Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anne Dittrich | -- | 2533 | 2023-07-07 04:25:46 | | | |
| 2 | Catherine Yang | Meta information modification | 2533 | 2023-07-07 04:28:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dittrich, A.T.M.; Janssen, E.J.M.; Geelen, J.; Bouman, K.; Ward, L.M.; Draaisma, J.M.T. Secondary Osteoporosis in Vulnerable Children. Encyclopedia. Available online: https://encyclopedia.pub/entry/46550 (accessed on 08 February 2026).
Dittrich ATM, Janssen EJM, Geelen J, Bouman K, Ward LM, Draaisma JMT. Secondary Osteoporosis in Vulnerable Children. Encyclopedia. Available at: https://encyclopedia.pub/entry/46550. Accessed February 08, 2026.
Dittrich, Anne T. M., Etienne J. M. Janssen, Joyce Geelen, Karlijn Bouman, Leanne M. Ward, Jos M. T. Draaisma. "Secondary Osteoporosis in Vulnerable Children" Encyclopedia, https://encyclopedia.pub/entry/46550 (accessed February 08, 2026).
Dittrich, A.T.M., Janssen, E.J.M., Geelen, J., Bouman, K., Ward, L.M., & Draaisma, J.M.T. (2023, July 07). Secondary Osteoporosis in Vulnerable Children. In Encyclopedia. https://encyclopedia.pub/entry/46550
Dittrich, Anne T. M., et al. "Secondary Osteoporosis in Vulnerable Children." Encyclopedia. Web. 07 July, 2023.
Copy Citation
By definition, children constitute a vulnerable population, especially when they are chronically ill and/or disabled. A characteristic of chronically ill and disabled children is that they also suffer from indirect effects of their disease, such as immobilization, chronic inflammation, reduced time outdoors in the sun, osteotoxic effects of disease-targeted therapy (like glucocorticoids), and poor nutrition. All these factors may lead to bone fragility due to secondary osteoporosis, a co-morbidity that may be overlooked in the context of serious underlying diseases.
secondary osteoporosis
prevention
screening
therapy
1. Risk Factors for Developing Secondary Osteoporosis
1.1. Immobilization and Secondary Osteoporosis
Immobilization is normal in children who are wheelchair-dependent. Cerebral palsy and neuromuscular disorders, such as Duchenne muscular dystrophy, are examples of diseases with immobilization. However, also severe neurodevelopmental disorders and spina bifida are frequent causes of immobilization.
The degree of immobilization in children with cerebral palsy is classified with the Gross Motor Functional Classification Scale (GMFCS) in five levels [1]. GMFCS level 4 means that a child can walk indoors for short distances with assistance but relies on a wheelchair outdoors. Children with GMFCS level 5 are wheelchair dependent. Immobilization in children with cerebral palsy reduces biomechanical bone loading, leading to thinner long bones and less trabecular bone formation [2]. In children aged 2–19 years with moderate to severe cerebral palsy, classified as GMFCS level 3–5, low BMD was found in 97% of children unable to stand and older than 9 years. This leads to reduced periosteal apposition in lower extremity bones, reducing cortical thickness. Consequently, fractures occur most commonly in the distal femur and tibia. Fractures occurred in 26% of children who were older than 10 years [3]. Other factors that contributed to low BMD (z score ≤−2.0] were feeding difficulty and the use of anticonvulsants. Other studies showed an incidence of fractures of 4% per year [4].
The major role that immobilization plays in secondary osteoporosis and low BMD is also illustrated by boys with Duchenne muscular dystrophy. In the era preceding treatment with glucocorticoids, Larson and Henderson found that BMD was only slightly decreased (z-score lumbar spine −0.8] when the boys were ambulatory but decreased significantly after the loss of ambulation (z-score lumbar spine −1.6] [5]. This was also shown by Crabtree et al. in boys with Duchenne muscular dystrophy treated with glucocorticoids who became non-ambulant [6]. They showed that 44% of the boys sustained a fracture. Two-thirds of these fractures involved the lower extremities, and there were no vertebral fractures. Moreover, 44% of the boys who walked with support at the time of fracture never resumed walking after the fracture. In addition, Joseph et al. showed an absence of clinical vertebral fractures in glucocorticoid naïve boys [7]. In the authors’ experience, vertebral fractures can occur in DMD among steroid-naïve patients if routine screening is part of the bone health evaluation; however, this occurrence is rarely related to boys with DMD who are receiving glucocorticoid therapy.
