Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Majid Moshirfar | -- | 1582 | 2023-07-06 22:20:58 | | | |
| 2 | Conner Chen | Meta information modification | 1582 | 2023-07-10 05:55:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Moshirfar, M.; Masud, M.; Harvey, D.H.; Payne, C.; Bruce, E.; Ronquillo, Y.C.; Hoopes, P.C. The Multifold Etiologies of Limbal Stem Cell Deficiency. Encyclopedia. Available online: https://encyclopedia.pub/entry/46541 (accessed on 07 February 2026).
Moshirfar M, Masud M, Harvey DH, Payne C, Bruce E, Ronquillo YC, et al. The Multifold Etiologies of Limbal Stem Cell Deficiency. Encyclopedia. Available at: https://encyclopedia.pub/entry/46541. Accessed February 07, 2026.
Moshirfar, Majid, Maliha Masud, Devon Hori Harvey, Carter Payne, Elayna Bruce, Yasmyne C. Ronquillo, Philip C. Hoopes. "The Multifold Etiologies of Limbal Stem Cell Deficiency" Encyclopedia, https://encyclopedia.pub/entry/46541 (accessed February 07, 2026).
Moshirfar, M., Masud, M., Harvey, D.H., Payne, C., Bruce, E., Ronquillo, Y.C., & Hoopes, P.C. (2023, July 06). The Multifold Etiologies of Limbal Stem Cell Deficiency. In Encyclopedia. https://encyclopedia.pub/entry/46541
Moshirfar, Majid, et al. "The Multifold Etiologies of Limbal Stem Cell Deficiency." Encyclopedia. Web. 06 July, 2023.
Copy Citation
A brief overview of Limbal Stem Cell Deficiency including normal limbal stem cell physiology, pathophysiology, incidence and prevalence, clinical presentation, diagnosis and treatment. Included is a comprehensive list of several genetic, acquired, and immunologic etiologies of the disease.
limbal stem cells
limbal stem cell deficiency
Palisades of Vogt
1. Introduction
Ocular homeostasis is maintained by several processes involving the eye’s structural layers, cell populations, and immunoregulatory responses. These processes involve the corneal stromal stem cells (CSSCs) and limbal epithelial stem cells (LESCs), each of which contributes to the regeneration of the corneal stromal layer and corneal surface, respectively [1]. Disruption to the corneal limbus, a well-defined layer of corneal stem cells between the sclera and cornea, often results in corneal epithelium irregularity and opacity, neovascularization, stromal scarring, and ulceration [2]. The likely etiology for this pathogenic process, termed limbal stem cell deficiency, includes a diverse array such as genetic, acquired, and immunologic. Etiologies of limbal stem cell deficiency have also been categorized as either LSC aplasia secondary to destruction or decreased function of LSC due to insufficient stromal support [3]. Figure 1 summarizes the many etiologies linked to LSCD.
