Two-dimensional liquid chromatography has deservedly won a dominant position in the family of (liquid chromatography) LC techniques. Even though liquid chromatography has great flexibility, regarding the different forms in which it has prevailed and the variety of samples it can analyze, it is unable to provide high resolving power in a short period of time. It is not able to deal with two types of mixtures: those consisting of thousands of analytes such as biological samples and those consisting of closely related compounds which cannot be separated by their physicochemical properties, such as enantiomers.
1. Historical Data
Two-dimensional separations are not a recent invention. The first mention of this type of chromatography was made in 1941 by Martin and Synge. They argued that two-dimensional separation of liquid samples, known as partition chromatography, depends on two factors: the rate at which the liquid mobile phase flows and the square of the diameter of the particles fixed in the separation column. After this paper, Dent and his colleagues used 2D paper for the isolation and separation of 19 amino acids from potatoes. The results of various subsequent attempts raised several questions in the scientific community, which was unable to provide a conclusive explanation of the divisions until 1967, when scientists discovered high-pressure liquid chromatography (HPLC), providing documented answers to key questions. In 1978, Erni and Frei conducted a very important experiment in which they tried to combine a gel permeation stationary phase with a reverse phase one, separating sienna glycoside
[1]. In 1990, Bushey and Jorgenson applied for the first time the functionally complete technique of two-dimensional liquid chromatography. They separated 14 components contained in a protein mixture, combining size-exclusion and ion-exchange chromatography. The applications that followed were based on homemade heart-cutting mode for most samples, except for proteomics. In proteomics, scientists attempted to apply the comprehensive technique. The first decade of 2000 was followed by papers that highlighted some weaknesses of the technique, such as undersampling. Others highlighted features that upgrade the analysis in terms of both accuracy and time. Among the properties investigated were that of maximum capacity, the application of gradient elution to the second column, and the statistical processing of data.
