expression of Sam68 or by high expression of cellular proteins with Rev-interacting domain (Risp), class I histone deacetylases (HDACs), and a lysine-specific histone methyltransferase, Su(var)3–9
[15][16][17][29,30,31]. Additionally, the microenvironment of the cell can also determine latency. Zhuang et al. observed that under hypoxic conditions, hypoxia-inducible factors could restrict HIV transcription by binding to the viral promoter regions
[18][32].
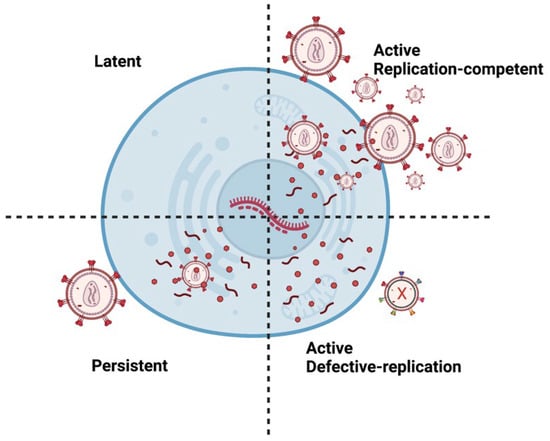
There are multiple types of HIV infections including latent, active, and persistent (
Figure 2). Persistent infection is believed to result from low ARV concentrations in anatomical HIV reservoirs. Within these reservoirs, HIV-infected cells can have transcriptionally active proviruses that are persistent (producing little virions) or latent (producing no virions)
[19][33]. Moreover, infected cells can contain replication-competent or defective proviruses
[20][34]. Replication-competent proviruses produce virions with the ability to replicate. On the other hand, defective proviruses produce imperfect proteins that prevent the virions from reproducing. Approximately 5–11% of CD4 T-cells within the reservoirs are replication-competent
[21][35].
Figure 2. Different types of HIV Infection. A latent provirus is transcriptionally silent and produces no virions. However, active provirus can produce virions. Replication-competent provirus produces virions that can replicate. Persistent proviruses produce a few virions. Defective proviruses contain many mutations that produce nonfunctional proteins that render the virions incapable of replicating. It is important to note that latent or persistent provirus can be replication-competent or defective. Image created with biorender.com and Adobe Illustrator/Photoshop.
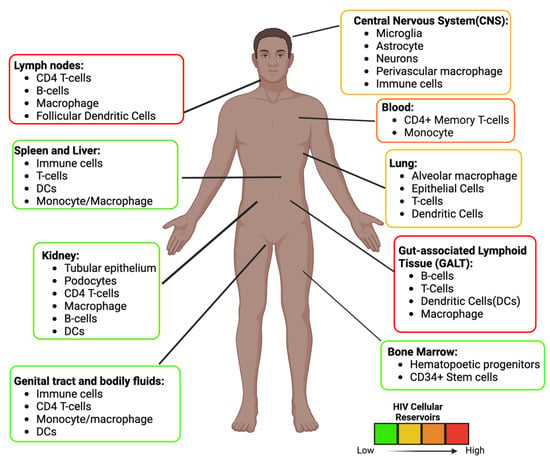
These reservoirs are established throughout the body where ARVs are limited
[22][36] (
Figure 3). In some unique microenvironments such as the CNS, compartmentalization can occur, resulting in HIV variants. In addition, infected cells can also undergo normal cellular division (clonal expansion), which replication-competent proviruses can increase in number
[23][37]. It is estimated that more than half of the latent cells are maintained by clonal expansion
[24][25][38,39]. Antigen-driven proliferation is considered one of the forces that drive clonal expansion. The location of these replication-competent cells needs to be identified to develop an effective strategy to prevent viral rebound. Specific tissues within the body serve as reservoirs and possess persistent and latently infected cells, described below.
Figure 3. HIV Anatomical and Cellular Reservoirs during ART. Estimation of the location and type of cells latently infected with HIV. The color depicts the high (red) to the low (green) concentration of latent cells. The GALT and the Lymph nodes are two of the major sites. The estimation and cellular reservoirs were obtained from multiple sources. Image created with biorender.com and Adobe Illustrator/Photoshop.
