Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Euis Siti Nurazizah | -- | 2078 | 2023-07-11 06:27:57 | | | |
| 2 | Conner Chen | Meta information modification | 2078 | 2023-07-12 05:23:28 | | | | |
| 3 | Conner Chen | -1 word(s) | 2077 | 2023-07-17 04:20:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nurazizah, E.S.; Aprilia, A.; Risdiana, R.; Safriani, L. Synthesis and Characteristics of PEDOT:PSS and PEDOT:Carrageenan. Encyclopedia. Available online: https://encyclopedia.pub/entry/46622 (accessed on 07 February 2026).
Nurazizah ES, Aprilia A, Risdiana R, Safriani L. Synthesis and Characteristics of PEDOT:PSS and PEDOT:Carrageenan. Encyclopedia. Available at: https://encyclopedia.pub/entry/46622. Accessed February 07, 2026.
Nurazizah, Euis Siti, Annisa Aprilia, Risdiana Risdiana, Lusi Safriani. "Synthesis and Characteristics of PEDOT:PSS and PEDOT:Carrageenan" Encyclopedia, https://encyclopedia.pub/entry/46622 (accessed February 07, 2026).
Nurazizah, E.S., Aprilia, A., Risdiana, R., & Safriani, L. (2023, July 11). Synthesis and Characteristics of PEDOT:PSS and PEDOT:Carrageenan. In Encyclopedia. https://encyclopedia.pub/entry/46622
Nurazizah, Euis Siti, et al. "Synthesis and Characteristics of PEDOT:PSS and PEDOT:Carrageenan." Encyclopedia. Web. 11 July, 2023.
Copy Citation
Poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS) has been mostly used as a counter electrode to give a high performance of dye-sensitized solar cell (DSSC). PEDOT doped by carrageenan, namely PEDOT:Carrageenan, was introduced as a new material to be applied on DSSC as an electrolyte. PEDOT:Carrageenan has a similar synthesis process as PEDOT:PSS, owing to their similar ester sulphate (-SO3H) groups in both PSS and carrageenan.
counter electrode
DSSC
electrolyte
PEDOT-PSS
PEDOT-Carrageenan
1. Synthesis and Characteristics of PEDOT:PSS
In 1988, PEDOT with cation of EDOT monomer (C2H4O2C4H2S)+ was first synthesized and commercialized by scientists in a German research laboratory [1]. PEDOT has succeeded in becoming one of the electronically conducting polymers (ECPs) that have immense potential applications. However, PEDOT was difficult to be synthesized by the standard chemo/electro-polymerization due to its insolubility and infusibility in many solvents. Therefore, PEDOT needs some materials as a dopant, which is a solvent-dispersible material, while maintaining the properties of PEDOT.
PSS with a chemical formula of C8H7SO3−, is a water-dispersible polyelectrolyte and a polymer surfactant that is able to be used as a dopant for PEDOT due to its -SO3H functional group as the hydrophilic part. PEDOT and PSS formed a complex material as PEDOT:PSS, which is a poly–ion complex by electrostatic interaction between PEDOT cation and PSS anion. It consists of both positive charges conjugated PEDOT and negative charge saturated PSS. The chemical structure of PEDOT, PSS and PEDOT:PSS is shown in Figure 1, and the structure identification was completed with the FTIR analysis in Table 1. In PEDOT:PSS, PSS has two jobs: (i) it works as a counter ion for doped-PEDOT stability, and (ii) it supplies a matrix to form an aqueous dispersion [2].
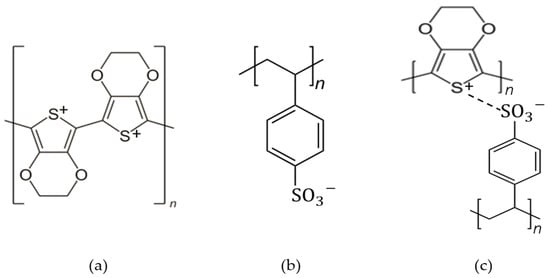
Figure 1. The chemical structure of (a) PEDOT, (b) PSS and (c) PEDOT:PSS.
