Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicolas King Goff | -- | 1659 | 2023-07-07 17:31:07 | | | |
| 2 | Jason Zhu | + 11 word(s) | 1670 | 2023-07-10 03:51:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Muzyka, L.; Goff, N.K.; Choudhary, N.; Koltz, M.T. Molecular Targeted Therapies for Adult-Type Diffuse Glioma. Encyclopedia. Available online: https://encyclopedia.pub/entry/46569 (accessed on 07 February 2026).
Muzyka L, Goff NK, Choudhary N, Koltz MT. Molecular Targeted Therapies for Adult-Type Diffuse Glioma. Encyclopedia. Available at: https://encyclopedia.pub/entry/46569. Accessed February 07, 2026.
Muzyka, Logan, Nicolas K. Goff, Nikita Choudhary, Michael T. Koltz. "Molecular Targeted Therapies for Adult-Type Diffuse Glioma" Encyclopedia, https://encyclopedia.pub/entry/46569 (accessed February 07, 2026).
Muzyka, L., Goff, N.K., Choudhary, N., & Koltz, M.T. (2023, July 07). Molecular Targeted Therapies for Adult-Type Diffuse Glioma. In Encyclopedia. https://encyclopedia.pub/entry/46569
Muzyka, Logan, et al. "Molecular Targeted Therapies for Adult-Type Diffuse Glioma." Encyclopedia. Web. 07 July, 2023.
Copy Citation
Gliomas are the most common brain tumor in adults, and molecularly targeted therapies to treat gliomas are becoming a frequent topic of investigation. The current state of molecular targeted therapy research for adult-type diffuse gliomas has yet to be characterized, particularly following the 2021 WHO guideline changes for classifying gliomas using molecular subtypes.
glioma
glioblastoma
molecular targeted therapy
WHO brain tumor guideline
1. Introduction
As the most common brain tumor in adults, gliomas have sustained the focus of scientific research for the past several decades. Recently, more attention has been drawn to the diagnostic criteria of gliomas with the restructured 2021 WHO Classification of Tumors of the Central Nervous System, specifically focusing more on molecular biomarkers as a means of categorization [1]. Within this classification adult-type diffuse gliomas are the most prevalent tumor types, defined on the basis of molecular expression of isocitrate dehydrogenase (IDH) and the 1p/19q codeletion. These glioma subtypes include astrocytoma (IDH-mutant astrocytoma), oligodendroglioma (IDH-mutant and 1p19q-codeleted), and glioblastoma (GBM) (IDH-wildtype) [1]. The typical management of adult-type diffuse glioma begins with a resection or biopsy, followed by possible radiotherapy and/or chemotherapy with the alkylating agent, temozolomide, or the combination procarbazine, lomustine, and vincristine (PCV) [2]. Even with this regimen, recurrence is prevalent, and the prognosis is dismal, particularly in GBM, which has an average survival of 14–16 months [3].
As gliomas are becoming more molecularly defined, so too is their treatment progressing more towards the targeting of molecular pathways [4]. Compared with traditional chemotherapeutic drugs, molecularly targeted antitumor therapy has the advantage of strong specificity with minimal damage to normal tissues. Molecular-targeted glioma therapies have gained traction in the scientific literature, with many analyses centered on identifying mechanisms pertinent to glioma growth [5]. The Raf/MEK/Erk pathway has been of particular interest as a targetable pathway due to its preponderance among gliomas [5]. Additionally, a systematic review by Da Silva et al. highlighted the molecular targeted therapies in clinical trials for GBM, identifying four categories of targets: targeting the potential for unlimited replication, growth autonomy and migration, cell cycle and apoptosis, and angiogenesis [6]. The figure below is a visual summary of some of the most common pathways where targeted therapies act.(Figure 1)
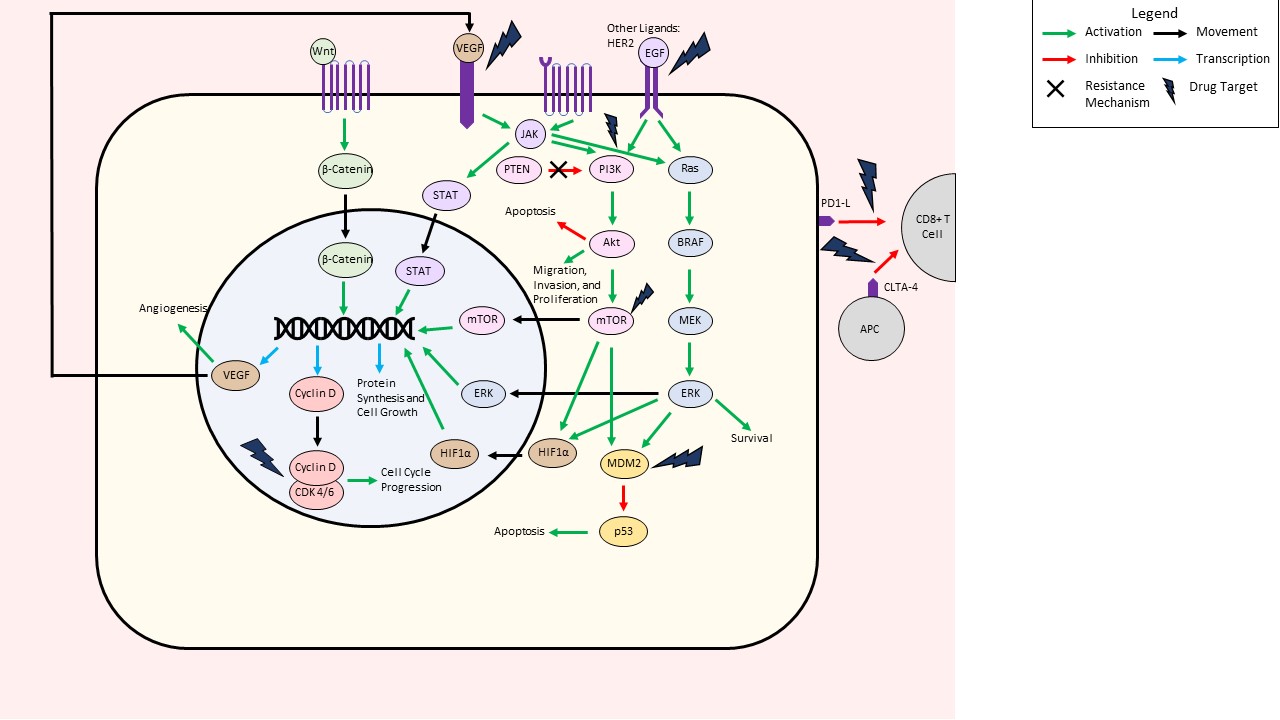
Figure 1. Summary of molecularly targeted pathways in adult-type diffuse glioma.
