Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Afzal Shah | -- | 1685 | 2023-07-11 17:14:03 | | | |
| 2 | Camila Xu | Meta information modification | 1685 | 2023-07-12 02:43:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sadiq, M.U.; Shah, A.; Haleem, A.; Shah, S.M.; Shah, I. Plant Extract-Mediated Synthesis and Characterization of Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/46654 (accessed on 05 March 2026).
Sadiq MU, Shah A, Haleem A, Shah SM, Shah I. Plant Extract-Mediated Synthesis and Characterization of Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/46654. Accessed March 05, 2026.
Sadiq, Muhammad Usman, Afzal Shah, Abdul Haleem, Syed Mujtaba Shah, Iltaf Shah. "Plant Extract-Mediated Synthesis and Characterization of Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/46654 (accessed March 05, 2026).
Sadiq, M.U., Shah, A., Haleem, A., Shah, S.M., & Shah, I. (2023, July 11). Plant Extract-Mediated Synthesis and Characterization of Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/46654
Sadiq, Muhammad Usman, et al. "Plant Extract-Mediated Synthesis and Characterization of Nanoparticles." Encyclopedia. Web. 11 July, 2023.
Copy Citation
Eucalyptus globulus (EG) is an endemic plant in Australia that is widely found throughout the world. It is the main source of botanical essential oils and is well-recognized in pharmacopeia around the globe. In the plant-assisted fabrication of nanoparticles, the salt solution and extract are simply mixed at room temperature or slightly high temperature, resulting in the synthesis of nanoparticles (NPs) within minutes.
Eucalyptus globulus
phytonanofactory
nanostructures
1. Introduction
The last two decades witnessed remarkable utilization of nanotechnology in every avenue of science and technology, which is currently regarded as one of the top and demanding research areas for the safety of the society and the environment. Nanotechnology is the manipulated reorganization and restructuring of materials at the nanoscale [1]. The physicochemical characteristics of nanoscale materials markedly differ from macroscale materials due to quantum effects, enhancement of the surface area, and dominance of interfacial processes [2][3]. Albeit nanotechnology flourished exponentially after the industrial revolution, the history of human exposure to nanomaterial can be traced back to the medieval ages when gold nanoparticles were used to color glass [4]. The term “nanometer” was initially proposed by Nobel Laureate Richard Zsigmondy in 1925. He invented the word “nanometer” to define particle size and was the first to use the microscope to quantify the size of particles like gold colloids [5]. Richard Feynman explicitly can be regarded as the father of nanotechnology, which proposed the hypothesis of manipulation of atoms and molecules to generate nanoscale materials during his revolutionary lecture entitled “There is plenty of room at the bottom” at the American Physical Society 1959 meeting. Although the term nanotechnology was first used by Norio Taniguchi in a publication in 1974, and Eric Drexler popularized this term to the world in his book “Engineer of Creation” in 1986 [6].
To date, a considerable number of nanostructures with a variety of applications in various fields have been prepared and reported by employing different physicochemical methods (thermal decomposition, chemical reduction, microwave synthesis, ion sputtering methods, and hydrothermal methods, among others). Nanomaterials synthesized using these methods have a broad range of applications, but all these mentioned methods are not environmentally benign due to the use of bio-hazardous chemicals, which limit their uses for biomedical and clinical applications. The use of hazardous and expensive chemicals has urged scientists to exploit a cost-effective and eco-friendly synthesis approach of nanomaterials that can be an efficient alternative to these conventional methods [7]. In this regard, green methods have emerged as a potent alternative because they are easy to handle, cheaper, and environmentally safe in comparison to physical and chemical methods. The fabrication of nanostructures based on biological sources can be broadly divided into two categories: Biotemplated forming synthesis and biological synthesis. In the biotemplating method, inorganic materials are incorporated on the substrate, followed by mineralization/fossilization through various physical or chemical deposition processes, which results in the fabrication of ordered nanostructures, whereas biological synthesis relies on the metabolites of materials that result in the production of randomly distributed nanomaterials [8][9]. Albeit arguably, large-scale production of nanostructure using the green method is a difficult task, in the future, with advances in studying the composition of biological extract and the nature of reaction with metal, large-scale production will be feasible [10]. The nanomaterials fabricated employing green methods have wide applications in various fields, such as contaminant remediation, antifungal, antibacterial, biomedical, and photocatalytic activity [11][12]. Green synthesis have multiple advantages as mentioned in Figure 1.
