Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Catarina Rodrigues Trindade | -- | 2936 | 2023-07-05 20:14:24 | | | |
| 2 | Catherine Yang | Meta information modification | 2936 | 2023-07-06 02:47:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Quelhas, A.R.; Trindade, A.C. Natural-Colored Photonic Structures with Cellulose-Based Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/46480 (accessed on 08 February 2026).
Quelhas AR, Trindade AC. Natural-Colored Photonic Structures with Cellulose-Based Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/46480. Accessed February 08, 2026.
Quelhas, Ana Rita, Ana Catarina Trindade. "Natural-Colored Photonic Structures with Cellulose-Based Materials" Encyclopedia, https://encyclopedia.pub/entry/46480 (accessed February 08, 2026).
Quelhas, A.R., & Trindade, A.C. (2023, July 05). Natural-Colored Photonic Structures with Cellulose-Based Materials. In Encyclopedia. https://encyclopedia.pub/entry/46480
Quelhas, Ana Rita and Ana Catarina Trindade. "Natural-Colored Photonic Structures with Cellulose-Based Materials." Encyclopedia. Web. 05 July, 2023.
Copy Citation
Structural coloration has become a fascinating field of research, inspiring scientists and engineers to explore the vibrant colors observed in nature and develop bio-inspired photonic structures for various applications. Cellulose-based materials derived from plant fibers offer a promising platform for mimicking natural photonic structures. Their abundance, renewability, and versatility in form and structure make them ideal for engineering specific optical properties.
cellulose nanocrystals (CNCs)
structurally colored CNC films
photonic properties
1. Photonic Structures in Nature
Structural coloration, observed in a wide range of organisms including animals, plants, and fruits, serves vital ecological functions. It plays a key role in attracting pollinators, signaling ripeness, deterring herbivores, gaining a competitive advantage, and manipulating light. This remarkable adaptation enhances reproductive success, species survival, and ecological interactions within diverse ecosystems. The phenomenon of structural coloration in natural systema has garnered significant attention from researchers and engineers in recent times, due to the captivating display of vibrant colors observed and the potential applications of bio-inspired functional photonic structures and materials. Numerous studies have been conducted to uncover and replicate the physical mechanisms responsible for the natural occurrence of structural colors in plants, fruits and animals [1][2][3][4] and revealed the self-assembling structural color in Nature [2][3].
Structural coloration in plants serves important functions related to their ecological interactions, and demonstrates their intricate interaction with their environment. From attracting pollinators to deterring herbivores and manipulating light, these visual cues contribute to the plant’s reproductive success, competitive advantage, and overall survival in their environment. The presence of vibrant and visually striking hues plays a crucial role in attracting pollinators, ensuring successful reproduction. Flowers employ structural colors, such as vivid petals and intricate patterns, as visual cues to signal the availability of nectar or pollen rewards, thus enticing pollinators like bees, butterflies, and birds. In 2009, Whitney et al. made a groundbreaking discovery regarding iridescence in Hibiscus trionum and revealed that the iridescence exhibited by this plant is a result of regular nanoscale patterns, such as striations or wrinkles, that are formed on the cuticle covering the flat epidermis of the petal’s surface [5][6]. These patterns act as diffraction gratings, leading to diffractive optical effects [5][7][8]. A similar iridescence phenomenon was observed in the H. trionum tulip species, where periodic striations are present on top of the purple-pigmented epidermis of the petal [9]. Conversely, the SEM image of the side of the tulip petal reveals an unorganized structure and lack of iridescence [10].
Whitney et al. emphasized the significance of these patterns in petals for biological purposes. They discovered that the iridescent signals produced by the H. trionum flower, through its diffraction gratings, allow it to interact with its main pollinators, particularly bumblebeesn [5]. Remarkably, the researchers successfully trained bumblebees to distinguish between replicas of iridescent H. trionum petals and identical non-iridescent replicas with smooth surfaces [5]. This is in accordance with the demonstration of Kevan and Lane that the microtextures present on the surface of flower petals serve as tactile cues for bees during pollination. They found that honeybees can differentiate between petals with distinct textures and detect variations in textures within petals of the same species [11].