Preclinical studies are useful for a more in-depth understanding of the relationship between immobilization and secondary osteoporosis. Animal models may be used to study immobilization and the cellular mechanisms of secondary osteoporosis. A recent systematic review gives an overview of known animal models [8].
1.2. Drug-Induced Secondary Osteoporosis
A myriad of drugs can lead to low BMD and secondary osteoporosis. The most well-known are glucocorticoids, anticonvulsants, and methotrexate [9]. Glucocorticoids are often used for prolonged periods of time in (chronic) diseases in children like systemic inflammatory and autoimmune diseases, renal diseases, after organ transplantation, leukemia, and Duchenne muscular dystrophy. These diseases in themselves may also lead to fragility fractures because of reduced bone strength, for example, due to the effect of the increased cytokines (like IL1, IL6, and tumor necrosis factor-alpha) in case of systemic inflammatory and autoimmune diseases on bone metabolism [10]. Much is known about the adverse effects of glucocorticoids on bone strength [11]. Glucocorticoids cause decreased bone formation, with an additional early and transient increase in bone resorption. The final effect is increased bone turnover with early onset bone loss [12]. BMD rapidly decreases in the first 2 weeks after the start of glucocorticoids, leading to significant bone loss in the first 3–6 months of therapy [13]. This loss diminishes with time and is replaced by a chronic phase of decreased bone formation. The ultimate effect is a reduction of BMD and altered bone microarchitecture, with a predilection for the trabecular-rich spine [11]. This deleterious effect of glucocorticoids on bone strength can be seen in children with Duchenne muscular dystrophy. Glucocorticoids are used to delay loss of ambulation, improve or retain pulmonary function with reduced need for assisted ventilation and delay cardiomyopathy [14]. Before corticosteroids were used, vertebral fractures were rarely seen in children with Duchenne muscular dystrophy, and most fractures involved the lower extremities [5]. In boys living with Duchenne muscular dystrophy who are treated with glucocorticoids, the prevalence of vertebral fractures is >50%, with a cumulative incidence of 28% over a median follow-up of 4 years after starting with glucocorticoids [7][15]. In Canada, an observational cohort study was performed to increase insight into glucocorticoid-induced osteoporosis in children [11]. The most important observations were that vertebral fractures are the hallmark of pediatric glucocorticoid-induced secondary osteoporosis, can occur in the first year of glucocorticoid treatment, and are frequently asymptomatic. However, some children have the growth-mediated ability to restore normal vertebral body dimensions following vertebral fractures. This is important to know since this may preclude the need for intravenous osteoporosis therapy [16]. Children with poor growth, older children (with less residual growth), and children with ongoing bone health threats have less potential for vertebral body reshaping, which can result in permanent vertebral deformity [17]. Therefore, timely intervention with intravenous osteoporosis therapy is paramount in children with vertebral fractures if they have persistent risk factors for ongoing spine collapse.
Although preclinical models may help to understand the effects of glucocorticoids on bone metabolism and bone strength, up to now, there is no robust animal model to evaluate known and new interventions [18].
2. Potential for Recovery
When vulnerable children receive a diagnosis of secondary osteoporosis, there is not always the need to treat with bone-targeted therapy because of the pediatric skeleton’s ability to undergo recovery in both bone mass/density and shape. Case in point, the growing skeleton has the potency to reconstitute normal heights of vertebral bodies following a vertebral collapse, a phenomenon known as “vertebral body reshaping” [11]. This has been illustrated in children with acute lymphoblastic leukemia and children with inflammatory bowel disease [17][19]. For example, many children with acute lymphoblastic leukemia will undergo vertebral body reshaping following vertebral fractures because most are diagnosed at a young age (and have significant residual growth potential), and the disease (and its treatment) are usually transient. In a series of children with acute lymphoblastic leukemia, the cumulative VF incidence over six years was 32.5% [17], and complete vertebral body reshaping occurred in 77.3% of these children. Notably, the children in which the reshaping was incomplete were older (and had less residual growth potential) and had more severe degrees of vertebral collapse.