2. Adult-Type Diffuse Glioma Subtypes
Though the overwhelming majority of studies centered on GBM, the literature shows that adult gliomas found more frequently in practice tend to harbor IDH mutations [7][8]. The reason for the overrepresentation of GBM-focused studies and the underrepresentation of IDH-mutant astrocytoma or oligodendroglioma is multifactorial. First off, the updated WHO classification is a recent development as of 2021; because the majority of the works occurred prior to the molecular subtype differentiation, there were likely studies that self-identified as GBM studies that may have included tumors with an IDH mutation or 1p19q co-deletion. Additionally, it is likely that GBM has received more research funding and scientific attention than other brain tumors, perhaps due to its more aggressive nature and mortality rates. Therefore, the funding for studies investigating IDH-mutant astrocytoma or oligodendroglioma may be less robust. Of note, the ongoing clinical trials for glioma vastly favor GBM as well, receiving the majority of funding from industry sources. Further studies to quantify the distribution of research funding between glioma subsets would be necessary to confirm this association. Lastly, the standard cell lines for all glioma research tend to be glioblastoma models, particularly U87, U373, and U251 [9].
3. Protein Kinase Pathways
In terms of molecular targets, protein kinase pathways—especially PI3K/Akt/mTOR and Ras/BRAF/Mek/Erk—were the most prevalent in the clinical and laboratory studies analyzing existing therapies and novel targets to treat adult-type diffuse glioma. These results are consistent with previous studies that have demonstrated a predominance in the PI3K/Akt/mTOR and Ras/BRAF/Mek/Erk protein kinase pathways in molecularly targeted glioma treatment [5][6]. The importance of these pathways in glioma has been well-described in the literature; ultimately, these tumors harbor mutations that continuously activate these protein kinase signaling pathways, leading to increased tumorigenesis and progression [10][11][12].
Both the PI3K/Akt/mTOR and Ras/BRAF/Mek/Erk protein kinase pathways are also downstream of receptors such as EGFR, one of the most significant signaling pathways clinically implicated in glioma [13]. A systematic review of molecular-targeted therapy clinical trials for GBM identified EGFR as the most prevalent molecular target [6]. Nonetheless, studies have demonstrated limited clinical benefit of anti-EGFR therapies, theorized to be secondary to PTEN-mediated resistance of GBM to this therapy type [14].
Similar to the published clinical studies on this topic, protein kinase pathways were by far the most predominant molecular targets tested in ongoing clinical trials. Interestingly, these therapeutics were also investigated much earlier on average. This finding is likely due to the fact that protein kinase inhibitors are some of the earliest molecular target therapies in the field of targeted oncologic interventions, thus being able to start clinical trials for the treatment of glioma as early as 2001 [15]. Perhaps, in the coming years, as the analysis of existing molecularly targeted therapies progresses from earlier stage clinical testing or laboratory testing, there will be a shift favoring more of the scientifically novel approaches—such as immunotherapeutics, cell cycle inhibitors, or more specifically localized targeting—in clinical trials.
Additional protein kinase pathways targeted in laboratory studies included HER2 receptors, epithelial membrane protein-2 (EMP2), and STAT3, to name a few [16][17][18]. HER2 expression tends to be low in GBM, and though one clinical trial examining a HER2 inhibitor has yet to show therapeutic gain, laboratory studies have promising evidence for efficacy [18][19]. EMP2 has been implicated in bevacizumab resistance and thus shows promise as a molecular target for preventing resistance in conjunction with this common therapeutic [17][20]. STAT3 plays a role in astrocyte development and has tumor-suppressive roles in glial malignancies; this target shows promise in laboratory research using tetrandrine as an inhibitor [16]. Despite varying clinical evidence of efficacy, protein kinase-targeted therapies remain a prevalent area of study for both individual inhibitors and combined therapies.