Figure 1. Advantages of green synthesis of nanoparticles.
2. Plant Extract-Mediated Synthesis and Characterization of Nanoparticles
A significant fraction of plants have been used in the synthesis of nanomaterials due to the reductive abilities of phytochemicals. Plants-assisted synthesis has attracted much attention as compared to other organisms due to the stable, faster, and large-scale production of nanomaterials [13]. Additionally, plants are accessible, easy to cultivate, and safe to handle. Various parts of plants (roots, fruits, peels, flowers, stems, and leaves) have been employed for the production of nanomaterials. However, leaf extracts are widely employed due to metabolite richness [14][15]. A new avenue of NPs manufacturing has emerged as a result of recent research on the biosynthesis of nanomaterials utilizing plant extracts.
Although phytochemical-mediated synthesis of NPs has been widely adopted by the scientific community around the globe, there is still no justified reason for the selection of a particular plant during their work. It can be speculated that the facile availability, traditional and herbal uses of plants are the determining factors for their choice. Since polyphenols, alkaloids, flavonoids, saponins, polysaccharides, and other plant compounds are excellent stabilizing and reducing agents, using plant materials abundant in these compounds increases the probability of success. In the plant-assisted fabrication of nanoparticles, the salt solution and extract are simply mixed at room temperature or slightly high temperature, resulting in the synthesis of NPs within minutes. The synthesis of NPs can be initially confirmed by observing the change in the color of the solution. A detailed schematic of NPs synthesis is presented in Figure 2.
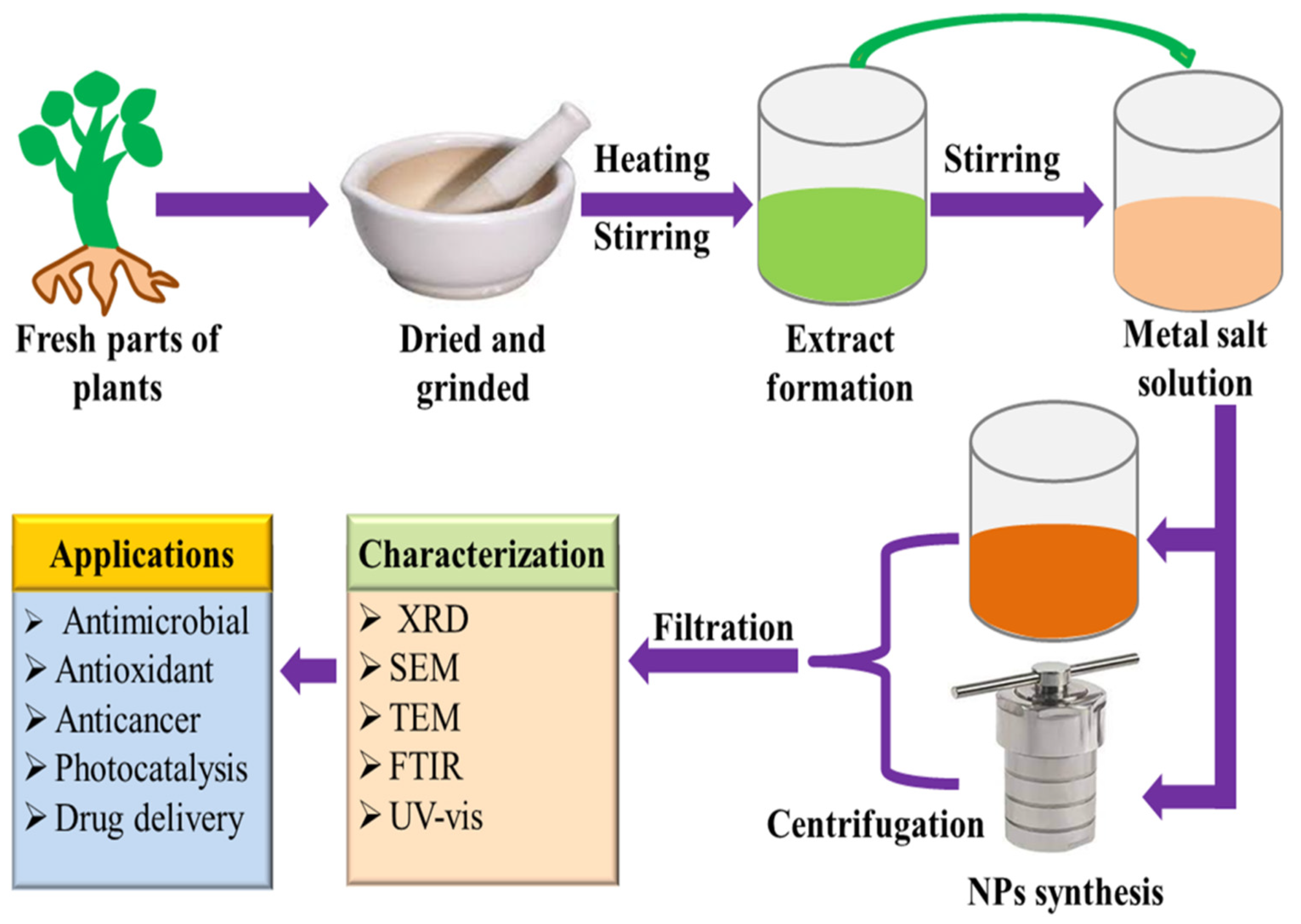
Figure 2. Proposed pathway of plant-assisted green synthesis of nanoparticles.
Plant extract is mostly prepared by dissolving leaves, flowers, roots, bark, etc., in a solvent (most commonly used water and ethanol), followed by heating at low temperatures. After that, the mixture is filtered, and the filtrate is used for the synthesis of NPs. For the synthesis of NPs, the plant extract is added to the salt solution of the respective metal, followed by heating. The metal salts in the solution dissociate into metal ions. These metal ions and phytochemicals are attracted by electrostatic interaction to form a metal-phytochemicals