
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | DANIELA Poenaru | -- | 2837 | 2023-07-11 17:41:59 | | | |
| 2 | Lindsay Dong | Meta information modification | 2837 | 2023-07-13 02:51:23 | | |
Video Upload Options
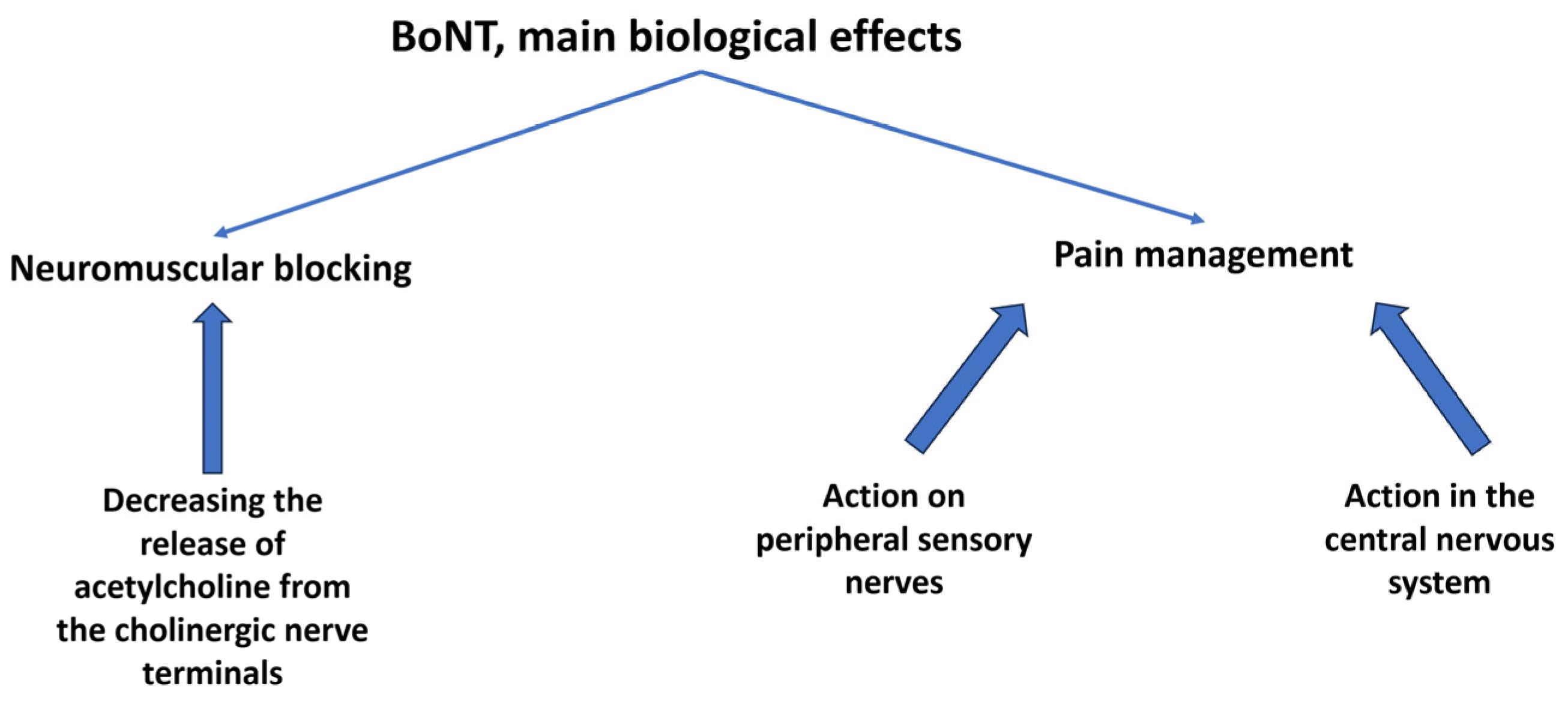
Botulinum neurotoxin (BoNT), a product of Clostridium botulinum, reversibly inhibits the presynaptic release of the neurotransmitter acetylcholine at the neuromuscular junction. In addition, BoNT blocks the transmission of other substances involved in pain perception and, together with a soft-tissue anti-inflammatory effect, may play a role in analgesia. When first-line treatment fails, second-line therapies might include BoNT. Studies on chronic and recurrent pain using different mechanisms offer heterogenous results that must be validated and standardized. Plantar fasciitis, severe knee osteoarthritis, painful knee and hip arthroplasty, antalgic muscular contractures, and neuropathic and myofascial pain syndromes may benefit from the administration of BoNT.