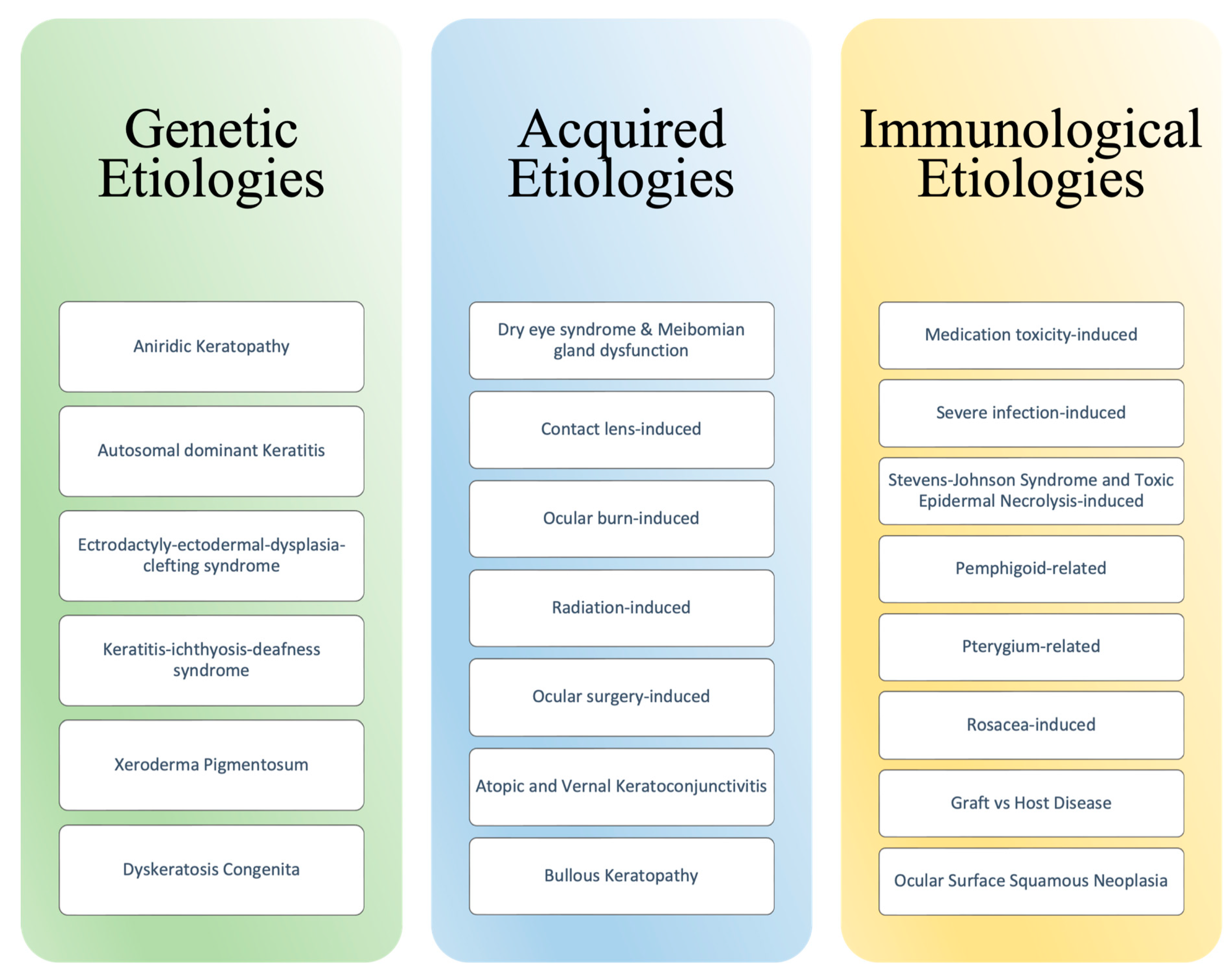
Figure 1. Etiologies of Limbal Stem Cell Deficiency.
2. Limbal Stem Cells
The cornea can be separated into five distinct layers: corneal epithelial layer, Bowman’s membrane, corneal stroma layer, Descemet’s membrane, and corneal endothelium, from superficial to deep [4]. The limbus is a highly vascular and cellular-rich layer at the interface of the scleral and corneal layers. Within the limbus, rippling and folding of the basement membrane reveal crypts of the pluripotent stem cells, termed the Palisades of Vogt [5]. These limbal epithelial crypts are concentrated in the superior and inferior limbi and contain a high density of limbal epithelial stem cells [6]. LESCs function to maintain and restore the corneal surface following physical trauma or chemical insult [7]. The proposed mechanism for cellular turnover, known as the XYZ hypothesis, suggests that limbal stem cells proliferate and differentiate to give rise to cells that migrate centripetally along the basement membrane to the basal layers of the cornea before moving superficially to replace the epithelial cells that are shed [3][8][9]. The division and migration of limbal epithelial crypts to the cornea form a barrier to prevent encroachment of the conjunctival epithelium, maintaining the avascular and transparent conditions vital to corneal homeostasis. Corneal stromal-derived mesenchymal stem cells (CS-MSCs) inhabit the basal layer of the corneal epithelium and promote stromal support via extracellular matrix deposition, the expression of anti-apoptotic transcription factors, and the reinforcement of reconstruction and corneal repair of the limbus [4]. These mesenchymal stem cells can differentiate into keratinocytes and are found adjacent to LSCs within the stromal layer [4]. Studies have shown their corneal protective actions and proximity to LSCs significantly influence the health of the LSC microenvironment. Thus, disruption of the limbus and subsequent stromal support via injury to the CS-MSCs impairs the repair and reconstruction of the cornea [4]. Following disruption to the delicate stromal layer, the corneal epithelium is displaced by conjunctival epithelial cells (conjunctivalization), leading to neovascularization, corneal opacity, and subsequent loss in visual acuity [2].