complex, from which metal oxide NPs can be fabricated by the high-temperature calcination treatment. However, in the case of metal NPs synthesis, the biomolecules present in plant extract reduce the metal ions to the metal atoms (M°), and nucleation of M° occurs. The small-size NPs combine to form larger particles which increase the thermodynamic stability. Biomolecules present in plant extracts play a role in stabilizing and reducing the metal ions in the solution. The plant extracts contain a wide variety of phytochemicals. It is difficult to determine the precise stabilizing and reducing agents for the formation of NPs [16]. Since the phytochemicals vary from one plant to another, as well as their locality, therefore, the nature and concentration of extract, pH, the concentration of salt, contact time, and temperature are credited to influence the rate of synthesis, quantity, and other properties of NPs. Vast research on plant extract-mediated fabrication of nanostructures has been reported. The green synthesis of silver nanostructures involves the reduction of a silver metal solution to nanoscale using different metabolites present in plant extracts. The variation from light yellow to brownish color solution is a visual indicator of NPs synthesis [17][18][19]. Stable gold NPs (Au NPs) with different morphologies have been synthesized using plant extracts. Green synthesized Au NPs have catalytic and biomedical applications [20][21]. The plant extract-mediated approach has been mostly employed to prepare an enormous number of metal oxide nanostructures that have applications such as the biomedical (anticancer, antioxidant, antibacterial, antifungal, etc.), agricultural, catalytic, contaminants mitigation, and energy sectors [22].
Characterization of Nanoparticles
The size and morphology (shape) of NPs are the most important parameters that substantially influence their physiochemical characteristics and applications [23]. These two parameters can be carefully regulated during the synthesis of NPs. The other factors which can also influence the characteristics of NPs are solubility, surface charges, surface area, porosity, zeta potential, particle aggregation, etc. Several electronic and spectroscopic techniques are utilized to evaluate the different properties (electronic, optical, mechanical, morphological, etc.) of NPs. The most widely employed techniques for the characterizations of NPs are precisely described below.