4. Cell Cycle/Apoptosis Pathways
Interestingly, a prevalent molecular target in laboratory studies—both testing existing therapies and identifying novel targets—were cell cycle/apoptosis pathways. This difference may be attributed to the fact that clinical studies tend to focus on targets with existing FDA-approved therapies or targets that are more well-established in the literature, while studies with the goal of establishing new targets or testing newly developed therapies can explore a wider range of targets with less established evidence.
The use of cell cycle or apoptosis pathways as targets stems from the use of these pathways in the treatment of other tumors, in particular. In the present study, only four clinical studies included cell cycle/apoptosis pathway inhibitors, namely the cyclin-dependent kinase (CDK) 4/6 inhibitor palbociclib, the mouse double minute 2 (MDM2) inhibitor idasanutlin, the ribonucleotide reductase inhibitor Motexafin Gadolinium, and the 26S proteasome inhibitor bortezomib [21][22][23][24]. CDK and MDM2 inhibitors were also prevalent in laboratory studies testing existing therapies [25][26][27][28][29][30]. Two CDKs were identified as novel molecular targets—namely CDK 5 and 10—and other novel targets include other apoptosis regulators such as E2F1, trichothiodystrophy group A protein (TTDA), and protease-activated receptor 2 (PAR2) [31][32][33][34][35].
5. Microenvironmental Pathways (Angiogenesis, Cell-Cell Adhesion, Ιron/Cation Regulation)
Anti-angiogenic therapies aim to compensate for the robust vascularity of gliomas, particularly GBM [36]. Specifically, vascular endothelial growth factor (VEGF) is overexpressed in GBM, providing the rationale for the thirteen published clinical trials investigating VEGF inhibitors. Specific inhibitors studied include cediranib, cabozantinib, apatinib, and bevacizumab, which appear to be well-tolerated by patients and, in many cases, portend progression-free survival [37][38][39][40][41][42][43][44][45][46][47][48]. Many laboratory studies also tested VEGF inhibitors—namely bevacizumab, axitinib, and apatinib—and all found promising results in vivo [49][50][51]. Other microenvironmental targets included mitochondrial transcription factor A (TFAM), transient receptor potential cation channel subfamily V member 4 (TRPV4), and HIF2α. These targets were acted on by melatonin, cannabidiol, and PT2385, respectively, all of which demonstrated antitumor effects [52][53][54]. Promising novel microenvironmental targets include miR-497, TWIST transcription factor, and tenascin-W, among others [55][56][57].
6. Immunotherapy Pathways
The immune checkpoint blockade adopted in the glioma therapeutics model follows treatment paradigms for melanoma, lung cancer, colon cancer, and hepatocellular carcinoma; the therapies used to treat these tumors tend to block programmed cell death protein 1 (PD1), a protein known for attenuating the host immune response to tumor cells, or cytotoxic T lymphocyte antigen-4 (CTLA-4), a molecule that inhibits T-cell activation [58][59][60][61]. The clinical studies identified in the present study investigating immunotherapeutic pathways targeted PD1 using the inhibitor nivolumab [62][63]. Other immunotherapy targets that were found to be effective in vivo included the inhibition of CD73 with antibodies, extracellular matrix metalloproteinase (EMMPRIN) with icaritin, and NFκB with BAY117082 [64][65][66]. Novel immunotherapeutics for GBM and oligodendroglioma include cluster of differentiation 204 (CD204), S100A, and the CE7 epitope of the L1-CAM adhesion molecule [67][68][69].
7. Wnt/β-Catenin Pathway
The wnt/β-catenin pathway was much more prevalent in earlier stages of laboratory research identifying new targets, likely because the role of wnt/β-catenin in glioma progression is a more recent scientific advancement [13][70][71][72][73][74][75][76]. It is likely that in the upcoming years, the distribution of molecular targets may shift from protein kinase pathway-targeted therapies towards the wnt/β-catenin pathway or a combinatory approach of the two. Ongoing clinical trials have yet to target these pathways, but it is likely that this will soon change.
8. Study Design
The majority of laboratory studies utilized GBM cell lines or GBM patient samples. The frequent use of the U87 cell line in laboratory studies may be attributed to its widely accepted use as a model for GBM [77]. The use of technology such as spheroid or 3D cell culture is highly relevant in the context of therapies for gliomas. These technologies more accurately represent the tumor microenvironment and allow for better design of patient-specific treatments. Nearly one-third of laboratory studies testing existing therapies utilized this technology, implying that these studies are likely closer to translation to human studies.
The use of patient-derived GBM and glioma samples also highlights the importance of personalized medicine approaches in glioma treatment; nonetheless, this use also limits the generalizability of the conclusions, as most of these studies did not investigate molecular subtypes.
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251.
- Jakola, A.S.; Skjulsvik, A.J.; Myrmel, K.S.; Sjåvik, K.; Unsgård, G.; Torp, S.H.; Aaberg, K.; Berg, T.; Dai, H.Y.; Johnsen, K.; et al. Surgical Resection versus Watchful Waiting in Low-Grade Gliomas. Ann. Oncol. 2017, 28, 1942–1948.