Pediatric patients with inflammatory bowel disease, in general, are older at diagnosis, and the disease is chronic with exacerbations. Vertebral fractures have been reported at diagnosis [20]. The direct effects of chronic inflammation, use of glucocorticoids, delayed puberty, and poor nutrition are contributing factors causing secondary osteoporosis. Optimizing disease control can help reshape vertebral fractures and specific bones and may be the only therapeutic intervention needed for the effective reshaping of vertebral bodies [20].
At the opposite end of the spectrum are children with chronic diseases and persistent risk factors for osteoporosis (i.e., due to presently incurable conditions), with the need for continuous treatment with glucocorticoids, for example, boys with Duchenne muscular dystrophy. In these children, there is no capacity for spontaneous vertebral body reshaping. For this reason, contemporary care includes monitoring for signs of osteoporosis at the time of initiation of glucocorticoid treatment and starting osteoporosis treatment at the first sign of a low-trauma long-bone fracture or VF [9][21][22][23]. In such children, strategies are currently being considered for the prevention of first-ever fractures.
These three different clinical scenarios show the importance of assessing whether the vulnerable child with a fragility fracture needs osteoporosis therapy. It is important to recognize that younger age, transient risk factors, normal growth and puberty, and less severe vertebral collapse are key factors for recovery without needing bone-targeted intervention (i.e., bisphosphonates).
3. Therapy
Currently, the most widely used agents for treating secondary osteoporosis in children are bisphosphonates [16]. Bisphosphonates are synthetic analogs of pyrophosphates. Classically, treatment with intravenous bisphosphonates should be considered in children with a formal diagnosis of secondary osteoporosis that manifests as at least one low-trauma vertebral or long bone fracture and limited potential for spontaneous (i.e., medication-unassisted) recovery due to older age and/or persistence of osteoporosis risk factors [23][24]. In previous years, the ISCD criteria for otherwise healthy children were erroneously applied to some children with risk factors for osteoporosis, which meant that bisphosphonate therapy was withheld from, for example, boys with DMD who had a single femur fracture (instead of two or more long-bone fractures by 10 years of age or three or more long bone fractures by 19 years of age plus a low BMD) [25][26]. However, it is now understood that these criteria were intended for otherwise healthy children so as not to over-diagnose osteoporosis in this population, and even a single low-trauma long bone fracture can represent an osteoporotic event in children with risk factors for osteoporosis [16]. Low-trauma long bone fractures and symptomatic vertebral fractures (or asymptomatic VF in high-risk settings such as CP, DMD, and glucocorticoid-treated disorders) are the most frequent indications for treatment with intravenous bisphosphonates. The primary function of bisphosphonates is to inactivate osteoclasts, resulting in cortical and trabecular bone thickening. This makes bones wider, denser, and stronger. There has been much discussion in the literature on the use of oral versus intravenous therapy with bisphosphonates. At this time, the scientific data support the use of the (more potent) intravenous bisphosphonates over oral bisphosphonates.
There are different regimens to prescribe the most-used intravenous bisphosphonate, pamidronate. The most frequently used regimen is 1 mg/kg/day for three days every four months (in total, 9 mg/kg/year). However, a regimen of 1 mg/kg every three months (in total, 4 mg/kg/year) in children with primary osteoporosis has shown a comparable effect on BMD and reduction of fragility fractures [27]. Other studies also showed a comparable effect [28][29]. A different intravenous bisphosphonate, zoledronic acid, is also used in children. Zoledronic acid can be given every six months and is preferred by some because of its greater convenience with respect to the shorter duration of the infusion and longer duration of action compared with pamidronate. Saraff et al. showed that zoledronic acid has a comparable efficacy compared to pamidronate in children with osteogenesis imperfecta [30]. Additionally, Nasomyont et al. found no difference in effect on BMD Z scores between Pamidronate and zoledronic acid in patients with primary, secondary, and glucocorticoid-induced osteoporosis [25]. Furthermore, the costs of zoledronic acid are lower [30]. Conversely, Pamidronate probably has fewer adverse drug reactions, predominantly hypocalcemia [25], nausea, and vomiting. It is important to note that the first-infusion reactions of intravenous bisphosphonate therapy can precipitate adrenal insufficiency in glucocorticoid-treated children. For this reason, steroid stress dosing is recommended in children at risk for adrenal suppression in this context. In a recent study of intravenous zoledronic acid versus intravenous placebo in glucocorticoid-treated children, 25% of children in the placebo group had at least one adverse event following the first zoledronic acid infusion [31]. This observation provided evidence that not all health events following the first intravenous bisphosphonate infusions are bisphosphonate-related but may be linked to underlying chronic illnesses.
