
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilya Akberdin | -- | 1341 | 2023-07-11 09:15:35 | | | |
| 2 | Camila Xu | Meta information modification | 1341 | 2023-07-11 09:42:00 | | |
Video Upload Options
Optimizing physical training regimens to increase muscle aerobic capacity requires an understanding of the internal processes that occur during exercise that initiate subsequent adaptation. During exercise, muscle cells undergo a series of metabolic events that trigger downstream signaling pathways and induce the expression of many genes in working muscle fibers. There are a number of studies that show the dependence of changes in the activity of AMP-activated protein kinase (AMPK), one of the mediators of cellular signaling pathways, on the duration and intensity of single exercises. The activity of various AMPK isoforms can change in different directions, increasing for some isoforms and decreasing for others, depending on the intensity and duration of the load.
1. Introduction
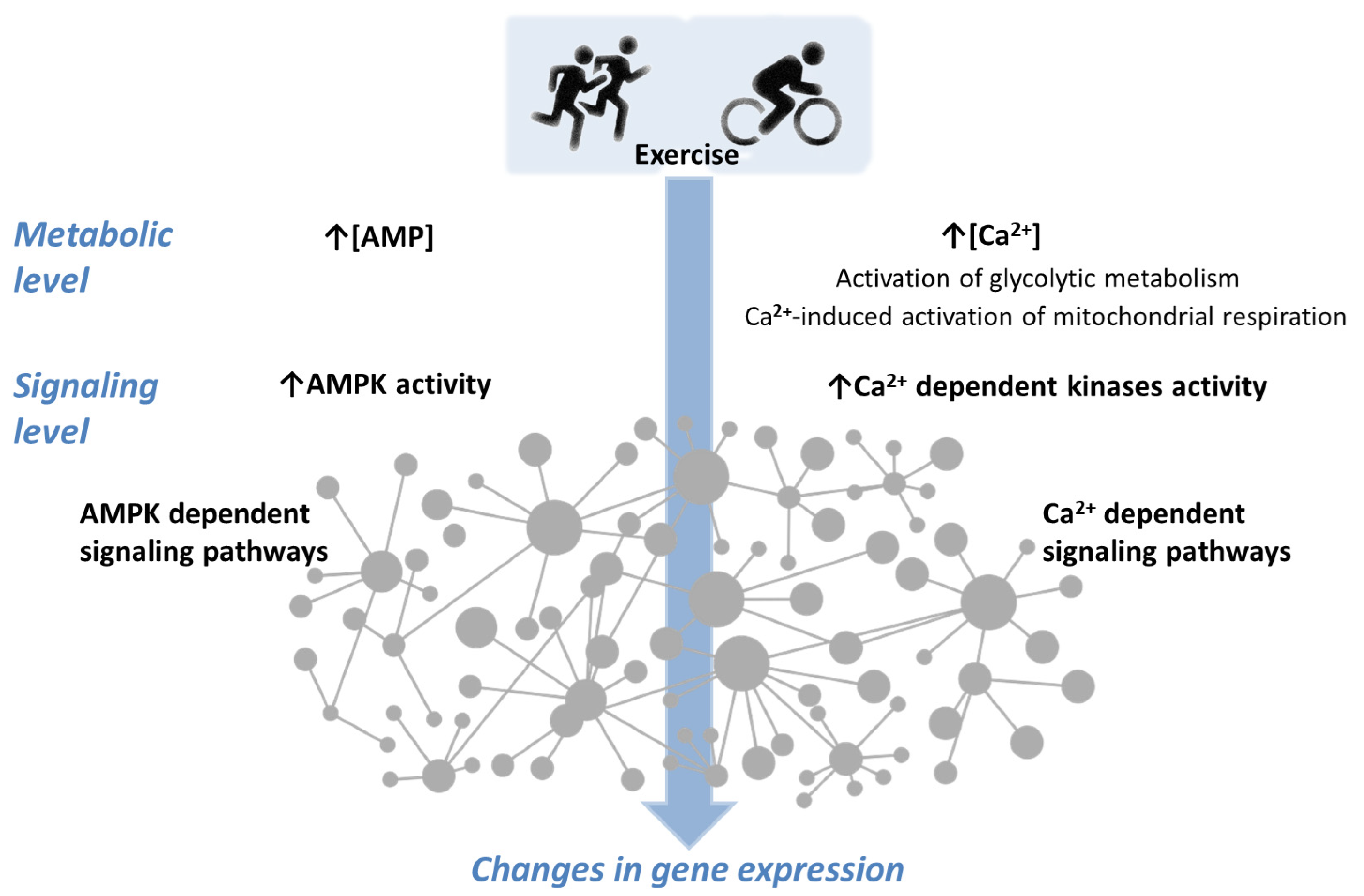
2. AMPK and Ca2+—Dependent Signaling Transients
2.1. Dynamics of AMPK and CaMKII Activity during Exercise
2.2. Possible Effects of Repeated Exercise
2.3. Simulations Based on the Current Model and Their Limitations
References
- Li, Y.; Dash, R.K.; Kim, J.; Saidel, G.M.; Cabrera, M.E.; White, A.T.; Schenk, S.; Solomon, T.P.J.; Haus, J.M.; Kirwan, J.P. Role of NADH/NAD+ transport activity and glycogen store on skeletal muscle energy metabolism during exercise: In silico studies. Am. J. Physiol.-Cell Physiol. 2009, 296, C25–C46.