- Chai, R.; Fang, S.; Pang, B.; Liu, Y.; Wang, Y.; Zhang, W.; Jiang, T. Molecular Pathology and Clinical Implications of Diffuse Glioma. Chin. Med. J. 2022, 135, 2914–2925.
- Whitfield, B.T.; Huse, J.T. Classification of Adult-type Diffuse Gliomas: Impact of the World Health Organization 2021 Update. Brain Pathol. 2022, 32, e13062.
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma Targeted Therapy: Insight into Future of Molecular Approaches. Mol. Cancer 2022, 21, 39.
- Cruz Da Silva, E.; Mercier, M.-C.; Etienne-Selloum, N.; Dontenwill, M.; Choulier, L. A Systematic Review of Glioblastoma-Targeted Therapies in Phases II, III, IV Clinical Trials. Cancers 2021, 13, 1795.
- Ichimura, K.; Pearson, D.M.; Kocialkowski, S.; Bäcklund, L.M.; Chan, R.; Jones, D.T.W.; Collins, V.P. IDH1 Mutations Are Present in the Majority of Common Adult Gliomas but Rare in Primary Glioblastomas. Neuro-Oncology 2009, 11, 341–347.
- Miller, J.J.; Gonzalez Castro, L.N.; McBrayer, S.; Weller, M.; Cloughesy, T.; Portnow, J.; Andronesi, O.; Barnholtz-Sloan, J.S.; Baumert, B.G.; Berger, M.S.; et al. Isocitrate Dehydrogenase (IDH) Mutant Gliomas: A Society for Neuro-Oncology (SNO) Consensus Review on Diagnosis, Management, and Future Directions. Neuro-Oncology 2023, 25, 4–25.
- Allen, M.; Bjerke, M.; Edlund, H.; Nelander, S.; Westermark, B. Origin of the U87MG Glioma Cell Line: Good News and Bad News. Sci. Transl. Med. 2016, 8, 354re3.
- Kenesei, Á.; Volkó, J.; Szalóki, N.; Mocsár, G.; Jambrovics, K.; Balajthy, Z.; Bodnár, A.; Tóth, K.; Waldmann, T.A.; Vámosi, G. IL-15 Trans-Presentation Is an Autonomous, Antigen-Independent Process. J. Immunol. 2021, 207, 2489–2500.
- Cole, D.E.; Lester-McCully, C.M.; Widemann, B.C.; Warren, K.E. Plasma and Cerebrospinal Fluid Pharmacokinetics of the Akt Inhibitor, Perifosine, in a Non-Human Primate Model. Cancer Chemother. Pharmacol. 2015, 75, 923–928.
- Britten, C.D. PI3K and MEK Inhibitor Combinations: Examining the Evidence in Selected Tumor Types. Cancer Chemother. Pharmacol. 2013, 71, 1395–1409.
- Paul, I.; Bhattacharya, S.; Chatterjee, A.; Ghosh, M.K. Current Understanding on EGFR and Wnt/β-Catenin Signaling in Glioma and Their Possible Crosstalk. Genes Cancer 2013, 4, 427–446.
- Mellinghoff, I.K.; Wang, M.Y.; Vivanco, I.; Haas-Kogan, D.A.; Zhu, S.; Dia, E.Q.; Lu, K.V.; Yoshimoto, K.; Huang, J.H.Y.; Chute, D.J.; et al. Molecular Determinants of the Response of Glioblastomas to EGFR Kinase Inhibitors. N. Engl. J. Med. 2005, 353, 2012–2024.
- National Institute of Health (NIH) 219 Studies Found for: Molecular Targeted Therapy OR Molecular Target OR Protein Kinase Inhibitors/Administration OR Mitogen-Activated Protein Kinase Kinases/Antagonists and Inhibitors OR Antineoplastic Combined Chemotherapy Protocols/Administration and Dosage|Glioma Glioblastoma Multiforme. Clinicaltrials.Gov. Available online: https://clinicaltrials.gov/ct2/results?cond=Glioma+Glioblastoma+Multiforme&term=molecular+targeted+therapy+OR+molecular+target+OR+Protein+Kinase+Inhibitors%2Fadministration+OR+Mitogen-Activated+Protein+Kinase+Kinases%2Fantagonists+and+inhibitors+OR+Antineoplastic+Combined+Chemotherapy+Protocols%2Fadministration+and+dosage&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort= (accessed on 29 May 2023).
- Ma, J.; Zhang, Y.; Li, R.; Ye, J.; Li, H.; Zhang, Y.; Ma, Z.; Li, J.; Zhong, X.; Yang, X. Tetrandrine Suppresses Human Glioma Growth by Inhibiting Cell Survival, Proliferation and Tumour Angiogenesis through Attenuating STAT3 Phosphorylation. Eur. J. Pharmacol. 2015, 764, 228–239.
- Qin, Y.; Fu, M.; Takahashi, M.; Iwanami, A.; Kuga, D.; Rao, R.G.; Sudhakar, D.; Huang, T.; Kiyohara, M.; Torres, K.; et al. Epithelial Membrane Protein-2 (EMP2) Activates Src Protein and Is a Novel Therapeutic Target for Glioblastoma. J. Biol. Chem. 2014, 289, 13974–13985.
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genßler, S.; Schönfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T.; et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J. Natl. Cancer Inst. 2016, 108, djv375.