Table 1. FTIR analysis of PEDOT:PSS [3].
| Wavenumber (cm−1) | Types of Atom Bonding | Identifications |
|---|---|---|
| 691 | C–S | Stretching of the thiophene ring in PEDOT |
| 840 | ||
| 936 | ||
| 983 | S–O | Stretching SO42− from oxide and the S-phenyl bond in PSS |
| 1145 | Stretching in PSS | |
| 1055 | S=O | Stretching antisymmetric SO42− from oxidant |
| 1198 | Stretching symmetric in PSS | |
| 1092 | C–O | Stretching in PEDOT |
| 1144 | ||
| 1340 | C–C | Stretching in the thiophene rings of PEDOT |
| 1518 | C=C | Stretching in the thiophene rings of PEDOT |
| 1640 | Stretching in the aromatic rings in PSS | |
| 2921 | C–H | Stretching of PEDOT and PSS |
| 3415 | O–H | Stretching in PSS |
PEDOT:PSS was also first synthesized by scientists in German research laboratories. PEDOT:PSS is the most successful commercially available in the form of an aqueous dispersion with high water-dispersibility, excellent miscibility, good transparency, high electrical conductivity, excellent flexibility and satisfactory stretch-ability. PEDOT:PSS is typically synthesized via oxidative polymerization in two simple ways: in-situ and post-polymerization [4][5]. The synthesis process is shown in Figure 2. In-situ polymerization has several steps to do as follows: First, monomer powder EDOT was added to an aqueous of PSS solution. Then, the mixtures were stirred vigorously in a water bath at room temperature under nitrogen. The oxidant agents (e.g., sodium persulfate (N2S2O8) and iron trichloride (FeCl3) or iron tri-sulfate (Fe2(SO4)3)) were immediately added to the mixture solution to produce a complex and stirred again at room temperature for 24 h. The precipitate was collected from the complex solution by centrifugation at a certain rpm and then rinsed with acetone and methanol at a certain ratio. The final result can be dispersed in water to be aqueous in a dark-blue solution or be dried in an oven at 60 °C for 24 h to get a black-powder. Post-polymerization includes the steps as follows: first, monomer powder EDOT was dispersed in water. The oxidant agents (e.g., N2S2O8 and FeCl3 or Fe2(SO4)3) were immediately added to EDOT solution and stirred at room temperature for 24 h. Then, the mixture was purified by mixing water and ethanol at a certain ratio. The PEDOT powder as a result was added to the PSS solution. The PEDOT:PSS solution was stirred at room temperature for 24 h. The final result was the PEDOT:PSS aqueous in a dark-blue solution.