In practice, there are still two other questions regarding the treatment with bisphosphonates in children with permanent risk factors: when should we start bisphosphonates, and how long should patients be on bisphosphonates therapy?
When should we start bisphosphonates? As said before, treatment with intravenous bisphosphonates should be considered in children fulfilling the ISCD criteria. However, in children with significant risk factors for bone fragility due to chronic illness, even a single low-trauma long bone or vertebral fracture (in the absence of a “low BMD”) may be sufficient to diagnose the child with osteoporosis, as discussed in a recent paper by Ward et al. [21]. recently Nasomyont et al. published a study of the treatment with bisphosphonates in 48 with primary osteoporosis, 46 with secondary osteoporosis without glucocorticoids and 29 children with glucocorticoid-induced osteoporosis [32]. They treated 19% of the patients with intravenous bisphosphonates without these patients fulfilling the ISCD criteria for osteoporosis. One of these indications was low, declining BMD without a history of fractures; another was low BMD in association with kidney stones in children with ongoing risk factors. In addition, Draaisma et al. treated children on a ketogenic diet with ongoing risk factors with bisphosphonates out of the ISCD criteria [33].
How long should patients be on bisphosphonate therapy? Some observations in children with both primary and secondary osteoporosis have provided answers to these questions [34]. After discontinuing bisphosphonate therapy in children with open growth plates and persistent risk factors for osteoporosis, the newly formed bone has a low density. This may cause a point of increased bone fragility because of the “density differential” between the high-density bone (formed under treatment with bisphosphonates) and the low-density bone [35], leading to fractures in the transition zone between treated and untreated bone [36][37]. So, if bisphosphonate therapy should be continued, for how long? A possible answer followed from a study in children with osteogenesis imperfecta [36]. In children with still open growth plates and discontinuing bisphosphonate therapy, the BMD z-scores declined. However, discontinuing therapy in children with closed growth plates had no effect on the BMD z-scores two years later. This has led to the recommendation of continuation of bisphosphonate therapy until the closure of the growth plates in those with persistent risk factors (such as congenital/genetic disorders and long-term acquired risk factors, such as chronic glucocorticoid therapy). Starting with a higher dose until the patient is clinically stable and tapering the dose or frequency is often implemented in order to avoid over-treatment in the context of long-term therapy considered [16][38].
In summary, treatment with intravenous bisphosphonates should be considered in children fulfilling the ISCD criteria (intended for otherwise healthy children with clinically significant fractures and low, appropriately size-adjusted BMD parameters). However, it should also be considered in high-risk patients outside of this conservative approach who have even a single-low-trauma long bone or vertebral fracture, a known increased risk for bone fragility, and limited potential for spontaneous recovery [16][33]. It is important to keep in mind that not all children with secondary osteoporosis require bisphosphonate therapy following a fragility fracture; those with transient risk factors for vertebral or long bone fractures and potential for spontaneous recovery due to younger age or less severe vertebral collapse typically do not need osteoporosis intervention, unless they have significant bone pain interfering with the quality of life.
A proposition for screening, prevention, and therapy of secondary osteoporosis is given in Figure 1.