- Thiessen, B.; Stewart, C.; Tsao, M.; Kamel-Reid, S.; Schaiquevich, P.; Mason, W.; Easaw, J.; Belanger, K.; Forsyth, P.; McIntosh, L.; et al. A Phase I/II Trial of GW572016 (Lapatinib) in Recurrent Glioblastoma Multiforme: Clinical Outcomes, Pharmacokinetics and Molecular Correlation. Cancer Chemother. Pharmacol. 2010, 65, 353–361.
- Patel, K.S.; Kejriwal, S.; Thammachantha, S.; Duong, C.; Murillo, A.; Gordon, L.K.; Cloughesy, T.F.; Liau, L.; Yong, W.; Yang, I.; et al. Increased Epithelial Membrane Protein 2 Expression in Glioblastoma after Treatment with Bevacizumab. Neuro-Oncol. Adv. 2020, 2, vdaa112.
- Wick, W.; Dettmer, S.; Berberich, A.; Kessler, T.; Karapanagiotou-Schenkel, I.; Wick, A.; Winkler, F.; Pfaff, E.; Brors, B.; Debus, J.; et al. N2M2 (NOA-20) Phase I/II Trial of Molecularly Matched Targeted Therapies plus Radiotherapy in Patients with Newly Diagnosed Non-MGMT Hypermethylated Glioblastoma. Neuro-Oncology 2019, 21, 95–105.
- Brachman, D.G.; Pugh, S.L.; Ashby, L.S.; Thomas, T.A.; Dunbar, E.M.; Narayan, S.; Robins, H.I.; Bovi, J.A.; Rockhill, J.K.; Won, M.; et al. Phase 1/2 Trials of Temozolomide, Motexafin Gadolinium, and 60-Gy Fractionated Radiation for Newly Diagnosed Supratentorial Glioblastoma Multiforme: Final Results of RTOG 0513. Int. J. Radiat. Oncol. 2015, 91, 961–967.
- Kubicek, G.J.; Werner-Wasik, M.; Machtay, M.; Mallon, G.; Myers, T.; Ramirez, M.; Andrews, D.; Curran, W.J.; Dicker, A.P. A Phase I Trial Using the Proteasome Inhibitor Bortezomib and Concurrent Temozolomide and Radiotherapy for CNS Malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 433–439.
- Lin, J.; Yu, L.; Fu, Y.; Chen, H.; Zheng, X.; Wang, S.; Gao, C.; Cao, Y.; Lin, L. A Refractory Case of CDK4-Amplified Spinal Astrocytoma Achieving Complete Response upon Treatment with a Palbociclib-Based Regimen: A Case Report. BMC Cancer 2020, 20, 630.
- Hamada, T.; Akahane, T.; Yokoyama, S.; Higa, N.; Kirishima, M.; Matsuo, K.; Shimokawa, M.; Yoshimoto, K.; Tanimoto, A. An Oncogenic Splice Variant of PDGFRα in Adult Glioblastoma as a Therapeutic Target for Selective CDK4/6 Inhibitors. Sci. Rep. 2022, 12, 1275.
- Li, C.; Shen, J.; Wei, X.; Xie, C.; Lu, W. Targeted Delivery of a Novel Palmitylated D-Peptide for Antiglioblastoma Molecular Therapy. J. Drug Target. 2012, 20, 264–271.
- Merlino, F.; Daniele, S.; La Pietra, V.; Di Maro, S.; Di Leva, F.S.; Brancaccio, D.; Tomassi, S.; Giuntini, S.; Cerofolini, L.; Fragai, M.; et al. Simultaneous Targeting of RGD-Integrins and Dual Murine Double Minute Proteins in Glioblastoma Multiforme. J. Med. Chem. 2018, 61, 4791–4809.
- Michaud, K.; Solomon, D.A.; Oermann, E.; Kim, J.-S.; Zhong, W.-Z.; Prados, M.D.; Ozawa, T.; James, C.D.; Waldman, T. Pharmacologic Inhibition of Cyclin-Dependent Kinases 4 and 6 Arrests the Growth of Glioblastoma Multiforme Intracranial Xenografts. Cancer Res. 2010, 70, 3228–3238.
- Punganuru, S.R.; Artula, V.; Zhao, W.; Rajaei, M.; Deokar, H.; Zhang, R.; Buolamwini, J.K.; Srivenugopal, K.S.; Wang, W. Targeted Brain Tumor Therapy by Inhibiting the MDM2 Oncogene: In Vitro and In Vivo Antitumor Activity and Mechanism of Action. Cells 2020, 9, 1592.
- Zhong, S.; Wu, B.; Dong, X.; Han, Y.; Jiang, S.; Zhang, Y.; Bai, Y.; Luo, S.X.; Chen, Y.; Zhang, H.; et al. Identification of Driver Genes and Key Pathways of Glioblastoma Shows JNJ-7706621 as a Novel Antiglioblastoma Drug. World Neurosurg. 2018, 109, e329–e342.
- Abe, T.; La, T.M.; Miyagaki, Y.; Oya, E.; Wei, F.-Y.; Sumida, K.; Fujise, K.; Takeda, T.; Tomizawa, K.; Takei, K.; et al. Phosphorylation of Cortactin by Cyclin-Dependent Kinase 5 Modulates Actin Bundling by the Dynamin 1-Cortactin Ring-like Complex and Formation of Filopodia and Lamellipodia in NG108-15 Glioma-Derived Cells. Int. J. Oncol. 2019, 54, 550–558.