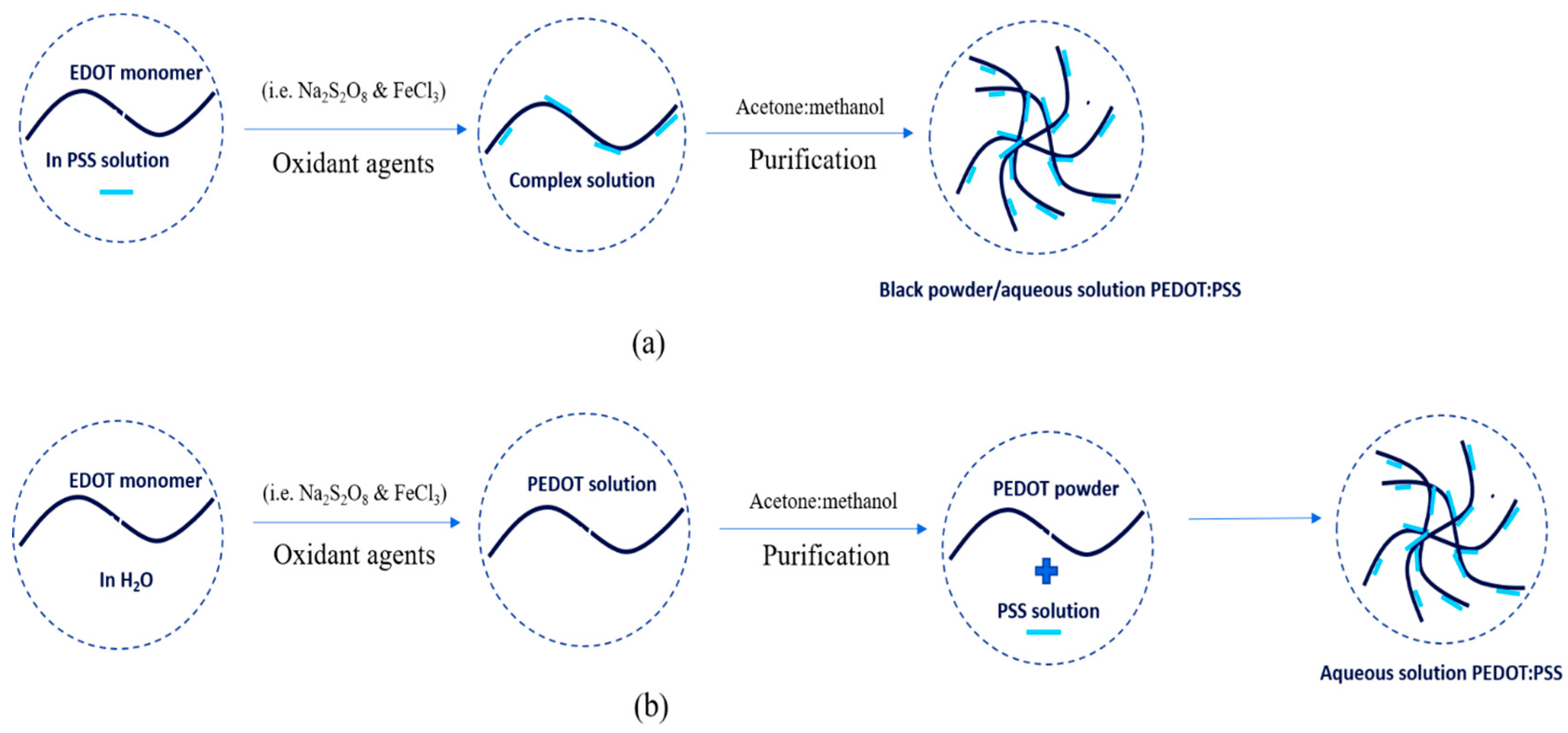
Figure 2. The synthesis process of PEDOT:PSS with (a) in-situ and (b) post-polymerization.
The properties of PEDOT:PSS are crucial things to consider in the synthesis process, especially for electrical conductivity. There are some factors that affect the electrical conductivity of PEDOT:PSS as shown in Figure 3. In the synthesis process, some parameters should be controlled, such as pH solution, temperature, humidity and polar solvent as additives or blending components. PSS has hygroscopic and corrosive properties due to its strong acid (pH < 2), which lowers the lifetime and performance of the application devices. The neutralizing of PSS can be attained by various alkaline, but this could change the structural and electrical conductivity resulting in PEDOT:PSS [6].
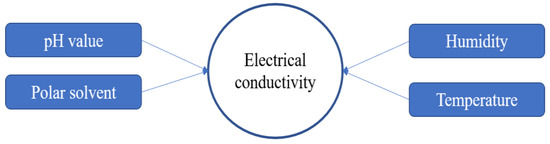
Figure 3. Factors affecting electrical conductivity.
Several studies showed that the higher the pH of the solution resulting from the synthesis process, for example, due to adding alkaline materials (e.g., sodium hydroxide (NaOH)), the more the electrical conductivity decreased and vice versa. If the resulting solution was in a lower pH, by adding acid materials (e.g., hydrochloride acid (HCl)) during the synthesis process, the electrical conductivity increased [1][6]. Mochizuki et al. have investigated the effect of adding NaOH concentration from 0.8 to 1.2 M with increasing pH of 2.5 to 11.7 to cause decreasing electrical conductivity of PEDOT:PSS from 10−2 to 10−4 S·cm−1 [6]. This is due to the removal of the insulting PSS from the surface of the colloidal particles and to the crystallization of the PEDOT molecule, which improves both intraparticle and interparticle transfer to charge carrier. The high electrical conductivity affected the higher carrier mobility and the structure of PEDOT:PSS. The lower pH and also adding polar solvents partially change PEDOT molecules with an amorphous form to a crystalline state by, i.e., ethylene glycol (EG), dimethyl sulfoxide (DMSO), dimethyl formamide (DMF), etc. that were added during the synthesis process.