1. Introduction
| Disease | Administration | Number of Studies |
|---|---|---|
| Plantar fasciitis | Intrafascial Intramuscular (short plantar muscles) Intramuscular (medial head gastrocnemius) |
10 |
| Knee osteoarthritis | Intra-articular | 6 |
| Painful knee arthroplasty | Intra-articular Intramuscular (for contractures) |
3 |
| Painful muscular contractures | Intramuscular | 3 |
| Chronic lateral epicondylitis | Intratendinous Intramuscular |
6 |
| Neuropathic pain | Intradermal, subcutaneous/submucosal Perinervous Sympathetic block |
16 |
| Carpal tunnel syndrome | Intracannalar Intramuscular |
1 |
| Morton neuroma | Perineural | 1 |
| Myofascial pain syndrome | Trigger point injection | 6 |
2. Chronic Musculoskeletal Disorders Pain Management by Botulinum Toxin
2.1. Plantar Fasciitis
2.2. Knee Osteoarthritis
2.3. Painful Knee Arthroplasty
2.4. Joint Contractures
2.5. Chronic Lateral Epicondylitis
2.6. Neuropathic Pain
2.7. Carpal Tunnel Syndrome
2.8. Morton Neuroma
2.9. Myofascial Pain Syndrome
3. Conclusions
References
- Lew, M.F. Review of the FDA-approved uses of botulinum toxins, including data suggesting efficacy in pain reduction. Clin. J. Pain 2002, 18 (Suppl. S6), S142–S146.
- Chen, S. Clinical uses of botulinum neurotoxins: Current indications, limitations and future developments. Toxins 2012, 4, 913–939.
- Wheeler, P.; Boyd, K.; Shipton, M. Surgery for patients with recalcitrant plantar fasciitis: Good results at short-, medium-, and long-term follow-up. Orthop. J. Sports Med. 2014, 2, 2325967114527901.
- Neufeld, S.K.; Cerrato, R. Plantar fasciitis: Evaluation and treatment. J. Am. Acad. Orthop. Surg. 2008, 16, 338–346.
- Díaz-Llopis, I.V.; Gómez-Gallego, D.; Mondéjar-Gómez, F.J.; López-García, A.; Climent-Barberá, J.M.; Rodríguez-Ruiz, C.M. Botulinum toxin type A in chronic plantar fasciitis: Clinical effects one year after injection. Clin. Rehabil. 2013, 27, 681–685.
- Babcock, M.S.; Foster, L.; Pasquina, P.; Jabbari, B. Treatment of pain attributed to plantar fasciitis with botulinum toxin A: A short-term, randomized, placebo-controlled, doubleblind study. Am. J. Phys. Med. Rehabil. 2005, 84, 649–654.
- Chou, L.W.; Hong, C.Z.; Wu, E.S.; Hsueh, W.H.; Kao, M.J. Serial Ultrasonographic Findings of Plantar Fasciitis After Treatment with Botulinum Toxin A: A Case Study. Arch. Phys. Med. Rehabil. 2011, 92, 316–319.
- Placzek, R.; Deuretzbacher, G.; Buttgereit, F.; Meiss, A.L. Treatment of chronic plantar fasciitis with botulinum toxin A: An open case series with a 1 year follow up. Ann. Rheum. Dis. 2005, 64, 1659–1661.
- Peterlein, C.D.; Funk, J.F.; Hölscher, A.; Schuh, A.; Placzek, R. Is Botulinum Toxin An Effective for the Treatment of Plantar Fasciitis? Clin. J. Pain 2012, 28, 527–533.
- Elizondo-Rodríguez, J.; Simental-Mendía, M.; Peña-Martínez, V.; Vilchez-Cavazos, F.; Tamez-Mata, Y.; Acosta-Olivo, C. Comparison of Botulinum Toxin A, Corticosteroid, and Anesthetic Injection for Plantar Fasciitis. Foot Ankle Int. 2021, 42, 305–313.
- Ahmad, J.; Ahmad, S.H.; Jones, K. Treatment of Plantar Fasciitis with Botulinum Toxin. Foot Ankle Int. 2017, 38, 1–7.
- Radovic, P. Treatment of “Plantar Fasciitis”/Plantar Heel Pain Syndrome with Botulinum Toxin- A Novel Injection Paradigm Pilot Study. Foot 2020, 45, 101711.
- Fonfria, E.; Maignel, J.; Lezmi, S.; Martin, V.; Splevins, A.; Shubber, S.; Kalinichev, M.; Foster, K.; Picaut, P.; Krupp, J. The Expanding Therapeutic Utility of Botulinum Neurotoxins. Toxins 2018, 10, 208.
- Kellgren, J.; Lawrence, J. Radiological assessment of osteoarthrosis. Ann. Rheum. Dis. 1957, 16, 494–502.
- Chou, C.L.; Lee, S.H.; Lu, S.Y.; Tsai, K.L.; Ho, C.Y.; Lai, H.C. Therapeutic effects of intra-articular botulinum neurotoxin in advanced knee osteoarthritis. J. Chin. Med. Assoc. 2010, 73, 573–580.