3. Pathophysiology
A deficiency of limbal epithelial stem cells occurs in two ways: first, as limbal stem cell aplasia secondary to destruction via chemical burns, contact lens use, Stevens–Johnson syndrome (SJS), microbial keratitis, multiple surgeries or procedures; and second, insufficient stromal support, or an “abnormal microenvironment”, causing the decreased function of the limbal epithelial crypts [10]. The latter is seen in conditions such as aniridia, peripheral inflammatory disorder, chronic limbitis, or neurotrophic keratopathy [3]. Classification of LSCD falls under partial and complete deficiency, depending on the amount of residual LSCs present in the stromal layer and the degree of conjunctivalization of the corneal surface. Partial LSCD is defined by the presence of residual LSCs within the stromal layer, maintaining little stromal function and partial conjunctivalization of the corneal surface. In contrast, complete LSCD is characterized by a complete lack of LSCs in the limbus and the complete conjunctivalization of the corneal surface [11]. The most reliable indicator of limbal stem cell deficiency, conjunctivalization, refers to the migration or overgrowth of the conjunctival epithelial and goblet cells on the corneal surface, resulting in opacification and vision loss [3][9]. Compromise of the avascular state of the cornea occurs with neovascularization, where the balance between pro-angiogenic and anti-angiogenic factors is disrupted, resulting in a shift towards a pro-angiogenic state [12]. Recurrent corneal erosions, ulcers, or perforation of the cornea may also be seen [2][13].
4. Incidence and Prevalence
The current literature on LSCD shows that the leading cause is ocular surface burns [14]. Global trends for LSCD show that unilateral LSCD is more common than bilateral LSCD, with the most common causes being ocular surface burns for unilateral LSCD, while allergic conjunctivitis, SJS, toxic epidermal necrolysis (TEN), aniridia, and mucous membrane pemphigoid are seen for bilateral LSCD [14]. Provided the diversity in etiology for unilateral and bilateral LSCD, specialized approaches to treatment are required [14]. Gender-specific prevalence for LSCD is not definitive, considering a lack of agreement on the disease’s definition and diagnostic criteria; however, a higher prevalence of disease in young males is documented, with a majority suffering from total LSCD (male 2:1). Significant male predominance for chemical and thermal causes and a female predominance for contact lens-associated LSCD are also reported in the literature [14]. Age-related prevalence of LSCD demonstrates that patients presenting with the disease are, on average, middle-aged (42.9 years) and range from 24 to 43 years old, without sex predominance [15].
5. Clinical Presentation
Presentation of LSCD differs according to the etiology, and symptoms are often due to poor epithelial healing, resulting in decreased vision, pain from epithelial breakdown, foreign body sensation, conjunctival redness, and tearing [2][16]. Early symptoms of LSCD include irregular corneal epithelium and changes to or loss of Palisades of Vogt [16]. Depending on the degree of the limbus and LSC destruction, termed partial and complete, patients may present asymptomatically (in the case of partial LSCD) or with severe damage to the entire corneal surface (complete), resulting in functional blindness [16]. Awareness of the following clinical signs of a possible LSCD diagnosis includes symptoms secondary to reduced corneal epithelial repair and erosions, such as chronic conjunctival redness, foreign body sensation, photophobia, tearing, discomfort/pain, and decreased visual acuity [17][18].
6. Diagnosis and Prognosis
Diagnostic tools for LSCD include patient history, impression cytology for the presence of goblet cells on the cornea, in vivo laser scanning confocal microscopy (IVCM) of the limbus, anterior segment optical coherence tomography (AS-OCT) to measure the epithelial thickness and assess corneal vasculature, and direct histological staining (H&E and Papanicolaou staining) to assess the morphology of the epithelium [2][16]. The severity of LSCD is determined using a staging model based on the extent of the corneal and limbal involvement upon examination [19]. In the first stage, only the peripheral cornea is involved. Stage two involves the peripheral cornea in addition to the central 5 mm of the cornea, and in stage three, the entire cornea is affected. The ocular examination includes whether the visual axis, central 5 mm of the cornea, is involved (stages II and III) and whether greater than 50% of the LSC are intact [19]. Suitable treatment plans can be made, provided the diagnosis and staging are precise in determining the amount of residual LSCs remaining. Studies show that host LSCs had reconstructed injured corneal epithelium following allogeneic LSC transplantation [2]. However, no definitive prognosis exists for LSCD, given the different etiologies present [16].