Besides pH and polar solvent, the humidity and temperature affected the electrical conductivity of PEDOT:PSS in thin layer form. The PEDOT:PSS can be applied in many application devices if it was made in thin layer form. Several methods, such as spin-coating, screen-printing, electrospinning, etc., are used as a technique for thin layer deposition. The electrical conductivity of PEDOT:PSS layer was changed in water. At first, the electrical conductivity initially increased significantly, but then it gradually decreased when its layer was slowly damaged, depending on the longer immersed time of PEDOT:PSS layer in water. Otherwise, when the temperature went down, the electrical conductivity of PEDOT:PSS layer started to decrease [1][2].
2. Synthesis and Characteristics of PEDOT:Carrageenan
Carrageenan comes from various forms of red algae (Rhodophyta) and is used as a general name for a poly-saccharides (sulphated galactans) family. Chemically, carrageenan is a linear polymer, which is arranged of alternating disaccharide repeating units of 3-linked β-D-galactopyranose (G-units) and 4-linked α-D-galactopyranose (D-Units) or 4-linked 3,6-anhydrogalactose (DA-Units) [7][8]. In 1862, the British pharmacist Stanford found the first called phycocolloid carrageenin by extracting it from Irish moss (Chandrus crispus) [8]. The name was later changed to carrageenan so as to fulfill with the “-an” suffix the names of polysaccharides that were accepted in the industry dates from the 1940s [9].
Carrageenan has three commercially important types according to the position and number of sulphate groups: kappa (κ)-, iota (ι)- and lambda (λ)-carrageenan. The κ-carrageenan with the chemical formula of C12H17O9SO3− is produced from the seaweed Kappaphycus alvarezii, known as Euchema cottonii (or simply cottonii). The ι-carrageenan with the chemical formula of C12H16O9(SO3−)2 is largely produced from Euchema denticulum, known as Euchemaspinosum (or simply spinosum species). The