3. Anti-HIV Therapy
3.1. Antiretroviral Therapy (ART)
Current anti-HIV therapies focus on inhibiting essential steps in the HIV life cycle; nonetheless, HIV can mutate, rendering these drugs useless if taken alone. HIV treatment is usually given in combination with two or three groups of ARVs, called cART. There are five classes of ARVs labeled as non-nucleoside reverse transcriptase inhibitors, protease inhibitors, entry/fusion inhibitors, integrase inhibitors, and nucleoside/nucleotide reverse transcriptase inhibitors. The three drugs of choice are an integrase strand transfer inhibitor and two nucleoside reverse transcriptase inhibitors
[26][76]. ARVs are administrated daily, which can make adherence difficult. Any interruption of this daily regimen can result in the virus rebound.
ARVs are administrated orally, making absorption the main route. Long-acting injectables (LAIs) such as Cabenuva on the other hand are administered via intramuscular injection. A higher concentration of the drug enters the systemic circulation via intramuscular injection than the oral route which gives LAIs and advantage over orally administrated drugs.
The biodistribution of the ARVs was also assessed. Labarthe et al. reported that in mice ARVs (tenofovir, emtricitabine, and dolutegravir) had the highest concentration in the digestive tract, liver, and kidneys but the lowest concentration in the brain
[27][77]. Although the brain is deemed to have a low ARV concentration compared to other organs, a recent study that was the first to assess ARV concentration from human brain tissues reported a higher concentration than any published concentration
[28][78]. Furthermore, different ARVs can be more concentrated in different tissues, indicating that certain steps in the HIV life cycle are not inhibited at certain reservoirs. For example, Rosen et al. also witnessed heterogeneous ARV disposition in lymph nodes
[29][79].
Most ARVs are metabolized in the liver after absorption via the cytochrome P450 enzymes. Therefore, ARV treatment consider the drug-to-drug interactions, which might increase drug toxicity. Additionally, some people living with HIV utilize marijuana, medically or recreationally, which can inhibit the cytochrome P450 enzymes
[30][31][32][80,81,82]. Ultimately this can lead to an increase in ARV concentration in the bloodstream increasing side effects and excretion rates. ARVs have a half-life in the body estimated to be between 2–9 h, so it is necessary to administer the drug daily to maintain a steady state. Most ARVs are eliminated via the kidney.
3.2. A Hematopoietic Stem-Cell Transplantation
After discontinuing ART in one year, a London and a Berlin patient were believed to be cured of HIV when clinicians observed no viral rebound
[33][34][35][83,84,85]. Both patients received chemotherapy for cancer treatment, followed by hematopoietic stem-cell transplantation with cells containing the 32 base-pair in the CCR5(Δ-32 bp)deletion gene. Two other HIV-infected patients in Boston received a similar treatment but did not receive implanted cells without the Δ-32 bp deletion in CCR5, resulting in a viral rebound after only 3 and 8 months
[35][85]. A mathematical model later demonstrated that these patient reservoirs had a two-log reduction compared to the Berlin patient’s 3.5-log reduction. This strategy shows that eradicating the viral reservoir is a possible route for a HIV cure; however, the method may be costly and impractical.
3.3. Shock and Kill/Kick and Kill
The shock and kill strategy functions to reactivate and eradicate latently infected cells. Latency reversal agents (LRAs) are drugs that reactivate latent cells. Consequently, these latent cells can be eliminated via virus-mediated cytolysis or immune-mediated clearance
[36][86]. Different LRAs can activate transcription, modify chromatins, or facilitate transcriptional elongation. In 2020, N-803, an interleukin-15 super agonist, activated latently infected cells successfully in mice and primate models; however, due to the level of toxicity, the study was deemed unsafe for clinical trials
[37][87]. In the RIVER study, a randomized clinical trial found no difference in replicated-competent provirus between HIV-positive patients on ART or their shock and kill treatment
[38][88]. The study used vorinostat (shock) and a viral vector vaccine (kill) to target latent cells; however, the clinical trial did not provide evidence for latency reversal or ART interruption to assess viral rebound
[39][89]. Overall, the shock and kill strategy lacks specificity. Current LRAs tend to cause global activation of both uninfected and infected T-cells. Moreover, eliminating the infected cells once activated is difficult. Improvements to the shock and kill strategy are needed for this form of therapy to be effective.