- Hsieh, L.F.; Wu, C.W.; Chou, C.C.; Yang, S.W.; Wu, S.H.; Lin, Y.J.; Hsu, W.C. Effects of Botulinum Toxin Landmark-Guided Intra-articular Injection in Subjects With Knee Osteoarthritis. PM R J. Inj. Funct. Rehabil. 2016, 8, 1127–1135.
- Najafi, S.; Sanati, E.; Khademi, M.; Abdorrazaghi, F.; Mofrad, R.K.; Rezasoltani, Z. Intra-articular botulinum toxin type A for treatment of knee osteoarthritis: Clinical trial. Toxicon 2019, 165, 69–77.
- Mendes, J.G.; Natour, J.; Nunes-Tamashiro, J.C.; Toffolo, S.R.; Rosenfeld, A.; Furtado, R.N.V. Comparison between intra-articular Botulinum toxin type A, corticosteroid, and saline in knee osteoarthritis: A randomized controlled trial. Clin. Rehabil. 2019, 33, 1015–1026.
- Zhai, S.; Huang, B.; Yu, K. The efficacy and safety of Botulinum Toxin Type A in painful knee osteoarthritis: A systematic review and meta-analysis. J. Int. Med. Res. 2020, 48, 300060519895868.
- Sun, S.F.; Hsu, C.W.; Lin, H.S.; Chou, Y.J.; Chen, J.Y.; Wang, J.L. Efficacy of intraarticular botulinum toxin A and intraarticular hyaluronate plus rehabilitation exercise in patients with unilateral ankle osteoarthritis: A randomized controlled trial. J. Foot Ankle Res. 2014, 7, 9.
- Singh, J.A.; Mahowald, M.L.; Noorbaloochi, S. Intraarticular botulinum toxin A for refractory painful total knee arthroplasty: A randomized controlled trial. J. Rheumatol. 2010, 37, 2377–2386, Erratum in J. Rheumatol. 2011, 38, 1534.
- Singh, J.A. Efficacy of Long-term Effect and Repeat Intraarticular Botulinum toxin in Patients with Painful Total Joint Arthroplasty: A Retrospective Study. Br. J. Med. Med. Res. 2014, 4, 139–148.
- Vahedi, H.; Khlopas, A.; Szymczuk, V.L.; Peterson, M.K.; Hammouda, A.I.; Conway, J.D. Treatment with posterior capsular release, botulinum toxin injection, hamstring tenotomy, and peroneal nerve decompression improves flexion contracture after total knee arthroplasty: Minimum 2-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2706–2714.
- Knobe, K.; Berntorp, E. Haemophilia and joint disease: Pathophysiology, evaluation, and management. J. Comorb. 2011, 1, 51–59.
- Daffunchio, C.; Caviglia, H.; Nassif, J.; Morettil, N.; Galatro, G. Knee flexion contracture treated with botulinum toxin type A in patients with haemophilia (PWH). Haemophilia 2015, 22, 134–141.
- Berend, M.E.; Smith, A.; Meding, J.B.; Ritter, M.A.; Lynch, T.; Davis, K. Long-term outcome and risk factors of proximal femoral fracture in uncemented and cemented total hip arthroplasty in 2551 hips. J. Arthroplast. 2006, 21, 53.
- Bhave, A.; Marker, D.R.; Seyler, T.M.; Ulrich, S.D.; Plate, J.F.; Mont, M.A. Functional problems and treatment solutions after total hip arthroplasty. J. Arthroplast. 2007, 22, 116–124.
- Bhave, A.; Zywiel, M.G.; Ulrich, S.D.; McGrath, M.S.; Seyler, T.M.; Marker, D.R.; Delanois, R.E.; Mont, M.A. Botulinum toxin type A injections for the management of muscle tightness following total hip arthroplasty: A case series. J. Orthop. Surg. Res. 2009, 4, 34.
- Wong, S.M.; Hui, A.C.F.; Tong, P.Y.; Poon, D.W.F.; Yu, E.; Wong, L.K.S. Treatment of Lateral Epicondylitis with Botulinum Toxin. Ann. Intern. Med. 2005, 143, 793.
- Placzek, R.; Drescher, W.; Deuretzbacher, G.; Hempfing, A.; Meiss, A.L. Treatment of Chronic Radial Epicondylitis with Botulinum Toxin A. J. Bone Jt. Surg. 2007, 89, 255–260.
- Hayton, M.J.; Santini, A.J.A.; Hughes, P.J.; Frostick, S.P.; Trail, I.A.; Stanley, J.K. Botulinum Toxin Injection in the Treatment of Tennis Elbow. J. Bone Jt. Surg. 2005, 87, 503–507.