λ-carrageenan with the chemical formula of C12H17O10(SO3−)3 is extracted from the species of Gigantana and Chondrus ginera. There are also several other carrageenan repeating units, e.g., mu (µ)-, nu (υ)-, xi(ξ)-, theta (θ)- and beta (β)-carrageenans [9][10][11]. Figure 4 shows all types of carrageenan. Based on IUPAC and the letter codes of carrageenans, which are shown in Table 2, their corresponding names of κ-, ι- and λ-carrageenan are carrageenase 2,40-disulphate (G4S-DA2S), carrageenase 40-sulphate (G4S-DA) and carrageenan 2,6,20-trisulphate (G2S-D2S,6S), respectively [9][10].
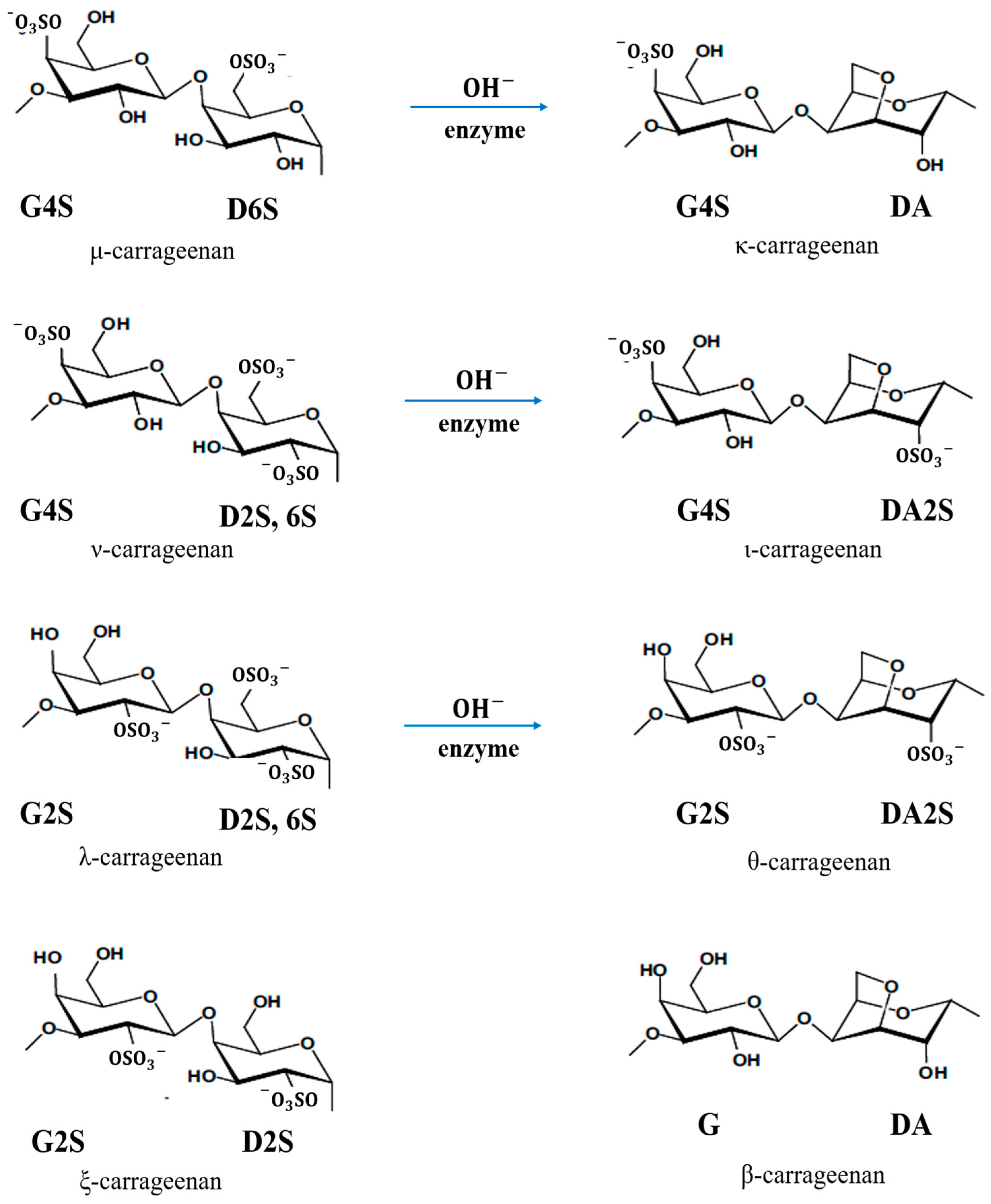
Figure 4. Chemical structure of all types of carrageenan.
| Letter Code | Carrageenan | IUPAC Name |
|---|---|---|
| D | Not Found | 4-Linked α-D-galactopyranose |
| D2S | ξ | 4-Linked α-D-galactopyranose 2-sulphate |
| D2S, 6S | λ, υ | 4-Linked α-D-galactopyranose 2,6-disulphate |
| D6S | μ | 4-Linked α-D-galactopyranose 6-sulphate |
| DA | κ, β | 4-Linked 3,6-anhydro-α-D-galactopyranose |
| DA2S | ι, θ | 4-Linked 3,6-anhydro-α-D-galactopyranose 2-sulphate |
| G | β | 3-Linked β-D-galactopyranose |
| G2S | λ, θ | 3-Linked β-D-galactopyranose 2-sulphate |
| G4S | κ, ι, μ, υ | 3-Linked β-D-galactopyranose 4-sulphate |
| S | κ, ι, λ, μ, υ, θ, ξ | Sulphate ester (O-SO3−) |
Carrageenan is one of the natural polymers, with ester sulphate (-SO3H) groups, that has the capability to produce thermo-reversible gels or high viscous solution. In a number of foods and pharmaceutical and cosmetic products, it is commonly used as gellifier, stabilizer and emulsifying agent [12]. Carrageenan has the similarity of ester sulphate (-SO3H) groups to PSS. PSS has been used as a dopant in complex material PEDOT:PSS; then, carrageenan also has the potential to be used as dopant to form a complex material PEDOT:Carrageenan. Figure 5 shows the complete chemical structure of PEDOT:Carrageenan, and the structure identification was completed with FTIR analysis in Table 3.