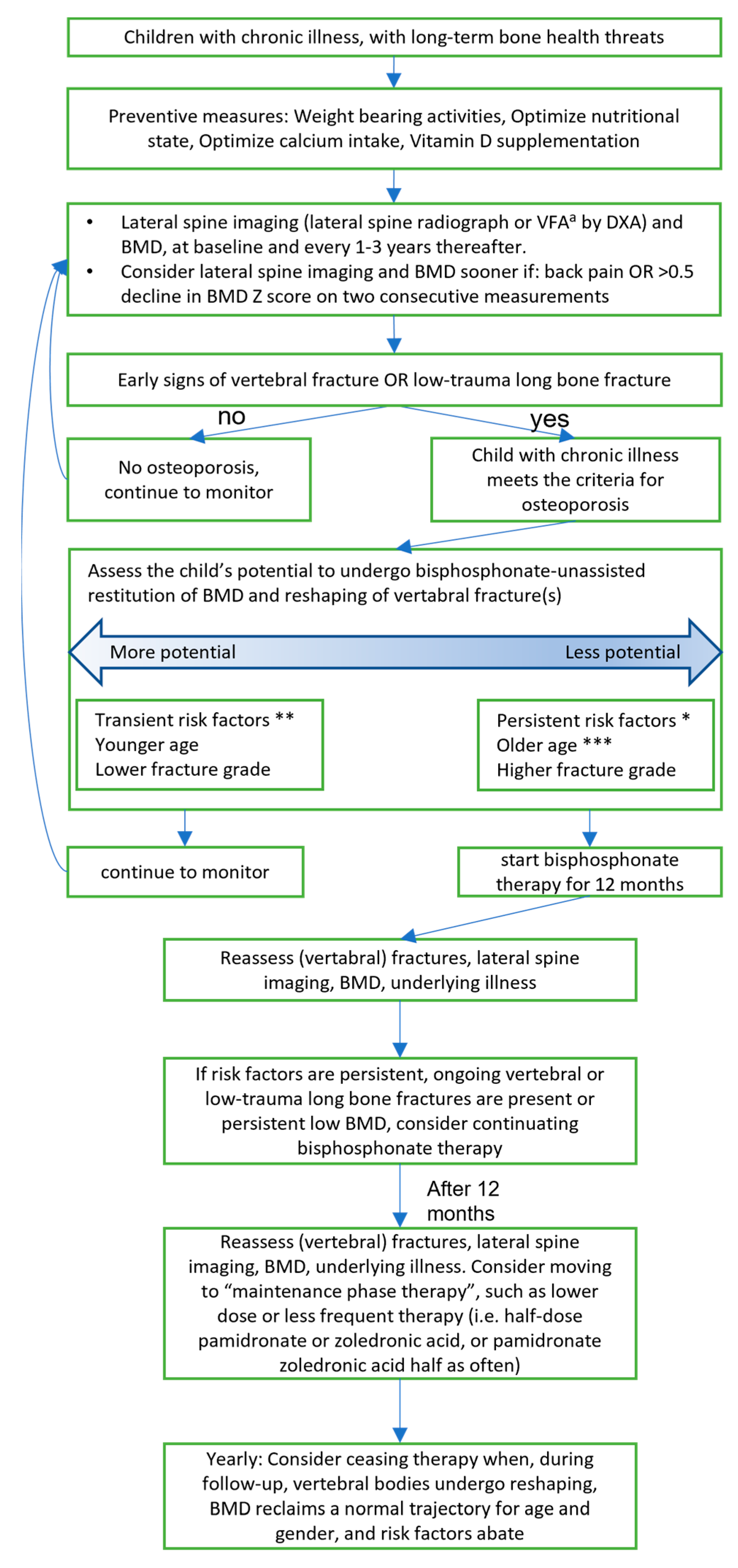
Figure 1. Recommendation for investigation and treatment of secondary osteoporosis in children. Partly based on the previous recommendation of papers of Ward [9][23], Fehlings et al. [39], and Simm et al. [38].a VFA = Vertebral Fracture Assessment * Persistent risk factors are >3 months steroids, sub-normal mobility, poorly controlled underlying disease ** Transient risk factors are <3months steroids, short-term immobilization (<2 weeks), well-controlled underlying disease *** Older age is defined as >8 years in girls and >9 years in boys.
References
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223.
- Saraff, V.; Högler, W. Endocrinology and Adolescence: Osteoporosis in children: Diagnosis and management. Eur. J. Endocrinol. 2015, 173, R185–R197.
- Henderson, R.C.; Lark, R.K.; Gurka, M.J.; Worley, G.; Fung, E.B.; Conaway, M.; Stallings, V.A.; Stevenson, R.D. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics 2002, 110, e5.
- Mergler, S.; Evenhuis, H.M.; Boot, A.M.; De Man, S.A.; Heus, K.G.B.-D.; Huijbers, W.A.; Penning, C. Epidemiology of low bone mineral density and fractures in children with severe cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2009, 51, 773–778.
- Larson, C.M.; Henderson, R.C. Bone Mineral Density and Fractures in Boys with Duchenne Muscular Dystrophy. J. Pediatr. Orthop. 2000, 20, 71–74.