2. Modes of 2D-LC
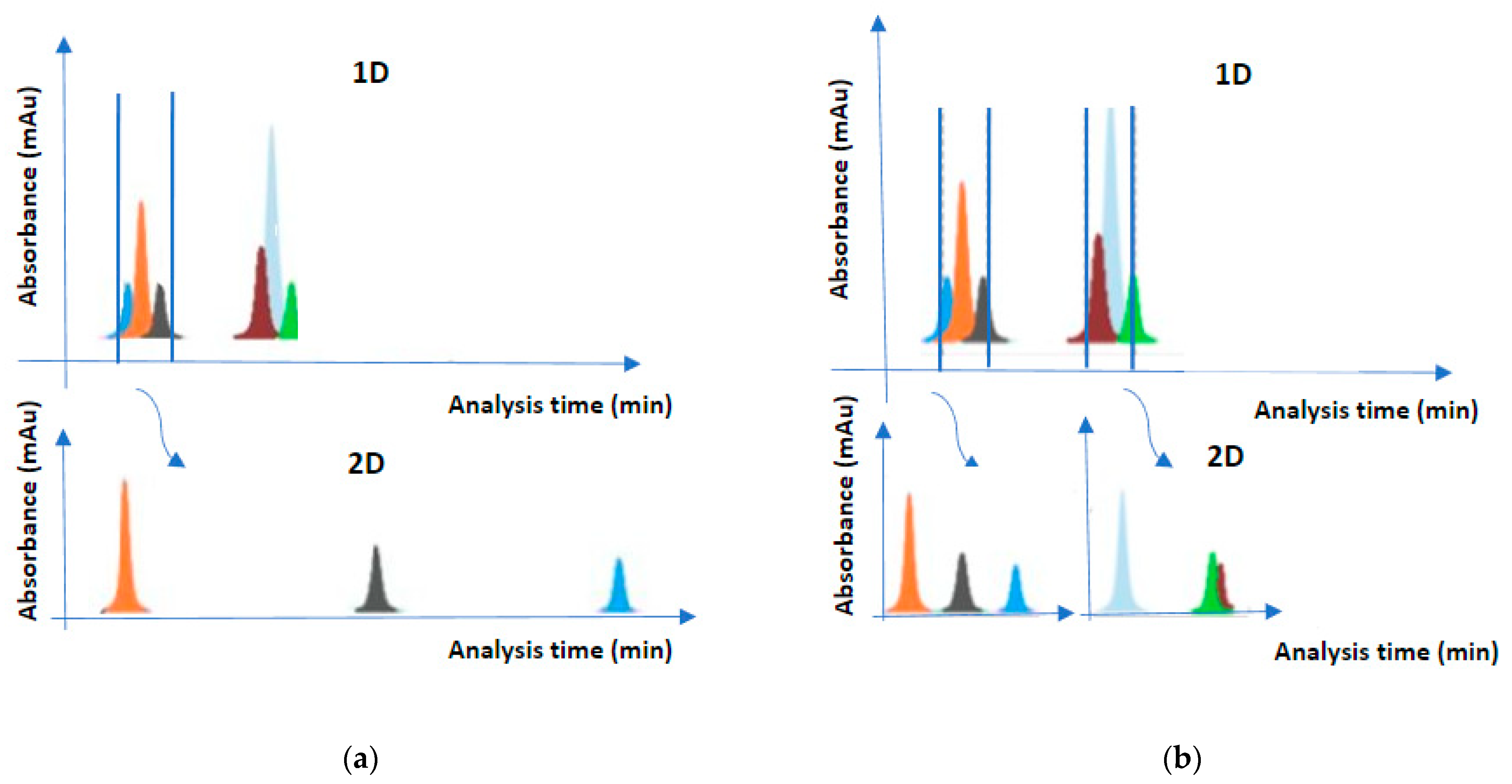
The heart-cutting mode is a simple and easy application of 2D-LC, which provides targeted analysis in a complex matrix (e.g., proteins in blood serum). The analytical process involves transferring one or even a small number of 1D fractions to the second column for further separation
[2]. By focusing on the compounds of interest, selectivity and sensitivity are enhanced, thus improving detection limits. The operating cost is relatively low as it does not require specialized instruments compared to comprehensive mode. This setup increases 2D sampling time from 1D runtime. Consequently, the separation power of the total chromatograph increases, and higher separation efficiency is achieved (
Figure 1). One disadvantage is the possible loss of important information due to selective transfer of compounds. The heart-cutting mode is very useful for identifying peaks, a very important tool for forensics
[3]. Conventionally it is denoted as LC–LC.
Figure 1. Heart-cutting mode (a) and multiple heart-cutting mode (b).
The multiple heart-cutting mode is an intermediate mode between comprehensive and heart-cutting mode which provides broader flexibility to the simple heart-cutting mode. More specifically, this mode focuses on multiple peaks of interest which are transferred sequentially to the 2D column for further separation (the total separation process lasts a few seconds or a minute). The looping systems are connected to specific valves to store and purify the 1D eluents before their reinjection. When only the 2D column is ready for a separation, the sampling loop will permit the transfer among columns
[2]. The multiple heart-cutting mode is the preferable method for the nonvolatile mobile phases with the ion source in mass spectroscopy
[4]. However multiple heart-cutting increases complexity
[5]. Conventionally, it is denoted as mLC–LC.