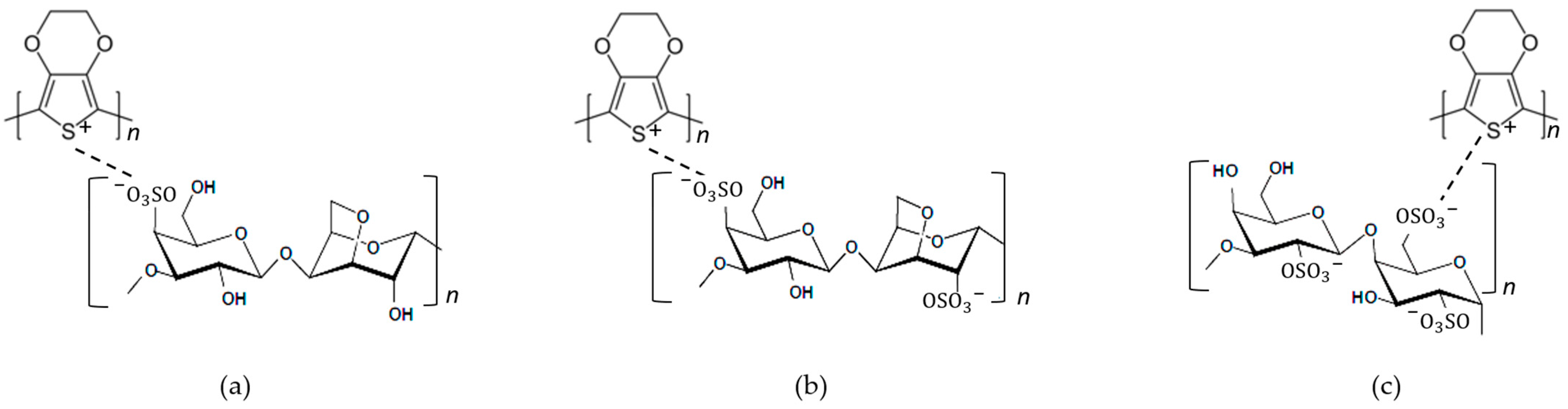
Figure 5. The chemical structure of (a) PEDOT:κ-Carrageenan, (b) PEDOT:ι-Carrageenan and (c) PEDOT:λ-Carrageenan.
| Wavenumber (cm−1) | Bond(s)/Group(s) | Letter Code | Type of Carrageenan |
|---|---|---|---|
| 1495 | C = C of thiophene ring | - | - |
| 1371 | C–C of thiophene ring | - | - |
| 1198, 1060 | C–O–C of stretching mode of the ethylene groups | - | - |
| 892 | C–H of PEDOT chains | - | - |
| 1210–1260 | S = O of sulphate ester | S | κ, ι, λ, μ, υ, θ, ξ |
| 970–975 | Galactose | G/D | κ, ι, μ, υ, θ, β |
| 928–933, 1070 (shoulder) |
C–O of 3,6-anhydro-D-galactose |
DA | κ, ι, θ, β |
| 890–900 | Unsulphated β-D-galactose | G/D | β |
| 840–850 | C–O–SO3 of D-galactose-4-sulphate |
G4S | κ, ι, μ, υ |
| 825–830 | C–O–SO3 of D-galactose-4-sulphate |
G/D2S | λ, υ, θ, ξ |
| 820, 825 (shoulder) |
C–O–SO3 of D-galactose-2,6-sulphate |
D2S,6S | λ, υ |
| 810–820, 867 (shoulder) |
C–O–SO3 of D-galactose-6-sulphate |
G/D6S | μ |
| 800–805, 905 (shoulder) |
C–O–SO3 of 3,6-anhydro-D-galactose-2-sulphate |
DA2S | ι, θ |
PEDOT:Carrageenan was synthesized via oxidative polymerization [13][14] as synthesis of PEDOT:PSS. The oxidative polymerization was done in two simple ways: in-situ and post-polymerization. The procedures of both two ways as similar as PEDOT:PSS, but the carrageenan powder was first diluted in water under stirring at 70 °C. Then, monomer EDOT, oxidant agents and surfactant were mixed together until the PEDOT:Carrageenan solution was obtained in homogenous solution. The process synthesis of PEDOT:Carrageenan is shown in Figure 6.