- Crabtree, N.J.; Roper, H.; Shaw, N.J. Cessation of ambulation results in a dramatic loss of trabecular bone density in boys with Duchenne muscular dystrophy (DMD). Bone 2022, 154, 116248.
- Joseph, S.; Wang, C.; Bushby, K.; Guglieri, M.; Horrocks, I.; Straub, V.; Ahmed, S.F.; Wong, S.C. Fractures and Linear Growth in a Nationwide Cohort of Boys with Duchenne Muscular Dystrophy with and Without Glucocorticoid Treatment: Results from the UK NorthStar Database. JAMA Neurol. 2019, 76, 701–709.
- Brent, M.B.; Brüel, A.; Thomsen, J.S. A Systematic Review of Animal Models of Disuse-Induced Bone Loss. Calcif. Tissue Int. 2021, 108, 561–575.
- Ward, L.M. Part I: Which Child with a Chronic Disease Needs Bone Health Monitoring? Curr. Osteoporos. Rep. 2021, 19, 278–288.
- Lorenzo, J. Cytokines and Bone: Osteoimmunology. Handb. Exp. Pharmacol. 2020, 262, 177–230.
- Ward, L.M. Glucocorticoid-Induced Osteoporosis: Why Kids Are Different. Front. Endocrinol. 2020, 11, 576.
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16.
- van Staa, T.P.; Leufkens, H.G.; Cooper, C. The epidemiology of corticosteroid-induced osteoporosis: A meta-analysis. Osteoporos. Int. 2002, 13, 777–787.
- Weber, F.J.; Latshang, T.D.; Blum, M.R.; Kohler, M.; Wertli, M.M. Prognostic factors, disease course, and treatment efficacy in Duchenne muscular dystrophy: A systematic review and meta-analysis. Muscle Nerve 2022, 66, 462–470.
- Singh, A.; Schaeffer, E.K.; Reilly, C.W. Vertebral Fractures in Duchenne Muscular Dystrophy Patients Managed With Deflazacort. J. Pediatr. Orthop. 2018, 38, 320–324.
- Ward, L.M.; Konji, V.N.; Ma, J. The management of osteoporosis in children. Osteoporos. Int. 2016, 27, 2147–2179.
- Ward, L.M.; Ma, J.; Lang, B.; Ho, J.; Alos, N.; Matzinger, M.A.; Shenouda, N.; Lentle, B.; Jaremko, J.L.; Wilson, B. Bone Morbidity and Recovery in Children with Acute Lymphoblastic Leukemia: Results of a Six-Year Prospective Cohort Study. J. Bone Miner. Res. 2018, 33, 1435–1443.
- Wood, C.L.; Soucek, O.; Wong, S.C.; Zaman, F.; Farquharson, C.; Savendahl, L.; Ahmed, S.F. Animal models to explore the effects of glucocorticoids on skeletal growth and structure. J. Endocrinol. 2018, 236, R69–R91.
- Digby, M.G.; Bishop, N.J.; Paggiosi, M.A.; Offiah, A.C. HR-pQCT: A non-invasive ‘biopsy’ to assess bone structure and strength. Arch. Dis. Child. Educ. Pract. Ed. 2016, 101, 268–270.
- Wong, S.C.; Catto-Smith, A.G.A.; Zacharin, M. Pathological fractures in paediatric patients with inflammatory bowel disease. Eur. J. Pediatr. 2014, 173, 141–151.
- Ward, L.M.; Weber, D.R.; Munns, C.F.; Högler, W.; Zemel, B.S. A Contemporary View of the Definition and Diagnosis of Osteoporosis in Children and Adolescents. J. Clin. Endocrinol. Metab. 2020, 105, e2088–e2097.
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadiyannakis, S.; Olson, A.K. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361.
- Ward, L.M. Part 2: When Should Bisphosphonates Be Used in Children with Chronic Illness Osteoporosis? Curr. Osteoporos. Rep. 2021, 19, 289–297.
- Bishop, N.; Arundel, P.; Clark, E.; Dimitri, P.; Farr, J.; Jones, G.; Makitie, O.; Munns, C.F.; Shaw, N. Fracture prediction and the definition of osteoporosis in children and adolescents: The ISCD 2013 Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 275–280.