- Kiselev, I.; Akberdin, I.; Vertyshev, A.; Popov, D.; Kolpakov, F. A Modular Visual Model of Energy Metabolism in Human Skeletal Muscle. Math. Biol. Bioinform. 2019, 14, 373–392.
- Akberdin, I.R.; Kiselev, I.N.; Pintus, S.S.; Sharipov, R.N.; Vertyshev, A.Y.; Vinogradova, O.L.; Popov, D.V.; Kolpakov, F.A. A Modular Mathematical Model of Exercise-Induced Changes in Metabolism, Signaling, and Gene Expression in Human Skeletal Muscle. Int. J. Mol. Sci. 2021, 22, 10353.
- Treebak, J.T.; Birk, J.B.; Rose, A.J.; Kiens, B.; Richter, E.A.; Wojtaszewski, J.F.P. AS160 phosphorylation is associated with activation of α2β2γ1- but not α2β2γ3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am. J. Physiol. Metab. 2007, 292, E715–E722.
- Birk, J.B.; Wojtaszewski, J.F.P. Kinase Activity Determination of Specific AMPK Complexes/Heterotrimers in the Skeletal Muscle. Methods Mol. Biol. 2018, 1732, 215–228.
- Wojtaszewski, J.F.P.; Nielsen, P.; Hansen, B.F.; Richter, E.A.; Kiens, B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J. Physiol. 2000, 528 Pt 1, 221–226.
- Wojtaszewski, J.F.; Mourtzakis, M.; Hillig, T.; Saltin, B.; Pilegaard, H. Dissociation of AMPK activity and ACCβ phosphorylation in human muscle during prolonged exercise. Biochem. Biophys. Res. Commun. 2002, 298, 309–316.
- Birk, J.B.; Wojtaszewski, J.F.P. Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J. Physiol. 2006, 577 Pt 3, 1021–1032.
- Chen, Z.-P.; McConell, G.K.; Michell, B.J.; Snow, R.J.; Canny, B.J.; Kemp, B.E. AMPK signaling in contracting human skeletal muscle: Acetyl-CoA carboxylase and NO synthase phosphorylation. Am. J. Physiol. Metab. 2000, 279, E1202–E1206.
- Nielsen, J.N.; Wojtaszewski, J.F.P.; Haller, R.G.; Hardie, D.G.; Kemp, B.E.; Richter, E.A.; Vissing, J. Role of 5′AMP-activated protein kinase in glycogen synthase activity and glucose utilization: Insights from patients with McArdle’s disease. J. Physiol. 2002, 541 Pt 3, 979–989.
- Nielsen, J.N.; Mustard, K.J.W.; Graham, D.A.; Yu, H.; MacDonald, C.S.; Pilegaard, H.; Goodyear, L.J.; Hardie, D.G.; Richter, E.A.; Wojtaszewski, J.F.P.; et al. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J. Appl. Physiol. 2003, 94, 631–641.
- Fujii, N.; Hayashi, T.; Hirshman, M.F.; Smith, J.T.; Habinowski, S.A.; Kaijser, L.; Mu, J.; Ljungqvist, O.; Birnbaum, M.J.; Witters, L.A.; et al. Exercise Induces Isoform-Specific Increase in 5′AMP-Activated Protein Kinase Activity in Human Skeletal Muscle. Biochem. Biophys. Res. Commun. 2000, 273, 1150–1155.
- Roepstorff, C.; Thiele, M.; Hillig, T.; Pilegaard, H.; Richter, E.A.; Wojtaszewski, J.F.P.; Kiens, B. Higher skeletal muscle α2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J. Physiol. 2006, 574 Pt 1, 125–138.