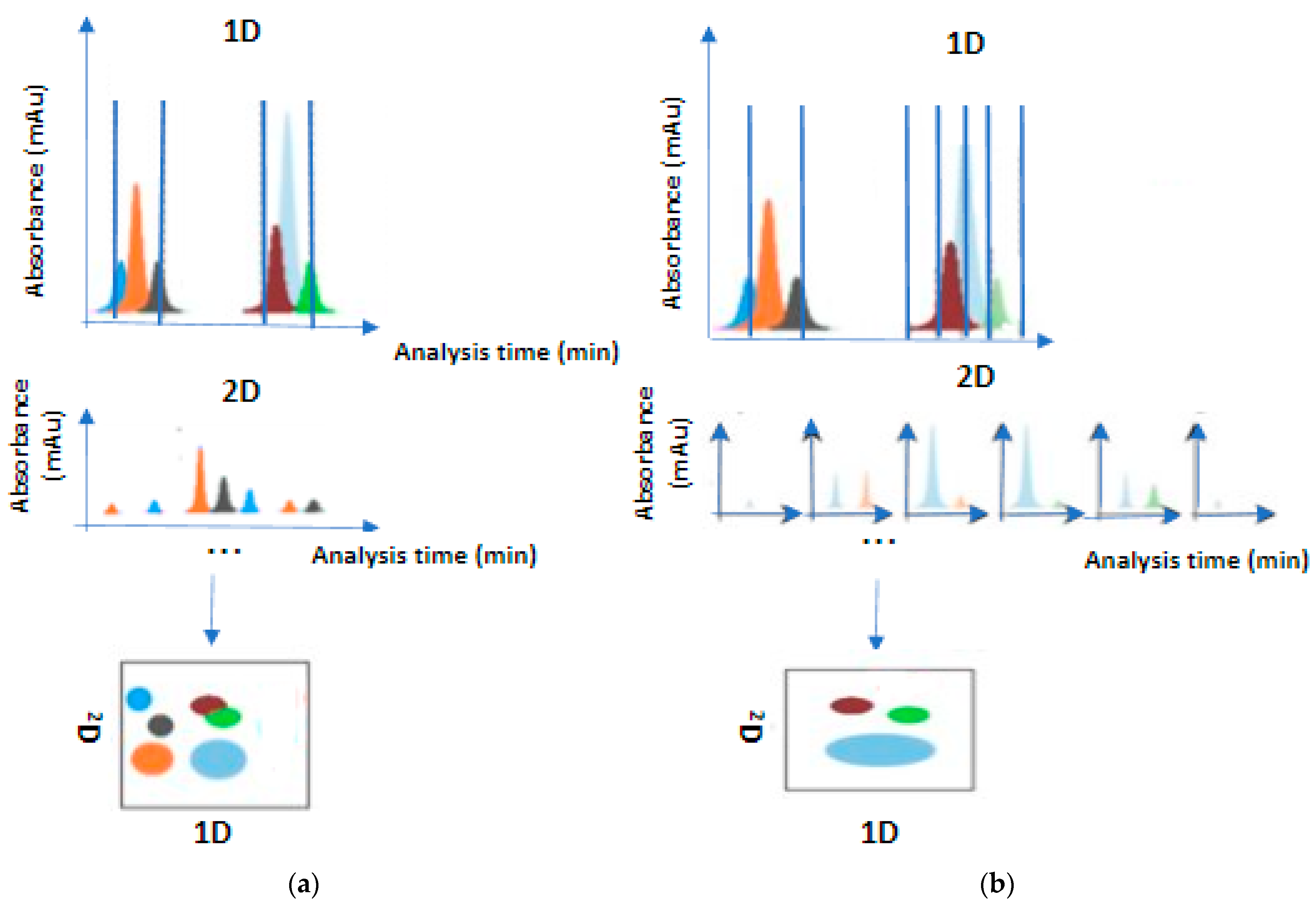
The comprehensive mode was proposed after the heart-cutting mode’s innovation
[6]. It expanded the field of applications and offered a total analysis of the sample, especially for the compounds consisting of thousands of metabolites or co-eluted 1D fractions. Every compound which is spotted in the sample is compulsorily passed through the whole analysis system
[7]. The 1D eluents are temporally stored in loops (the most common form)
[8] until the 2D column performs further separations (
Figure 2). The 2D separations must be faster than those of 1D. The primary goal of a complex fraction is to obtain as much information as possible about the sample by extracting a two-dimensional chromatogram
[2]. In simple case studies, the goal of the technique is the successful separation of all components. Sampling time is frequent to avoid remixing fractions that have been successfully separated in the first dimension. Thus, this setup requires short columns with a significant rate difference between columns, where the 2D rate must be higher. This technique is particularly widespread in the fields of omics technology
[9]. It is very useful for composite samples consisting of nonvolatile samples. It involves a more extensive separation process, resulting in a higher overall analysis time
[3]. It requires specialized operation and application of chemometric methods, increasing the operating cost
[10]. A significant advantage of the comprehensive mode is the ability to be combined with mass spectroscopy, eliminating the problems arising from conventional LC–MS. LC × LC/MS is a three-dimensional separation system which prevents matrix effects, offering quantitative analysis and identification of even unknown compounds
[11]. Conventionally, it is denoted as LC × LC.