- Nasomyont, N.; Hornung, L.N.; Gordon, C.M.; Wasserman, H. Outcomes following intravenous bisphosphonate infusion in pediatric patients: A 7-year retrospective chart review. Bone 2019, 121, 60–67.
- Morris, H.F. Veterans Administration Cooperative Studies Project No. 147. Part VI: Laboratory costs of castings from noble and alternative ceramic metal alloys. J. Prosthet. Dent. 1988, 60, 164–171.
- Martinez-Soto, T.; Pacaud, D.; Stephure, D.; Trussell, R.; Huang, C. Treatment of symptomatic osteoporosis in children: A comparison of two pamidronate dosage regimens. J. Pediatr. Endocrinol. Metab. 2011, 24, 271–274.
- Chattopadhyay, A.; Bhansali, A.; Mohanty, S.K.; Khandelwal, N.; Mathur, S.K.; Dash, R.J. Hypophosphatemic rickets and osteomalacia in polyostotic fibrous dysplasia. J. Pediatr. Endocrinol. Metab. 2003, 16, 893–896.
- Steelman, J.; Zeitler, P. Treatment of symptomatic pediatric osteoporosis with cyclic single-day intravenous pamidronate infusions. J. Pediatr. 2003, 142, 417–423.
- Saraff, V.; Sahota, J.; Crabtree, N.; Sakka, S.; Shaw, N.J.; Högler, W. Efficacy and treatment costs of zoledronate versus pamidronate in paediatric osteoporosis. Arch. Dis. Child. 2018, 103, 92–94.
- Ward, L.M.; Choudhury, A.; Alos, N.; Cabral, D.A.; Rodd, C.; Sbrocchi, A.M.; Taback, S.; Padidela, R.; Shaw, N.J.; Hosszu, E. Zoledronic Acid vs Placebo in Pediatric Glucocorticoid-induced Osteoporosis: A Randomized, Double-blind, Phase 3 Trial. J. Clin. Endocrinol. Metab. 2021, 106, e5222–e5235.
- Hough, J.P.; Boyd, R.N.; Keating, J.L. Systematic review of interventions for low bone mineral density in children with cerebral palsy. Pediatrics 2010, 125, e670–e678.
- Draaisma, J.M.; Hampsink, B.M.; Janssen, M.; van Houdt, N.B.; Linders, E.T.; Willemsen, M.A. The Ketogenic Diet and Its Effect on Bone Mineral Density: A Retrospective Observational Cohort Study. Neuropediatrics 2019, 50, 353–358.
- Robinson, M.-E.; Trejo, P.; Palomo, T.; Glorieux, F.H.; Rauch, F. Osteogenesis Imperfecta: Skeletal Outcomes After Bisphosphonate Discontinuation at Final Height. J. Bone Miner. Res. 2019, 34, 2198–2204.
- Rauch, F.; Munns, C.; Land, C.; Glorieux, F.H. Pamidronate in children and adolescents with osteogenesis imperfecta: Effect of treatment discontinuation. J. Clin. Endocrinol. Metab. 2006, 91, 1268–1274.
- Rauch, F.; Cornibert, S.; Cheung, M.; Glorieux, F.H. Long-bone changes after pamidronate discontinuation in children and adolescents with osteogenesis imperfecta. Bone 2007, 40, 821–827.
- Harcke, H.T.; Stevenson, K.L.; Kecskemethy, H.H.; Bachrach, S.J.; Grissom, L.E. Fracture after bisphosphonate treatment in children with cerebral palsy: The role of stress risers. Pediatr. Radiol. 2012, 42, 76–81.
- Simm, P.J.; Biggin, A.; Zacharin, M.R.; Rodda, C.P.; Tham, E.; Siafarikas, A.; Jefferies, C.; Hofman, P.L.; Jensen, D.E.; Woodhead, H. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J. Paediatr. Child Heal. 2018, 54, 223–233.
- Fehlings, D.; Switzer, L.; Agarwal, P.; Wong, C.; Sochett, E.; Stevenson, R.; Sonnenberg, L.; Smile, S.; Young, E.; Huber, J. Informing evidence-based clinical practice guidelines for children with cerebral palsy at risk of osteoporosis: A systematic review. Dev. Med. Child Neurol. 2012, 54, 106–116.
More
Information
Subjects:
Orthopedics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
638
Revisions:
2 times
(View History)
Update Date:
07 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