X-ray crystallography or diffraction (XRD) is employed to study the structural aspects (crystallinity, particle size, lattice dimension) of NPs. The full-width half maximum (FWHM) values of diffraction peaks are utilized to deduce the concerned information from the XRD spectrum. The diffraction peaks correspond to distinct crystallographic orientations of crystallites in NPs, thus FWHM is affected by the atoms participating in scattering events, and the contributing atoms are related to the size of the crystal plane that produces the particular reflection. The Debye–Scherrer equation is used to determine the mean crystallite size of NPs [24].

where D is the average crystallite size, λ is the X-rays wavelength, K is the crystallite shape factor, θ is the diffraction angle, and β is the FWHM. The crystalline structure of NPs can be estimated by comparing the intensity and, particularly, the position of peaks with the standard patterns established by the International Center of Diffraction Data (ICDD). Despite its wide utilization, this technique is not appropriate for amorphous materials and also for particles having a size less than 3 nm [25].
Scanning electron microscopy (SEM) is a versatile technique to study the surface morphology, electrical behavior, and chemical composition (SEM coupled energy dispersive X-rays analysis) of NPs. When a beam of the electron is directed on the surface of the sample (NPs), secondary electrons are emitted, which are then detected by the SEM to generate an image of the sample [25]. Contrary to SEM, transmission electron microscopy (TEM) uses the transmitted electrons to generate images of NPs. TEM can analyze the crystal structure, shape, and size at a single particle level with a capability of 200-million-fold magnification [26]. Fourier transform infrared spectroscopy (FTIR) is an environment-friendly and non-destructive technique used to determine functional groups based on how materials respond to infrared (IR) light. The molecules to be analyzed by FTIR spectroscopy must have a dipole moment. The FTIR analysis of NPs synthesized using plant extracts is mostly used to carry out to investigate the phytochemical makeup of extracts that act as capping agents [27]. UV-visible spectroscopy (UV-Vis) is widely employed to investigate the optical properties (absorption and band gap) of NPs. The absorption pattern obtained using UV-Vis spectroscopy is used to characterize the NPs. Furthermore, the band gap is used to analyze the photocatalytic behavior of NPs. Thermogravimetric analysis (TGA) is employed to study the thermal stability of nanostructures. X-ray photoelectron spectroscopy (XPS) is employed to determine the purity of NPs by analyzing the binding energy peaks and chemical states of the materials. Typically, XPS can probe the sample up to 10 nm [28]. Furthermore, Brunauer–Emmett–Teller (BET) study is employed to investigate the porosity of NPs.
References
- Shume, W.M.; Murthy, H.A.; Zereffa, E.A. A review on synthesis and characterization of Ag2O nanoparticles for photocatalytic applications. J. Chem. 2020, 2020, 5039479.
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.; Xu, M.; Wu, M.J.; Bao, S. Biocompatibility of nanomaterials and their immunological properties. Biomed. Mater. 2021, 16, 042005.
- Danish, M.S.S.; Estrella-Pajulas, L.L.; Alemaida, I.M.; Grilli, M.L.; Mikhaylov, A.; Senjyu, T. Green synthesis of silver oxide nanoparticles for photocatalytic environmental remediation and biomedical applications. Metals 2022, 12, 769.
- Freestone, I.; Meeks, N.; Sax, M.; Higgitt, C. The Lycurgus cup—A roman nanotechnology. Gold Bull. 2007, 40, 270–277.
- Khalaj, M.; Kamali, M.; Costa, M.E.V.; Capela, I. Green synthesis of nanomaterials—A scientometric assessment. J. Clean. Prod. 2020, 267, 122036.
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112.
- Kanwar, R.; Rathee, J.; Salunke, D.B.; Mehta, S.K. Green nanotechnology-driven drug delivery assemblies. ACS Omega 2019, 4, 8804–8815.