- Bai, H.-L.; Kang, C.-M.; Sun, Z.-Q.; Li, X.-H.; Dai, X.-Y.; Huang, R.-Y.; Zhao, J.-J.; Bei, Y.-R.; Huang, X.-Z.; Lu, Z.-F.; et al. TTDA Inhibited Apoptosis by Regulating the P53-Bax/Bcl2 Axis in Glioma. Exp. Neurol. 2020, 331, 113380.
- Godoy, P.R.D.V.; Donaires, F.S.; Montaldi, A.P.L.; Sakamoto-Hojo, E.T. Anti-Proliferative Effects of E2F1 Suppression in Glioblastoma Cells. Cytogenet. Genome Res. 2021, 161, 372–381.
- Li, H.; You, Y.; Liu, J. Cyclin-Dependent Kinase 10 Prevents Glioma Metastasis via Modulation of Snail Expression. Mol. Med. Rep. 2018, 18, 1165–1170.
- Luo, R.; Wang, X.; Dong, Y.; Wang, L.; Tian, C. Activation of Protease-Activated Receptor 2 Reduces Glioblastoma Cell Apoptosis. J. Biomed. Sci. 2014, 21, 25.
- Wirsching, H.-G.; Weller, M. The Role of Molecular Diagnostics in the Management of Patients with Gliomas. Curr. Treat. Options Oncol. 2016, 17, 51.
- Schiff, D.; Desjardins, A.; Cloughesy, T.; Mikkelsen, T.; Glantz, M.; Chamberlain, M.C.; Reardon, D.A.; Wen, P.Y. Phase 1 Dose Escalation Trial of the Safety and Pharmacokinetics of Cabozantinib Concurrent with Temozolomide and Radiotherapy or Temozolomide after Radiotherapy in Newly Diagnosed Patients with High-Grade Gliomas. Cancer 2016, 122, 582–587.
- Badruddoja, M.A.; Pazzi, M.; Sanan, A.; Schroeder, K.; Kuzma, K.; Norton, T.; Scully, T.; Mahadevan, D.; Ahmadi, M.M. Phase II Study of Bi-Weekly Temozolomide plus Bevacizumab for Adult Patients with Recurrent Glioblastoma. Cancer Chemother. Pharmacol. 2017, 80, 715–721.
- Brown, N.; McBain, C.; Nash, S.; Hopkins, K.; Sanghera, P.; Saran, F.; Phillips, M.; Dungey, F.; Clifton-Hadley, L.; Wanek, K.; et al. Multi-Center Randomized Phase II Study Comparing Cediranib plus Gefitinib with Cediranib plus Placebo in Subjects with Recurrent/Progressive Glioblastoma. PLoS ONE 2016, 11, e0156369.
- Clarke, J.L.; Molinaro, A.M.; Phillips, J.J.; Butowski, N.A.; Chang, S.M.; Perry, A.; Costello, J.F.; DeSilva, A.A.; Rabbitt, J.E.; Prados, M.D. A Single-Institution Phase II Trial of Radiation, Temozolomide, Erlotinib, and Bevacizumab for Initial Treatment of Glioblastoma. Neuro-Oncology 2014, 16, 984–990.
- D’Alessandris, Q.G.; Montano, N.; Cenci, T.; Martini, M.; Lauretti, L.; Bianchi, F.; Larocca, L.M.; Maira, G.; Fernandez, E.; Pallini, R. Targeted Therapy with Bevacizumab and Erlotinib Tailored to the Molecular Profile of Patients with Recurrent Glioblastoma. Preliminary Experience. Acta Neurochir. 2013, 155, 33–40.
- Desjardins, A.; Reardon, D.A.; Coan, A.; Marcello, J.; Herndon II, J.E.; Bailey, L.; Peters, K.B.; Friedman, H.S.; Vredenburgh, J.J. Bevacizumab and Daily Temozolomide for Recurrent Glioblastoma. Cancer 2012, 118, 1302–1312.
- Lassen, U.; Chinot, O.L.; McBain, C.; Mau-Sørensen, M.; Larsen, V.A.; Barrie, M.; Roth, P.; Krieter, O.; Wang, K.; Habben, K.; et al. Phase 1 Dose-Escalation Study of the Antiplacental Growth Factor Monoclonal Antibody RO5323441 Combined with Bevacizumab in Patients with Recurrent Glioblastoma. Neuro-Oncology 2015, 17, 1007–1015.
- Lu, Y.; Qi, S.; Ouyang, H.; Li, Z.; Yin, Y.; Shi, J.; Qiu, X.; Mo, Y. Preliminary clinical evaluations of bevacizumab for recurrent malignant glioma in Chinese patients. Zhonghua Yi Xue Za Zhi 2014, 94, 1165–1168.
- Vaccaro, V.; Fabi, A.; Vidiri, A.; Giannarelli, D.; Metro, G.; Telera, S.; Vari, S.; Piludu, F.; Carosi, M.A.; Villani, V.; et al. Activity and Safety of Bevacizumab plus Fotemustine for Recurrent Malignant Gliomas. BioMed Res. Int. 2014, 2014, 351252.