- Kristensen, D.E.; Albers, P.H.; Prats, C.; Baba, O.; Birk, J.B.; Wojtaszewski, J.F.P. Human muscle fibre type-specific regulation of AMPK and downstream targets by exercise. J. Physiol. 2015, 593, 2053–2069.
- McConell, G.K.; Wadley, G.D.; Le Plastrier, K.; Linden, K.C. Skeletal muscle AMPK is not activated during 2 h of moderate intensity exercise at ∼65% VO2 peak in endurance trained men. J. Physiol. 2020, 598, 3859–3870.
- Rose, A.J.; Kiens, B.; Richter, E.A. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J. Physiol. 2006, 574 Pt 3, 889–903.
- Bangsbo, J.; Krustrup, P.; González-Alonso, J.; Saltin, B. ATP production and efficiency of human skeletal muscle during intense exercise: Effect of previous exercise. Am. J. Physiol. Metab. 2001, 280, E956–E964.
- Burnley, M.; Doust, J.H.; Ball, D.; Jones, A.M. Effects of prior heavy exercise on VO(2) kinetics during heavy exercise are related to changes in muscle activity. J. Appl. Physiol. 2002, 93, 167–174.
- Gurd, B.J.; Scheuermann, B.W.; Paterson, D.H.; Kowalchuk, J.M.; Niemeijer, V.M.; Spee, R.F.; Schoots, T.; Wijn, P.F.F.; Kemps, H.M.C.; Williams, A.M.; et al. Prior heavy-intensity exercise speeds VO2 kinetics during moderate-intensity exercise in young adults. J. Appl. Physiol. 2005, 98, 1371–1378.
- Niemeyer, M.; Leithäuser, R.; Beneke, R. Effect of intensive prior exercise on muscle fiber activation, oxygen uptake kinetics, and oxygen uptake plateau occurrence. Eur. J. Appl. Physiol. 2020, 120, 2019–2028.
- Parolin, M.L.; Chesley, A.; Matsos, M.P.; Spriet, L.L.; Jones, N.L.; Heigenhauser, G.J.F. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am. J. Physiol. Metab. 1999, 277, E890–E900.
- Tupling, A.R.; Green, H.J.; Roy, B.D.; Grant, S.; Ouyang, J. Paradoxical effects of prior activity on human sarcoplasmic reticulum Ca2+-ATPase response to exercise. J. Appl. Physiol. 2003, 95, 138–144.
- Gurd, B.J.; Peters, S.J.; Heigenhauser, G.J.F.; LeBlanc, P.J.; Doherty, T.J.; Paterson, D.H.; Kowalchuk, J.M. Prior heavy exercise elevates pyruvate dehydrogenase activity and speeds O2 uptake kinetics during subsequent moderate-intensity exercise in healthy young adults. J. Physiol. 2006, 577 Pt 3, 985–996.
- Rico-Sanz, J. Progressive decrease of intramyocellular accumulation of H+ and Pi in human skeletal muscle during repeated isotonic exercise. Am. J. Physiol.-Cell Physiol. 2003, 284, C1490–C1496.
- Jones, A.M.; Fulford, J.; Wilkerson, D.P. Influence of prior exercise on muscle and deoxygenation kinetics during high-intensity exercise in men. Exp. Physiol. 2008, 93, 468–478.
- Layec, G.; Bringard, A.; Le Fur, Y.; Vilmen, C.; Micallef, J.-P.; Perrey, S.; Cozzone, P.J.; Bendahan, D. Effects of a prior high-intensity knee-extension exercise on muscle recruitment and energy cost: A combined local and global investigation in humans. Exp. Physiol. 2009, 94, 704–719.
- Jensen, T.E.; Wojtaszewski, J.F.P.; Richter, E.A. AMP-activated protein kinase in contraction regulation of skeletal muscle metabolism: Necessary and/or sufficient? Acta Physiol. 2009, 196, 155–174.
- Ross, F.A.; Rafferty, J.N.; Dallas, M.L.; Ogunbayo, O.; Ikematsu, N.; McClafferty, H.; Tian, L.; Widmer, H.; Rowe, I.C.M.; Wyatt, C.N.; et al. Selective Expression in Carotid Body Type I Cells of a Single Splice Variant of the Large Conductance Calcium- and Voltage-activated Potassium Channel Confers Regulation by AMP-activated Protein Kinase. J. Biol. Chem. 2011, 286, 11929–11936.
- Xiao, B.; Sanders, M.J.; Underwood, E.; Heath, R.; Mayer, F.V.; Carmena, D.; Jing, C.; Walker, P.A.; Eccleston, J.F.; Haire, L.F.; et al. Structure of mammalian AMPK and its regulation by ADP. Nature 2011, 472, 230–233.