The optical properties of the flower petals depend on the shape of the epithelial cells. Kourounioti et al. discovered that in order to generate iridescence through diffraction gratings, the epithelial cells must be planar and exhibit regular striations within the petal cuticle, with appropriate spacing between them. These striations or wrinkles can be either parallel or perpendicular to the long axis of the cells. Such variations in orientation can be observed within the same plant species and across different plant species. For example, the striations in H. trionum and Kalanchoe blossfeldiana were found to be parallel, while those in Yunnan rhododendron, Ursinia calendulifolia, and daisy were found to be perpendicular to the long axis of the cells [5][10][12].
Additionally, structural coloration in fruits serves as a signaling mechanism for ripeness. As fruits mature, they develop distinct hues that catch the attention of fruit-eating animals, indicating their readiness for consumption. This coloration facilitates seed dispersal, as animals consume the fruit and spread the enclosed seeds to different locations, contributing to the plant’s reproductive success. As structural colors do not fade, this bright coloration on fruits is maintained after the fruit is picked or has fallen from the plant, increasing its probability of being further dispersed [13][14][15].
Animals possess structural coloration for various purposes, including communication, camouflage, mate attraction, warning signals, and thermoregulation. Structural coloration serves as visual signals for species recognition, social interactions, and reproductive success. It aids in camouflage by blending with the environment or enhancing hunting abilities. In mate attraction, vibrant colors and patterns play a role in sexual selection. Some animals use bright colors as warning signals to deter predators, while structural coloration also assists in thermoregulation. These adaptations contribute to the survival, reproduction, and ecological interactions of animals. Similarly to plants and fruits, the mechanism behind structural colors in animals is based on diffraction and specular reflection and on nano and microscopic-scale patterns [16][17][18][19][20][21]. The majority of