
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kamla Pathak | -- | 2060 | 2023-07-12 08:11:47 | | | |
| 2 | Fanny Huang | Meta information modification | 2060 | 2023-07-13 05:35:05 | | |
Video Upload Options
Chitosan is a polycationic polymer generated from chitin with various characteristics such as biocompatibility, biodegradability, non-toxicity, and mucoadhesiveness, making it an ideal polymer to fabricate drug delivery systems. However, chitosan is poorly soluble in water and soluble in acidic aqueous solutions. Furthermore, owing to the presence of reactive amino groups, chitosan can be chemically modified to improve its physiochemical properties. Chitosan and its modified derivatives can be employed to fabricate nanoparticles, which are used most frequently in the pharmaceutical sector due to their possession of various characteristics such as nanosize, appropriate pharmacokinetic and pharmacodynamic properties, non-immunogenicity, improved stability, and improved drug loading capacity. Furthermore, it is capable of delivering nucleic acids, chemotherapeutic medicines, and bioactives using modified chitosan. Chitosan and its modified derivative-based nanoparticles can be targeted to specific cancer sites via active and passive mechanisms. Based on chitosan drug delivery systems, many anticancer drugs now have better effectiveness, potency, cytotoxicity, or biocompatibility.
1. Introduction
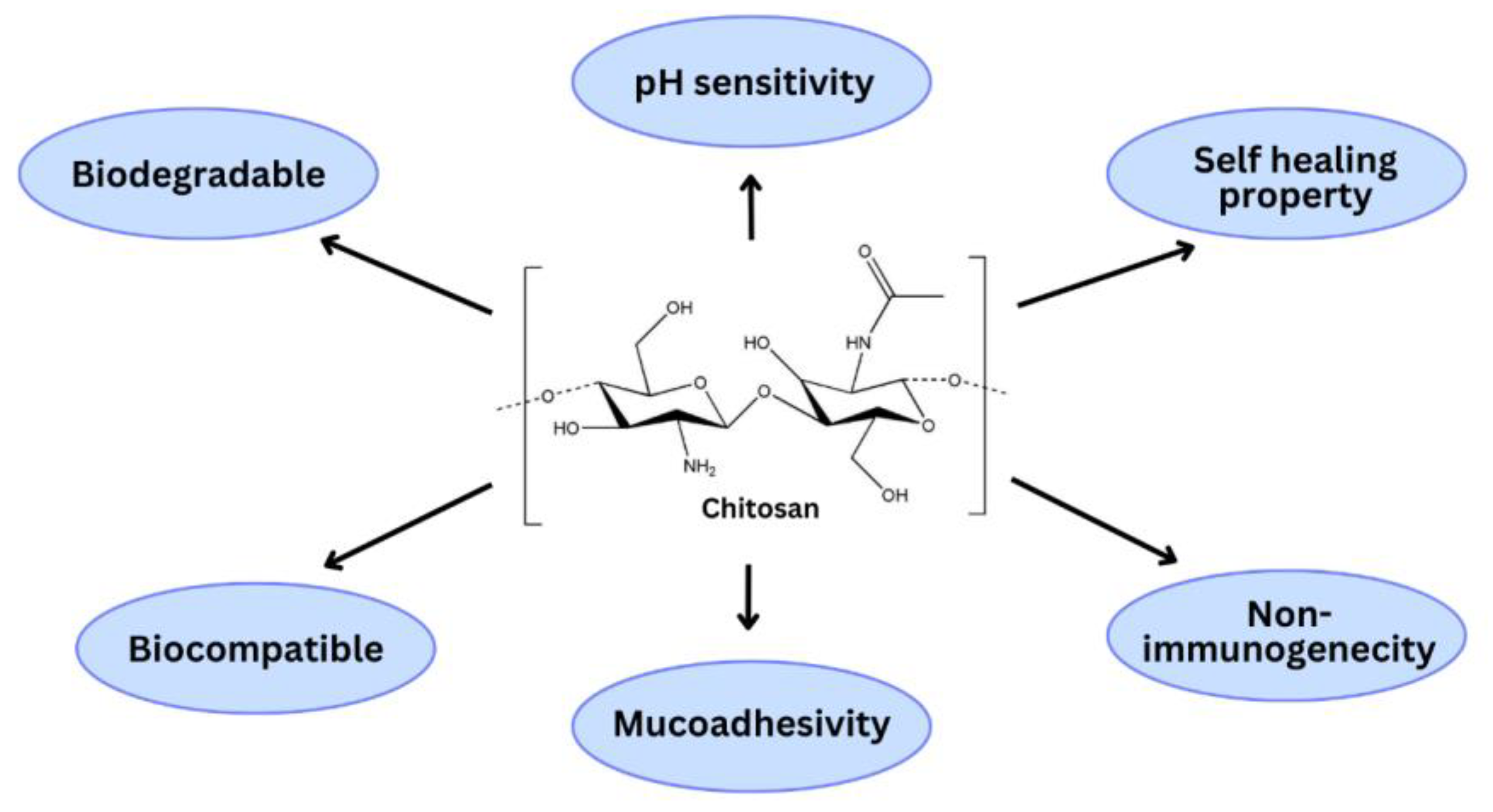
2. Chitosan and Its Derivatives for Anticancer Drug Delivery
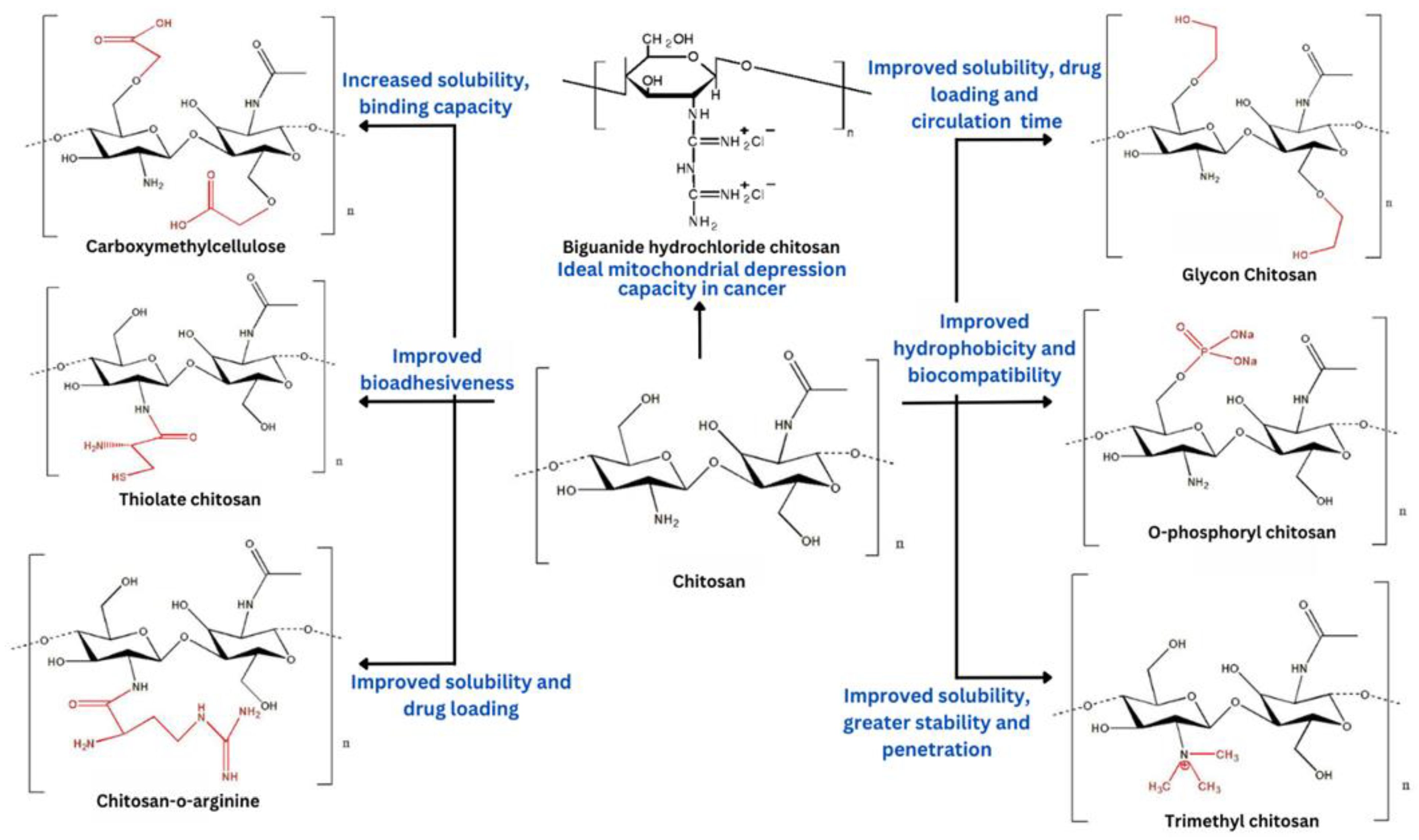
References
- Grover, M.; Behl, T.; Virmani, T.; Sanduja, M.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Bungau, S.G. Exploration of Cytotoxic Potential of Longifolene/Junipene Isolated from Chrysopogon Zizanioides. Molecules 2022, 27, 5764.
- Joyce Nirmala, M.; Kizhuveetil, U.; Johnson, A.; Balaji, G.; Nagarajan, R.; Muthuvijayan, V. Cancer Nanomedicine: A Review of Nano-Therapeutics and Challenges Ahead. RSC Adv. 2023, 13, 8606–8629.