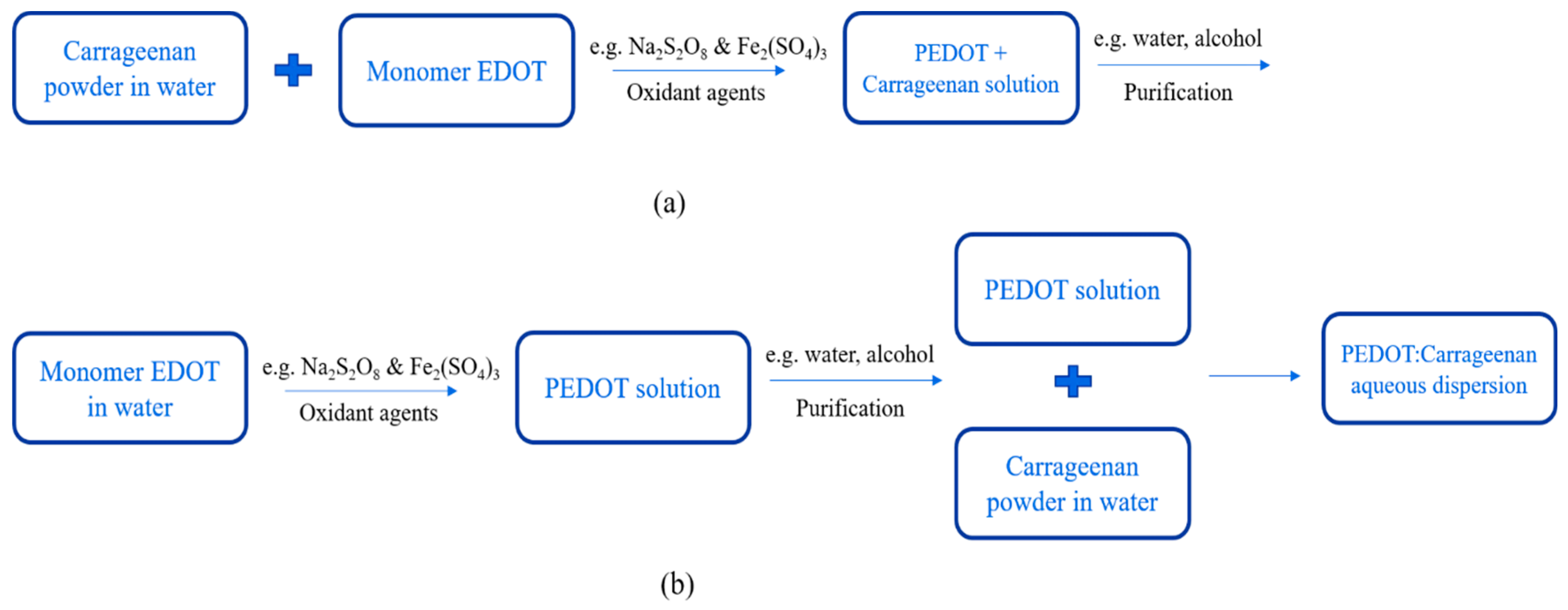
Figure 6. Synthesis process of PEDOT:Carrageenan with (a) in-situ and (b) post-polymerization.
In summary, PEDOT:PSS and PEDOT:Carrageenan has similar synthesis process because both PSS and Carrageenan has ester sulphate (-SO3H) functional group that cause them as a dopant for PEDOT. However, PSS and Carrageenan have different polymer structure that affects the molecular weight of both PEDOT:PSS and PEDOT:Carrageenan. By analyzing the chemical structure of their monomer as shown in Figure 1 and Figure 5, the molecular weight of both PEDOT:PSS and PEDOT:Carrageenan could be calculated by adding up the molecular weight of their monomer. It depends on their chain numbers (n). PEDOT:PSS has EDOT monomer and styrene sulfonate (SS) monomer with a molecular weight of 142 and 183 g/mol, respectively. Three types of PEDOT:Carrageenan, namely PEDOT:κ-Carrageenan, PEDOT:ι-Carrageenan and PEDOT:λ-Carrageenan, consist of EDOT monomer and carrageenan monomer (κ-, ι- and λ-carrageenan). Molecular weight of κ-, ι- and λ-carrageenan monomer is 385, 464 and 561 g/mol, respectively.