- Creuzé, A.; Petit, H.; de Sèze, M. Short-Term Effect of Low-Dose, Electromyography-Guided Botulinum Toxin A Injection in the Treatment of Chronic Lateral Epicondylar Tendinopathy: A Randomized, Double-Blinded Study. J. Bone Jt. Surg. Am. 2018, 100, 818–826.
- Cogné, M.; Creuzé, A.; Petit, H.; Delleci, C.; Dehail, P.; de Seze, M. Number of botulinum toxin injections needed to stop requests for treatment for chronic lateral epicondylar tendinopathy. A 1-year follow-up study. Ann. Phys. Rehabil. Med. 2019, 62, 336–341.
- Lin, Y.C.; Wu, W.T.; Hsu, Y.C.; Han, D.S.; Chang, K.V. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: A systematic review and meta-analysis. Clin. Rehabil. 2017, 32, 131–145.
- Wu, S.; Lian, Y.; Zhang, H.; Chen, Y.; Wu, C.; Li, S.; Zheng, Y.; Wang, Y.; Cheng, W.; Huang, Z. Botulinum Toxin Type A for refractory trigeminal neuralgia in older patients: A better therapeutic effect. J. Pain Res. 2019, 12, 2177–2186.
- Zúñiga, C.; Piedimonte, F.; Díaz, S.; Micheli, F. Acute treatment of trigeminal neuralgia with onabotulinum toxin A. Clin. Neuropharmacol. 2013, 36, 146–150.
- Zhang, H.; Lian, Y.; Ma, Y.; Chen, Y.; He, C.; Xie, N.; Wu, C. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: Observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J. Headache Pain 2014, 15, 65.
- Xiao, L.; Mackey, S.; Hui, H.; Xong, D.; Zhang, Q.; Zhang, D. Subcutaneous injection of botulinum toxin a is beneficial in postherpetic neuralgia. Pain Med. 2010, 11, 1827–1833.
- Apalla, Z.; Sotiriou, E.; Lallas, A.; Lazaridou, E.; Ioannides, D. Botulinum Toxin A in Postherpetic Neuralgia. Clin. J. Pain 2013, 29, 857–864.
- Shackleton, T.; Ram, S.; Black, M.; Ryder, J.; Clark, G.T.; Enciso, R. The efficacy of botulinum toxin for the treatment of trigeminal and postherpetic neuralgia: A systematic review with meta-analyses. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 61–71.
- Finnerup, N.B.; Sindrup, S.H.; Jensen, T.S. Recent advances in pharmacological treatment of neuropathic pain. F1000 Med. Rep. 2010, 2, 52.
- Lee, Y.; Lee, C.J.; Choi, E.; Lee, P.B.; Lee, H.J.; Nahm, F.S. Lumbar Sympathetic Block with Botulinum Toxin Type A and Type B for the Complex Regional Pain Syndrome. Toxins 2018, 10, 164.
- Tsai, C.P.; Liu, C.Y.; Lin, K.P.; Wang, K.C. Efficacy of botulinum toxin type a in the relief of Carpal tunnel syndrome: A preliminary experience. Clin. Drug Investig. 2006, 26, 511–515.
- Hablas, S.A.; Nada, D.W.; Alashkar, D.S.; Elsharkawy, A.A. The effect of botulinum toxin type A injection in decreasing intratunnel tendon tension in carpal tunnel syndrome: A randomized controlled trial for efficacy and safety. Egypt. Rheumatol. Rehabil. 2019, 46, 299–303.
- Climent, J.M.; Mondéjar-Gómez, F.; Rodríguez-Ruiz, C.; Díaz-Llopis, I.; Gómez-Gallego, D.; Martín-Medina, P. Treatment of Morton Neuroma with Botulinum Toxin A: A Pilot Study. Clin. Drug Investig. 2013, 33, 497–503.
- Ojala, T.; Arokoski, J.P.A.; Partanen, J. The Effect of Small Doses of Botulinum Toxin A on Neck-Shoulder Myofascial Pain Syndrome: A Double-Blind, Randomized, and Controlled Crossover Trial. Clin. J. Pain 2006, 22, 90–96.
- Kamanli, A.; Kaya, A.; Ardicoglu, O.; Ozgocmen, S.; Zengin, F.O.; Bayık, Y. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol. Int. 2004, 25, 604–611.
- Göbel, H.; Heinze, A.; Reichel, G.; Hefter, H.; Benecke, R. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport®) for the relief of upper back myofascial pain syndrome: Results from a randomized double-blind placebo-controlled multicentre study. Pain 2006, 125, 82–88.
- Graboski, C.L.; Gray, S.D.; Burnham, R.S. Botulinum toxin A versus bupivacaine trigger point injections for the treatment of myofascial pain syndrome: A randomised double blind crossover study. Pain 2005, 118, 170–175.