- Gong, D.; Sun, L.; Li, X.; Zhang, W.; Zhang, D.; Cai, J. Micro/Nanofabrication, Assembly, and Actuation Based on Microorganisms: Recent Advances and Perspectives. Small Struct. 2023, 2200356.
- Herrera-Beurnio, M.C.; Hidalgo-Carrillo, J.; López-Tenllado, F.J.; Martin-Gómez, J.; Estévez, R.C.; Urbano, F.J.; Marinas, A. Bio-templating: An emerging synthetic technique for catalysts. A review. Catalysts 2021, 11, 1364.
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336.
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374.
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559.
- Ahmed, A.; Usman, M.; Ji, Z.; Rafiq, M.; Yu, B.; Shen, Y.; Cong, H. Nature-inspired biogenic synthesis of silver nanoparticles for antibacterial applications. Mater. Today Chem. 2023, 27, 101339.
- Nadeem, M.; Khan, R.; Afridi, K.; Nadhman, A.; Ullah, S.; Faisal, S.; Mabood, Z.U.; Hano, C.; Abbasi, B.H. Green synthesis of cerium oxide nanoparticles (CeO2 NPs) and their antimicrobial applications: A review. Int. J. Nanomed. 2020, 15, 5951–5961.
- Rakib-Uz-Zaman, S.; Hoque Apu, E.; Muntasir, M.N.; Mowna, S.A.; Khanom, M.G.; Jahan, S.S.; Akter, N.; R. Khan, M.A.; Shuborna, N.S.; Shams, S.M. Biosynthesis of silver nanoparticles from Cymbopogon citratus leaf extract and evaluation of their antimicrobial properties. Challenges 2022, 13, 18.
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 2021, 11, 902.
- Oves, M.; Rauf, M.A.; Aslam, M.; Qari, H.A.; Sonbol, H.; Ahmad, I.; Zaman, G.S.; Saeed, M. Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci. 2022, 29, 460–471.
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805.
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124.
- Teimuri-Mofrad, R.; Hadi, R.; Tahmasebi, B.; Farhoudian, S.; Mehravar, M.; Nasiri, R. Green synthesis of gold nanoparticles using plant extract: Mini-review. Nanochem. Res. 2017, 2, 8–19.
- Santhosh, P.B.; Genova, J.; Chamati, H. Green synthesis of gold nanoparticles: An eco-friendly approach. Chemistry 2022, 4, 345–369.
- Samuel, M.S.; Ravikumar, M.; John, J.A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A review on green synthesis of nanoparticles and their diverse biomedical and environmental applications. Catalysts 2022, 12, 459.
- Ridolfo, R.; Tavakoli, S.; Junnuthula, V.; Williams, D.S.; Urtti, A.; van Hest, J.C. Exploring the impact of morphology on the properties of biodegradable nanoparticles and their diffusion in complex biological medium. Biomacromolecules 2020, 22, 126–133.
- Rajendran, N.K.; George, B.P.; Houreld, N.N.; Abrahamse, H. Synthesis of zinc oxide nanoparticles using Rubus fairholmianus root extract and their activity against pathogenic bacteria. Molecules 2021, 26, 3029.
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934.
- Jian, N.; Dowle, M.; Horniblow, R.D.; Tselepis, C.; Palmer, R.E. Morphology of the ferritin iron core by aberration corrected scanning transmission electron microscopy. Nanotechnology 2016, 27, 46LT02.
- Fowsiya, J.; Madhumitha, G.; Al-Dhabi, N.A.; Arasu, M.V. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B 2016, 162, 395–401.
- Zhang, H.; Wang, J.; Zeng, Y.; Wang, G.; Han, S.; Yang, Z.; Li, B.; Wang, X.; Gao, J.; Zheng, L. Leucine-coated cobalt ferrite nanoparticles: Synthesis, characterization and potential biomedical applications for drug delivery. Phys. Lett. A 2020, 384, 126600.
More
Information
Subjects:
Biochemical Research Methods
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.4K
Revisions:
2 times
(View History)
Update Date:
13 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