7. Treatment Overview
Management of LSCD primarily follows a stepwise approach, focused on addressing the standard presentations seen in the disease and employing less invasive strategies first. Treatment starts with the discontinuation or limitation of the offending agent (e.g., contact lenses, medication, irritant exposures); next is the administration of corticosteroids for ocular surface inflammation, and thereafter, support to the residual limbal stem cells is offered via preservative-free lubricants and amniotic membrane transplants, and in severe cases, the restoration of stem cell reserves via a limbal stem cell transplant and penetrating keratoplasty is attempted [20]. LSC transplantations can be autologous from the fellow eye or allogenic from a donor. The graft may be directly transplanted in a single-stage procedure, or cells may be cultivated in a lab to be expanded and then transplanted at a later date in a two-stage procedure [21]. Furthermore, grafts may be obtained from various tissues, including a conjunctival limbal graft, keratolimbal graft, and simple limbal epithelial graft [21]. The specific details for which graft and surgery to implement will be indicated by the underlying etiology and stage of progression in each patient. Investigations on LSC transplantation and avenues of improvement are ongoing. Masood et al. described several therapeutic strategies to improve LSCD interventions, including the use of non-limbal stem cells to potentially restore LSC function. They described the potential to reconstitute mature corneal epithelial cells into LSC-like cells for transplantation via autologous cultivation [22]. An area of recent inquiry is the use of simple LSC transplantation vs. cultivated LSC transplantation. Both have been shown to have similar clinical efficacy [23]. However, Thokala et al. propose that simple LSC transplantation is superior and will be more common in the future compared to cultivated LSC transplantation due to the difficulties that accompany tissue cultures, including facilities, commercial cell-culture services, and the costs to maintain and expand cultures [24]. Despite the difficulties in cell cultivation, Jurkunas et al. have developed a novel and consistent manufacturing process for cultivated LSC transplantation, which may prove beneficial in the culture process [25]. In addition to the standard of care, therapies that specifically address the cause of the LSCD can be added to the management plan to further promote resolution. Etiologies and their additional therapies are discussed below.
References
- Nurkovic, J.S.; Vojinovic, R.; Dolicanin, Z. Corneal Stem Cells as a Source of Regenerative Cell-Based Therapy. Stem Cells Int. 2020, 2020, 8813447.
- Le, Q.; Xu, J.; Deng, S.X. The diagnosis of limbal stem cell deficiency. Ocul. Surf. 2018, 16, 58–69.
- Puangsricharern, V.; Tseng, S.C.G. Cytologlogic Evidence of Corneal Diseases with Limbal Stem Cell Deficiency. Ophthalmology 1995, 102, 1476–1485.
- Liu, X.N.; Mi, S.L.; Chen, Y.; Wang, Y. Corneal stromal mesenchymal stem cells: Reconstructing a bioactive cornea and repairing the corneal limbus and stromal microenvironment. Int. J. Ophthalmol. 2021, 14, 448–455.
- Funderburgh, J.L.; Funderburgh, M.L.; Du, Y. Stem Cells in the Limbal Stroma. Ocul. Surf. 2016, 14, 113–120.
- Gonzalez, G.; Sasamoto, Y.; Ksander, B.R.; Frank, M.H.; Frank, N.Y. Limbal stem cells: Identity, developmental origin, and therapeutic potential. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e303.
- Altshuler, A.; Amitai-Lange, A.; Tarazi, N.; Dey, S.; Strinkovsky, L.; Hadad-Porat, S.; Bhattacharya, S.; Nasser, W.; Imeri, J.; Ben-David, G.; et al. Discrete limbal epithelial stem cell populations mediate corneal homeostasis and wound healing. Cell Stem Cell 2021, 28, 1248–1261.e8.