Table 4 shows the difference in the electrical and optical properties of PEDOT:PSS and PEDOT:Carrageenan. PEDOT:PSS has excellent conductivity of >4000 S·cm−1 with a low sheet resistance of <100 Ω·sq−1 and high transparency of 80–95% [1]. With high conductivity, PEDOT:PSS has ionic and electronic mobility of 2.2 × 10−3 and 1.3 cm2v−1s−1, respectively [15], with carrier density of 4 × 1020 cm−3 at approximately +0.5 Volt [16]. However, PEDOT:Carrageenan has conductivity of 16.23 S·cm−1, and it was measured for PEDOT:κ-Carrageenan [17]. The value of sheet resistance, transparency, ionic and electron mobility and carrier density of PEDOT:Carrageenan has not been available since the research of PEDOT:Carrageenan is still very limited, especially in analyzing the electrical and optical properties that are the basis for the application of a material in electronic devices.
Table 4. Electrical and optical properties of PEDOT:PSS and PEDOT:Carrageenan.
References
- Wen, Y.; Xu, J. Scientific Importance of Water-Processable PEDOT–PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1121–1150.
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2018, 31, 1806133.
- Sakunpongpitiporn, P.; Phasuksom, K.; Paradee, N.; Sirivat, A. Facile synthesis of highly conductive PEDOT:PSS via surfactant templates. RSC Adv. 2019, 9, 6363.
- Louwet, F.; Grooenendal, L.; Dhaen, J.; Manca, J.; Luppen, J.V.; Verdonck, E.; Leenders, L. PEDOT/PSS: Synthesis, characterization, properties and application. Synth. Met. 2003, 135–136, 115–117.
- Panigrahy, S.; Kandasubramanian, B. Polymeric thermoelectric PEDOT: PSS & composites: Synthesis, progress, and applications. Eur. Polym. J. 2020, 132, 109726.
- Mochizuki, Y.; Horii, T.; Okuzaki, H. Effect of pH on Structure and Conductivity of PEDOT/PSS. Trans. Mater. Res. Soc. Jpn. 2012, 37, 307–310.
- van de Velde, F.; Antipova, A.S.; Rollema, H.S.; Burova, T.V.; Grinberg, N.V.; Pereira, L.; Gilsenan, P.M.; Tromp, R.H.; Rudolph, B.; Grinberg, V.Y. The structure of κ/i-hybrid carrageenans II. Coil–helix transition as a function of chain composition. Carbohydr. Res. 2005, 340, 1113–1129.
- Thrimawithana, T.R.; Young, S.; Dunstan, D.E.; Alany, R.G. Texture and rheological characterization of kappa and iota carrageenan in the presence of counter ions. Carbohydr. Polym. 2010, 82, 69–77.
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Rebeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909.
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180.
- Volery, P.; Besson, R.; Schaffer-Lequart, C. Characterization of Commercial Carrageenans by Fourier Transform Infrared Spectroscopy Using Single-Reflection Attenuated Total Reflection. J. Agric. Food Chem. 2004, 52, 7457–7463.
- Webber, V.; de Carvalho, S.M.; Ogliari, P.J.; Hayashi, L.; Barreto, P.L.M. Optimization of the extraction of carrageenan from Kappaphycus alvarezii using response surface methodology. Ciênc. Tecnol. Aliment. Camp. 2012, 32, 812–828.
- Diah, A.W.M.; Wirayudha, A.; Kandolia, T.V.; Pujianti, N.K.T.; Widyakirana, I.; Hasriani, N.A.S.; Saehana, S.; Holdsworth, C.I. The Effect of Synthetic Conditions on the Characteristics of Carrageenan-Doped Poly(3,4-ethylenedioxythiophene). Macromol. Symp. 2020, 391, 1900162.
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520.
- Rivnay, J.; Inal, S.; Collins, B.A.; Sessolo, M.; Stavrinidou, E.; Strakosas, X.; Tassone, C.; Delongchamp, D.M.; Malliaras, G.G. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 2016, 7, 11287.
- Imae, I.; Yamane, H.; Imato, K.; Ooyama, Y. Thermoelectric properties of PEDOT:PSS/SWCNT composite films with controlled carrier density. Compos. Commun. 2021, 27, 100897.
- Ng, C.A.; Camacho, D.H. Polymer electrolyte system based on carrageenan-poly(3,4-ethylenedioxythiophene) (PEDOT) composite for dye sensitized solar cell. IOP Conf. Ser. Mater. Sci. Eng. 2015, 79, 012020.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.4K
Revisions:
3 times
(View History)
Update Date:
17 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