Figure 2. Comprehensive mode (a) and selective comprehensive mode (b).
A basic prerequisite for the application of the comprehensive technique is that the separation of 2D fractions and the sampling time of the next 1D eluent should be carried out simultaneously. Such limitations complicate the technique’s implementation compared to one-off heart cutting. Time constraints become sampling limitations, since the volume of sample that can be hosted in loop is specific and limited
[2]. Under perfect conditions, each 1D fraction should be transferred to the second dimension in three or four consecutive fractions. The maximum allowable pressure in both dimensions determines the time of a full LC × LC analysis. The selective comprehensive mode is a hybrid one that excellently combines the principles of the heart and comprehensive techniques. It can perform comprehensive separations, while still focusing on specific analytes. This specific feature has a double-positive impact: it restricts the problem of undersampling peaks in the first dimension, and it shortens the analysis time without taking under consideration the empty content of 1D separation. The selective comprehensive mode is used for quantitative purposes due to the ability to provide high resolution
[12][13]. It cannot be compared with the multiple heart-cutting setup, although similar instrument hardware can be used
[2]. Conventionally, it is denoted as sLC × LC.
Scientists developed the hybrid multiple heart-cutting and selective comprehensive mode to overcome the difficulties that arise when applying traditional methods. This approach can perform the basic concepts of a conventional 2D-LC method, while offering some extra advantages. However, its application is not always possible. Table 1 summarizes the benefits and drawbacks of two-dimensional liquid chromatography.
Table 1. Benefits and drawbacks of hybrid modes of two-dimensional liquid chromatography.
3. Classification of Two-Dimensional Techniques Based on Temporal Transfer of Fractions
In offline mode, fractions from the first column are processed before being injected into the second column. The storage of fractions in vials for reinjection is a factor contributing to contamination or sample loss. It can be performed with an LC system using the same instrument if there are complementary mechanisms among the two dimensions. The first dimension operates continuously while the analysis time of the second column does not affect the total analysis time
[2][6]. This is a significant benefit over the other modes as it can be very convenient for a variety of applications. However, the offline mode is time-consuming and cannot be performed in an automated manner
[7].
In online mode, the technique can be fully automated until the data processing stage and is much faster than offline mode. The two columns are combined via a specific interface (a two-position switching valve of eight or six ports equipped with two storage loops of the same volume), responsible for the direct/continuous transfer of eluents between columns. An important advantage of online modes is full control of the sample, as well as the fact that there is no possibility of loss. Nevertheless, the implementation of online mode requires complex settings for the utilization of data, and it offers lower peak capacity than offline mode
[2][6].
The stop-and-go approach is a compromise in terms of time and power of analysis compared to the previous approaches. The analytical procedures among the two dimensions are carried out alternately. When a specific volume of mobile phase has passed through the 1D column, the flow of the 1D mobile phase is temporarily stopped, allowing the fractions to be retained in the 1D column. Then, the 2D column is ready to start the separation without using sampling loops
[2].