- Grover, M.; Behl, T.; Virmani, T. Phytochemical Screening, Antioxidant Assay and Cytotoxic Profile for Different Extracts of Chrysopogon Zizanioides Roots. Chem. Biodivers 2021, 18, e2100012.
- Shah, S.C.; Kayamba, V.; Peek, R.M.; Heimburger, D. Cancer Control in Low- and Middle-Income Countries: Is It Time to Consider Screening? J. Glob. Oncol. 2019, 5, JGO.18.00200.
- Brinks, J.; Fowler, A.; Franklin, B.A.; Dulai, J. Lifestyle Modification in Secondary Prevention: Beyond Pharmacotherapy. Am. J. Lifestyle Med. 2017, 11, 137–152.
- Gao, Q.; Feng, J.; Liu, W.; Wen, C.; Wu, Y.; Liao, Q.; Zou, L.; Sui, X.; Xie, T.; Zhang, J.; et al. Opportunities and Challenges for Co-Delivery Nanomedicines Based on Combination of Phytochemicals with Chemotherapeutic Drugs in Cancer Treatment. Adv. Drug Deliv. Rev. 2022, 188, 114445.
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2022, 10, 1367–1401.
- Kumar, G.; Virmani, T.; Sharma, A.; Pathak, K. Codelivery of Phytochemicals with Conventional Anticancer Drugs in Form of Nanocarriers. Pharmaceutics 2023, 15, 889.
- Shakil, M.S.; Mahmud, K.M.; Sayem, M.; Niloy, M.S.; Halder, S.K.; Hossen, M.S.; Uddin, M.F.; Hasan, M.A. Using Chitosan or Chitosan Derivatives in Cancer Therapy. Polysaccharides 2021, 2, 795–816.
- Kumar, G.; Khar, R.K.; Virmani, T.; Jogpal, V.; Virmani, R. Comparative Evaluation of Fast Dissolving Tablet of Atorvastatin Calcium Using Natural and Synthetic Super Disintegrating Agents. Res. J. Pharm. Technol. 2018, 11, 5001.
- Kumar, G.; Virmani, T.; Pathak, K.; Alhalmi, A. A Revolutionary Blueprint for Mitigation of Hypertension via Nanoemulsion. BioMed Res. Int. 2022, 2022, e4109874.
- Virmani, T.; Kumar, G.; Pathak, K. Non-Aqueous Nanoemulsions: An Innovative Lipid-Based Drug Carrier. Available online: https://www.igi-global.com/chapter/non-aqueous-nanoemulsions/www.igi-global.com/chapter/non-aqueous-nanoemulsions/300404 (accessed on 19 April 2022).
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and Its Biomedical Applications in Health and Diseases: Special Focus on Drug Delivery. Environ. Sci. Pollut. Res. 2020, 27, 19151–19168.
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology Based Drug Delivery System: Current Strategies and Emerging Therapeutic Potential for Medical Science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J Nanobiotechnol. 2018, 16, 71.
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A Promising Approach for Delivery of Neuroprotective Drugs. Front. Neurosci. 2020, 14, 494.
- Virmani, T.; Kumar, G.; Virmani, R.; Sharma, A.; Pathak, K. Nanocarrier-Based Approaches to Combat Chronic Obstructive Pulmonary Disease. Nanomedicine 2022, 17, 1833–1854.
- Kumar, G.; Virmani, T.; Pathak, K.; Kamaly, O.A.; Saleh, A. Central Composite Design Implemented Azilsartan Medoxomil Loaded Nanoemulsion to Improve Its Aqueous Solubility and Intestinal Permeability: In Vitro and Ex Vivo Evaluation. Pharmaceuticals 2022, 15, 1343.
- Perumal, S. Polymer Nanoparticles: Synthesis and Applications. Polymers 2022, 14, 5449.
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731.
- Colone, M.; Calcabrini, A.; Stringaro, A. Drug Delivery Systems of Natural Products in Oncology. Molecules 2020, 25, 4560.
- Chang, D.; Ma, Y.; Xu, X.; Xie, J.; Ju, S. Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 707319.
- Virmani, T.; Kumar, G.; Virmani, R.; Sharma, A.; Pathak, K. Xanthan Gum-Based Drug Delivery Systems for Respiratory Diseases. In Natural Polymeric Materials Based Drug Delivery Systems in Lung Diseases; Springer Nature Singapore: Singapore, 2023; pp. 279–295. ISBN 978-981-19765-5-1.
- Polat, M.; Polat, H. Recent Advances in Chitosan-Based Systems for Delivery of Anticancer Drugs. In Functional Chitosan: Drug Delivery and Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 191–228. ISBN 9789811502620.
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatment. Polymers 2021, 13, 1717.