- Vredenburgh, J.J.; Desjardins, A.; Kirkpatrick, J.P.; Reardon, D.A.; Peters, K.B.; Herndon, J.E.; Marcello, J.; Bailey, L.; Threatt, S.; Sampson, J.; et al. Addition of Bevacizumab to Standard Radiation Therapy and Daily Temozolomide Is Associated with Minimal Toxicity in Newly Diagnosed Glioblastoma Multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 58–66.
- Wang, F.; Huang, Q.; Zhou, L.-Y. Analysis of the Treatment of Gliomas with SEC Therapy Combined with Radiochemotherapy. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2400–2405.
- Nayak, L.; Molinaro, A.M.; Peters, K.; Clarke, J.L.; Jordan, J.T.; de Groot, J.; Nghiemphu, L.; Kaley, T.; Colman, H.; McCluskey, C.; et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 1048–1057.
- Huveldt, D.; Lewis-Tuffin, L.J.; Carlson, B.L.; Schroeder, M.A.; Rodriguez, F.; Giannini, C.; Galanis, E.; Sarkaria, J.N.; Anastasiadis, P.Z. Targeting Src Family Kinases Inhibits Bevacizumab-Induced Glioma Cell Invasion. PLoS ONE 2013, 8, e56505.
- Lu, L.; Saha, D.; Martuza, R.L.; Rabkin, S.D.; Wakimoto, H. Single Agent Efficacy of the VEGFR Kinase Inhibitor Axitinib in Preclinical Models of Glioblastoma. J. Neurooncol. 2015, 121, 91–100.
- Xia, L.; Gong, M.; Zou, Y.; Wang, Z.; Wu, B.; Zhang, S.; Li, L.; Jin, K.; Sun, C. Apatinib Induces Ferroptosis of Glioma Cells through Modulation of the VEGFR2/Nrf2 Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 9925919.
- Franco, D.G.; Moretti, I.F.; Marie, S.K.N. Mitochondria Transcription Factor A: A Putative Target for the Effect of Melatonin on U87MG Malignant Glioma Cell Line. Molecules 2018, 23, 1129.
- Huang, T.; Xu, T.; Wang, Y.; Zhou, Y.; Yu, D.; Wang, Z.; He, L.; Chen, Z.; Zhang, Y.; Davidson, D.; et al. Cannabidiol Inhibits Human Glioma by Induction of Lethal Mitophagy through Activating TRPV4. Autophagy 2021, 17, 3592–3606.
- Renfrow, J.J.; Soike, M.H.; West, J.L.; Ramkissoon, S.H.; Metheny-Barlow, L.; Mott, R.T.; Kittel, C.A.; D’Agostino, R.B.J.r.; Tatter, S.B.; Laxton, A.W.; et al. Attenuating Hypoxia Driven Malignant Behavior in Glioblastoma with a Novel Hypoxia-Inducible Factor 2 Alpha Inhibitor. Sci. Rep. 2020, 10, 15195.
- Lan, J.; Xue, Y.; Chen, H.; Zhao, S.; Wu, Z.; Fang, J.; Han, C.; Lou, M. Hypoxia-Induced MiR-497 Decreases Glioma Cell Sensitivity to TMZ by Inhibiting Apoptosis. FEBS Lett. 2014, 588, 3333–3339.
- Li, C.; Chen, Y.; Zhang, Q.; Guo, C.; Chen, F.; Xi, S.; Zeng, J.; Ke, C.; Sharma, H.S.; Chen, Z. Expression of Twist Associated to Microcirculation Patterns of Human Glioma Correlated with Progression and Survival of the Patient—Novel Therapeutic Advances In Glioblastoma. In International Review of Neurobiology; Academic Press Ltd.—Elsevier Science Ltd.: London, UK, 2020; Volume 151, ISBN 0074-7742.
- Martina, E.; Degen, M.; Rueegg, C.; Merlo, A.; Lino, M.M.; Chiquet-Ehrismann, R.; Brellier, F. Tenascin-W Is a Specific Marker of Glioma-Associated Blood Vessels and Stimulates Angiogenesis in Vitro. FASEB J. 2010, 24, 778–787.
- Wang, J.Y.; Bettegowda, C. Genetics and Immunotherapy: Using the Genetic Landscape of Gliomas to Inform Management Strategies. J. Neurooncol. 2015, 123, 373–383.
- Xiong, W.; Zhao, Y.; Du, H.; Guo, X. Current Status of Immune Checkpoint Inhibitor Immunotherapy for Lung Cancer. Front. Oncol. 2021, 11, 704336.
- Han, J.W.; Park, S.-H. Advances in Immune Checkpoint Inhibitors for Hepatocellular Carcinoma. J. Liver Cancer 2021, 21, 139–145.
- Zhang, H.; Dai, Z.; Wu, W.; Wang, Z.; Zhang, N.; Zhang, L.; Zeng, W.-J.; Liu, Z.; Cheng, Q. Regulatory Mechanisms of Immune Checkpoints PD-L1 and CTLA-4 in Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 184.