- Pajoohesh-Ganji, A.; Pal-Ghosh, S.; Tadvalkar, G.; Stepp, M.A. Corneal goblet cells and their niche: Implications for corneal stem cell deficiency. Stem Cells 2012, 30, 2032–2043.
- Ramos, T.; Scott, D.; Ahmad, S. An Update on Ocular Surface Epithelial Stem Cells: Cornea and Conjunctiva. Stem Cells Int. 2015, 2015, 601731.
- Ahmad, S. Concise Review: Limbal Stem Cell Deficiency, Dysfunction, and Distress. Stem Cells Transl. Med. 2012, 1, 110–115.
- Shanbhag, S.S.; Chanda, S.; Donthineni, P.R.; Basu, S. Surgical Management of Unilateral Partial Limbal Stem Cell Deficiency: Conjunctival Autografts versus Simple Limbal Epithelial Transplantation. Clin. Ophthalmol. 2021, 15, 4389–4397.
- Lim, P.; Fuchsluger, T.A.; Jurkunas, U.V. Limbal stem cell deficiency and corneal neovascularization. Semin. Ophthalmol. 2009, 24, 139–148.
- Tseng, S.C. Concept and application of limbal stem cells. Eye 1989, 3, 141–157.
- Vazirani, J.; Nair, D.; Shanbhag, S.; Wurity, S.; Ranjan, A.; Sangwan, V. Limbal Stem Cell Deficiency-Demography and Underlying Causes. Am. J. Ophthalmol. 2018, 188, 99–103.
- Cheung, A.Y.; Sarnicola, E.; Denny, M.R.; Sepsakos, L.; Auteri, N.J.; Holland, E.J. Limbal Stem Cell Deficiency: Demographics and Clinical Characteristics of a Large Retrospective Series at a Single Tertiary Referral Center. Cornea 2021, 40, 1521–1531.
- Bonnet, C.; Roberts, J.S.; Deng, S.X. Limbal stem cell diseases. Exp. Eye Res. 2021, 205, 108437.
- Lee, S.C.; Hyon, J.Y.; Jeon, H.S. Contact Lens Induced Limbal Stem Cell Deficiency: Clinical Features in Korean Patients. Korean J. Ophthalmol. 2019, 33, 500–505.
- Osei-Bempong, C.; Figueiredo, F.C.; Lako, M. The limbal epithelium of the eye–A review of limbal stem cell biology, disease and treatment. BioEssays 2013, 35, 211–219.
- Deng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea 2019, 38, 364–375.
- Kate, A.; Basu, S. A Review of the Diagnosis and Treatment of Limbal Stem Cell Deficiency. Front. Med. 2022, 9, 836009.
- Le, Q.; Chauhan, T.; Yung, M.; Tseng, C.H.; Deng, S.X. Outcomes of Limbal Stem Cell Transplant. JAMA Ophthalmol. 2020, 138, 660.
- Masood, F.; Chang, J.-H.; Akbar, A.; Song, A.; Hu, W.-Y.; Azar, D.T.; Rosenblatt, M.I. Therapeutic Strategies for Restoring Perturbed Corneal Epithelial Homeostasis in Limbal Stem Cell Deficiency: Current Trends and Future Directions. Cells 2022, 11, 3247.
- Gupta, N.; Joshi, J.; Farooqui, J.; Mathur, U. Results of simple limbal epithelial transplantation in unilateral ocular surface burn. Indian J. Ophthalmol. 2018, 66, 45.
- Thokala, P.; Singh, A.; Singh, V.K.; Rathi, V.M.; Basu, S.; Singh, V.; MacNeil, S.; Sangwan, V.S. Economic, clinical and social impact of simple limbal epithelial transplantation for limbal stem cell deficiency. Br. J. Ophthalmol. 2022, 106, 923–928.
- Jurkunas, U.; Johns, L.; Armant, M. Cultivated Autologous Limbal Epithelial Cell Transplantation: New Frontier in the Treatment of Limbal Stem Cell Deficiency. Am. J. Ophthalmol. 2022, 239, 244–268.
More
Information
Subjects:
Ophthalmology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
517
Revisions:
2 times
(View History)
Update Date:
10 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