- Almutairi, F.M.; Abd-Rabou, A.A.; Mohamed, M.S. Raloxifene-Encapsulated Hyaluronic Acid-Decorated Chitosan Nanoparticles Selectively Induce Apoptosis in Lung Cancer Cells. Bioorganic Med. Chem. 2019, 27, 1629–1638.
- Ullah, S.; Azad, A.K.; Nawaz, A.; Shah, K.U.; Iqbal, M.; Albadrani, G.M.; Al-Joufi, F.A.; Sayed, A.A.; Abdel-Daim, M.M. 5-Fluorouracil-Loaded Folic-Acid-Fabricated Chitosan Nanoparticles for Site-Targeted Drug Delivery Cargo. Polymers 2022, 14, 2010.
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256.
- Lim, C.; Hwang, D.S.; Lee, D.W. Intermolecular Interactions of Chitosan: Degree of Acetylation and Molecular Weight. Carbohydr. Polym. 2021, 259, 117782.
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of Chitosan Composites and Chitosan Nanoparticle Composites on Various Drug Delivery Systems: A Review. J. Food Drug Anal. 2015, 23, 619–629.
- Chen, Y.-Z.; Huang, Y.-K.; Chen, Y.; Ye, Y.-J.; Lou, K.-Y.; Gao, F. Novel Nanoparticles Composed of Chitosan and β-Cyclodextrin Derivatives as Potential Insoluble Drug Carrier. Chin. Chem. Lett. 2015, 26, 909–913.
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652.
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963.
- Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460.
- Ways, T.M.; Lau, W.; Khutoryanskiy, V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267.
- Kumar, S.; Deepak, V.; Kumari, M.; Dutta, P. Antibacterial Activity of Diisocyanate-Modified Chitosan for Biomedical Applications. Int. J. Biol. Macromol. 2015, 84, 349–353.
- Xing, L.; Fan, Y.-T.; Zhou, T.-J.; Gong, J.-H.; Cui, L.-H.; Cho, K.-H.; Choi, Y.-J.; Jiang, H.-L.; Cho, C.-S. Chemical Modification of Chitosan for Efficient Vaccine Delivery. Molecules 2018, 23, 229.
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393.
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in Research of Chitosan Chemical Modification Technologies and Their Applications. Mar. Drugs 2022, 20, 536.
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan Based Hydrogels and Their Applications for Drug Delivery in Wound Dressings: A Review. Carbohydr. Polym. 2018, 199, 445–460.
- Ali, A.; Ahmed, S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286.
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and Its Derivatives for Controlled Drug Release Applications—A Review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659.
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and Its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519.
- Arya, G.; Gupta, N.; Nimesh, S. 8—Chitosan Nanoparticles for Therapeutic Delivery of Anticancer Drugs. In Polysaccharide Nanoparticles; Micro and Nano Technologies; Venkatesan, J., Kim, S.-K., Anil, S.P.d.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–230. ISBN 978-0-12-822351-2.
- Kurczewska, J. Chitosan-Based Nanoparticles with Optimized Parameters for Targeted Delivery of a Specific Anticancer Drug—A Comprehensive Review. Pharmaceutics 2023, 15, 503.
- Botelho Da Silva, S.; Krolicka, M.; Van Den Broek, L.A.M.; Frissen, A.E.; Boeriu, C.G. Water-Soluble Chitosan Derivatives and PH-Responsive Hydrogels by Selective C-6 Oxidation Mediated by TEMPO-Laccase Redox System. Carbohydr. Polym. 2018, 186, 299–309.
- Babu, A.; Ramesh, R. Multifaceted Applications of Chitosan in Cancer Drug Delivery and Therapy. Mar. Drugs 2017, 15, 96.
- Jaiswal, S.; Dutta, P.K.; Kumar, S.; Chawla, R. Chitosan Modified by Organo-Functionalities as an Efficient Nanoplatform for Anti-Cancer Drug Delivery Process. J. Drug Deliv. Sci. Technol. 2021, 62, 102407.
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor CGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608.
- Li, W.; Zhu, X.; Zhou, X.; Wang, X.; Zhai, W.; Li, B.; Du, J.; Li, G.; Sui, X.; Wu, Y.; et al. An Orally Available PD-1/PD-L1 Blocking Peptide OPBP-1-Loaded Trimethyl Chitosan Hydrogel for Cancer Immunotherapy. J. Control. Release 2021, 334, 376–388.
- Esmaily, M.; Masjedi, A.; Hallaj, S.; Nabi Afjadi, M.; Malakotikhah, F.; Ghani, S.; Ahmadi, A.; Sojoodi, M.; Hassannia, H.; Atyabi, F.; et al. Blockade of CTLA-4 Increases Anti-Tumor Response Inducing Potential of Dendritic Cell Vaccine. J Control. Release 2020, 326, 63–74.