- Anghileri, E.; Di Ianni, N.; Paterra, R.; Langella, T.; Zhao, J.; Eoli, M.; Patanè, M.; Pollo, B.; Cuccarini, V.; Iavarone, A.; et al. High Tumor Mutational Burden and T-Cell Activation Are Associated with Long-Term Response to Anti-PD1 Therapy in Lynch Syndrome Recurrent Glioblastoma Patient. Cancer Immunol. Immunother. CII 2021, 70, 831–842.
- Restrepo, P.; Yong, R.; Laface, I.; Tsankova, N.; Nael, K.; Akturk, G.; Sebra, R.; Gnjatic, S.; Hormigo, A.; Losic, B. Tumoral and Immune Heterogeneity in an Anti-PD-1-Responsive Glioblastoma: A Case Study. Cold Spring Harb. Mol. Case Stud. 2020, 6, a004762.
- Goswami, S.; Walle, T.; Cornish, A.E.; Basu, S.; Anandhan, S.; Fernandez, I.; Vence, L.; Blando, J.; Zhao, H.; Yadav, S.S.; et al. Immune Profiling of Human Tumors Identifies CD73 as a Combinatorial Target in Glioblastoma. Nat. Med. 2020, 26, 39–46.
- Xu, B.; Jiang, C.; Han, H.; Liu, H.; Tang, M.; Liu, L.; Ji, W.; Lu, X.; Yang, X.; Zhang, Y.; et al. Icaritin Inhibits the Invasion and Epithelial-to-Mesenchymal Transition of Glioblastoma Cells by Targeting EMMPRIN via PTEN/AKt/HIF-1α Signalling. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1296–1307.
- Zanotto-Filho, A.; Braganhol, E.; Schröder, R.; de Souza, L.H.; Dalmolin, R.J.; Pasquali, M.A.; Gelain, D.P.; Battastini, A.M.; Moreira, J.C. NFκB Inhibitors Induce Cell Death in Glioblastomas. Biochem. Pharmacol. 2011, 81, 412–424.
- Hong, H.; Stastny, M.; Brown, C.; Chang, W.C.; Ostberg, J.R.; Forman, S.J.; Jensen, M.C. Diverse Solid Tumors Expressing a Restricted Epitope of L1-CAM Can Be Targeted by Chimeric Antigen Receptor Redirected T Lymphocytes. J. Immunother. 2014, 37, 93–104.
- Yuan, Y.; Zhao, Q.; Zhao, S.; Zhang, P.; Zhao, H.; Li, Z.; Du, Y.; Tian, X.; Lu, J. Characterization of Transcriptome Profile and Clinical Features of a Novel Immunotherapy Target CD204 in Diffuse Glioma. Cancer Med. 2019, 8, 3811–3821.
- Zhang, Y.; Yang, X.; Zhu, X.L.; Bai, H.; Wang, Z.Z.; Zhang, J.J.; Hao, C.Y.; Duan, H.B. S100A Gene Family: Immune-Related Prognostic Biomarkers and Therapeutic Targets for Low-Grade Glioma. Aging 2021, 13, 15459–15478.
- Chen, T.; Zhang, F.; Liu, J.; Huang, Z.; Zheng, Y.; Deng, S.; Liu, Y.; Wang, J.; Sun, X. Dual Role of WNT5A in Promoting Endothelial Differentiation of Glioma Stem Cells and Angiogenesis of Glioma Derived Endothelial Cells. Oncogene 2021, 40, 5081–5094.
- Di, C.; Liang, J.; Wang, Y.; Zhao, G.; Zhao, Y. SPZ1 Promotes Glioma Aggravation via Targeting CXXC4. J. BUON 2021, 26, 373–379.
- Friedmann-Morvinski, D.; Bhargava, V.; Gupta, S.; Verma, I.M.; Subramaniam, S. Identification of Therapeutic Targets for Glioblastoma by Network Analysis. Oncogene 2016, 35, 608–620.
- Guo, G.; Liu, J.; Ren, Y.; Mao, X.; Hao, Y.; Zhong, C.; Chen, X.; Wang, X.; Wu, Y.; Lian, S.; et al. FRAT1 Enhances the Proliferation and Tumorigenesis of CD133+ Nestin+ Glioma Stem Cells In Vitro and In Vivo. J. Cancer 2020, 11, 2421–2430.
- Lan, J.; Guo, P.; Lin, Y.; Mao, Q.; Guo, L.; Ge, J.; Li, X.; Jiang, J.; Lin, X.; Qiu, Y. Role of Glycosyltransferase PomGnT1 in Glioblastoma Progression. Neuro-Oncology 2015, 17, 211–222.
- Mizobuchi, Y.; Matsuzaki, K.; Kuwayama, K.; Kitazato, K.; Mure, H.; Kageji, T.; Nagahiro, S. REIC/Dkk-3 Induces Cell Death in Human Malignant Glioma. Neuro-Oncology 2008, 10, 244–253.
- Zhou, X.; Ren, Y.; Zhang, J.; Zhang, C.; Zhang, K.; Han, L.; Kong, L.; Wei, J.; Chen, L.; Yang, J.; et al. HOTAIR Is a Therapeutic Target in Glioblastoma. Oncotarget 2015, 6, 8353–8365.
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2006–2010. Neuro-Oncology 2013, 15, ii1–ii56.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
652
Revisions:
2 times
(View History)
Update Date:
10 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