3.4. Block and Lock
The block and lock strategy aims to silence HIV provirus permanently via many mechanisms permanently; nonetheless, most techniques have only temporarily silenced HIV transcription. These mechanisms occurred by several molecules such as didehydro-cortistatin A, LEDGINs curaxin CBL0100, HSP90 Inhibitor, Jak-STAT Inhibitors, and ZL058 Tat inhibitor, all of which were shown to delay viral rebound
[40][90]. These techniques are not specific to HIV proviral DNA and might alter other cellular functions. Therefore, researchers have used small interfering RNA (siRNA) to target regions in HIV LTR to epigenetically silence HIV transcription via histone deacetylation
[41][91]. Overall, it is questionable whether the block and lock strategy can have specificity to lock HIV provirus permanently
[40][90].
3.5. Anti-HIV Vaccines
The development of broadly neutralizing vaccines against HIV have been been disappointing. For the past 30 years, HIV vaccines have shown low efficacy and were strain-specific
[42][92]. In 2014, the HIV Vaccine Trials Network (HVTN) outlined large-scale clinical trial directions to improve vaccine efficacy, but no successful vaccine has been developed. Moreover, it is questionable how vaccines will address HIV latency. In one study, a vaccine was paired with an LRA, resulting in 33% of the infected primates being able to control their infection virologically
[43][93]. In 2023, the Phase 3 Mosaico HIV vaccine clinical trial with 3900 volunteers of men who have sex with men, the vaccine was deemed safe but ineffective. The vaccine was composed of multiple HIV subtypes. The vaccine candidate used the common-cold virus adenovirus serotype 26 for delivery. The study resulted in a similar infection rate between the placebo and vaccine groups. Since the success of the anti- SARS-CoV2 mRNA vaccine, researchers have shifted the HIV vaccine strategy to focus on the mRNA vaccine technology. This strategy uses lipid nanovesicles to transport mRNA which code for specific viral proteins. This may be a promising strategy for an HIV vaccine.
4. Nanomedicine in HIV Therapeutics
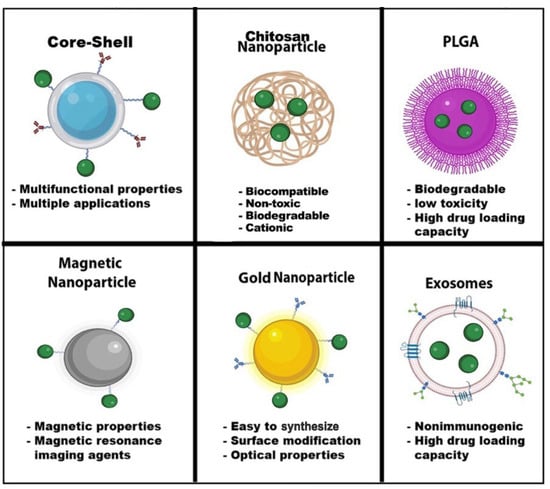
Nanomedicine has grown considerably during the last two decades. Nanoparticles typically range from l to 100 nanometers. In addition, the composition of nanoparticles can be inorganic or organic. Nanoparticles can have a variety of shapes to meet the experiment’s needs. Moreover, each type of nanoparticle offers a unique property with extraordinary functionalities. Some nanoparticle applications can range from biosensing to imaging and drug delivery (
Figure 4). The HIV nanotherapeutics was summarized in
Table 1. Nanoparticles’ unique functionalities are being exploited to treat HIV, which are mentioned below:
Figure 4. Nanoparticle Advantages. Nanoparticles have a wide range of advantages and applications in the field of nanomedicine. The shape, size, and composition give these nanoparticles unique properties for biosensors, drug delivery, MRI, diagnostic and therapeutics, optical imaging, and energy transfer. Some nanoparticles can have multiple functions, such as core-shell nanoparticles. Green dots—drugs. Image created with biorender.com and Adobe Illustrator/Photoshop.
Table 1.
Summary of Nanoparticle type used in therapeutics against HIV infection and latency.