the incoming light travels through the biopolymeric formations largely unhindered; nonetheless, a specific set of wavelengths (those with specific ratios to the size of the structure’s periodic patterns) are selectively bounced back and generate angle-dependent iridescent shades found in various insects, birds, and marine creatures. Common examples of structural colors that appears in animals are peacock feathers [22][23][24][25], colorful birds [26][27][28][29], butterfly wings [30][31][32] and beetle exoskeletons [20][33][34][35][36][37].
These natural structures, such as the iridescent colors found in flowers and animals, are, as known, often created through mechanisms of scatter, diffraction, polarization and interference of light interacting with periodic micro- and nano-scale structures in the materials (Figure 1).
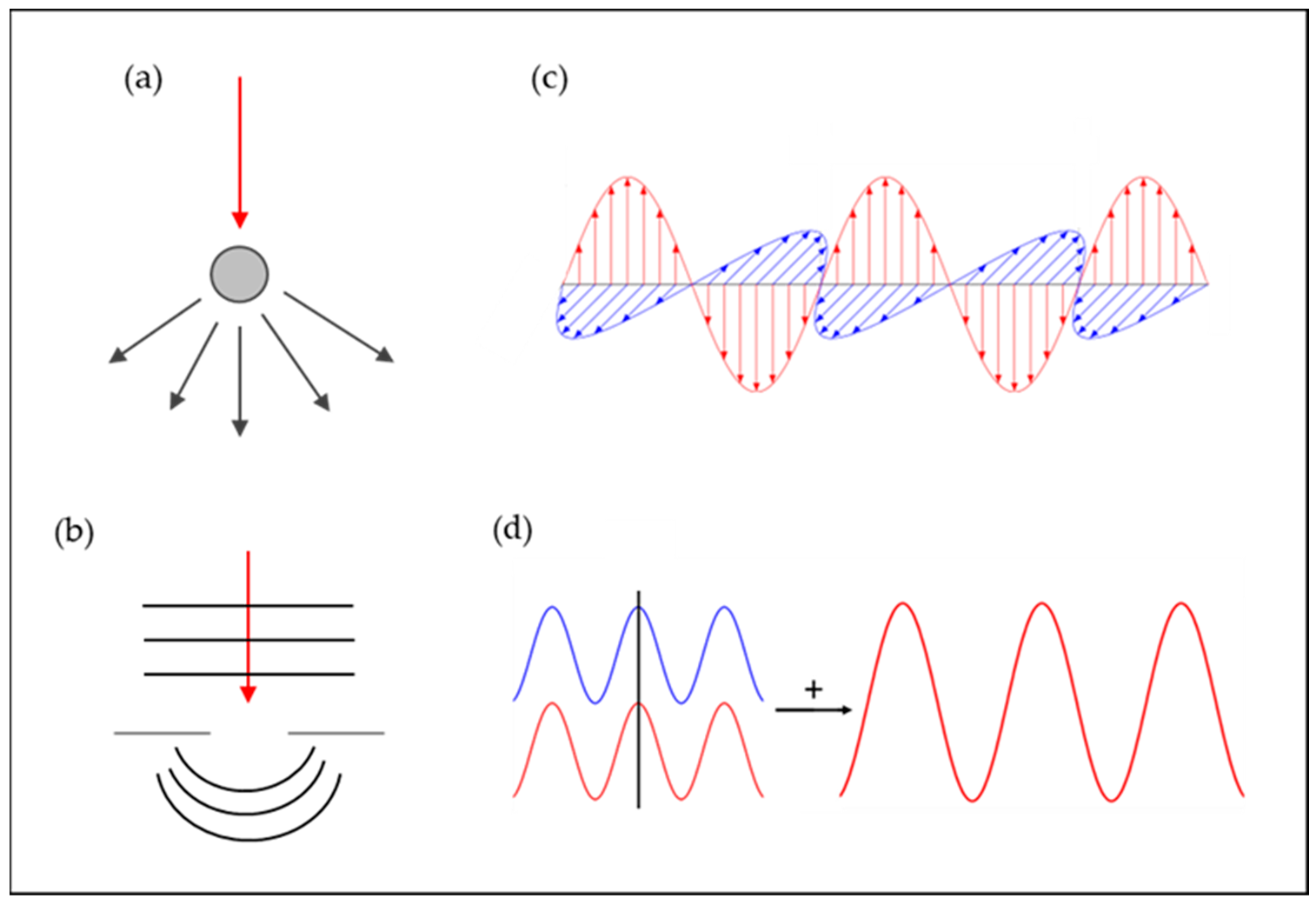
Figure 1. Illustration of examples of optical mechanisms of color: (a) scatter (adapted from [38]); (b) diffraction (adapted from [39]); (c) polarization (adapted from [40]); and (d) interference (adapted from [41]).
2. Photonic Structures in Cellulose-Based Materials
Natural structural colors serve as fascinating examples of nature’s ability to achieve functionality by shaping and molding inherently non-functional compounds into precisely defined structures [4][16]. Similar principles of creating functionality through structural design have become fundamental in the field of nanomaterials and nanotechnology over the past 40 years [42]. The resulting materials, often referred to as metamaterials, consist of conventional substances such as metals, semiconductors, ceramics, or polymers, yet they exhibit unconventional properties due to their nanostructures. For instance, by synthesizing semiconductors in the form of nanometer-sized spheres or rods, their electronic and optical properties can be dramatically altered. Similarly, transforming a transparent ceramic into a colored coating can be achieved by structuring it with a three-dimensional periodic architecture featuring a lattice spacing of a few hundred nanometers. Alternatively, a polymer film can be converted into a super-hydrophobic, self-cleaning surface by introducing hierarchically organized nano-to-micrometer-sized protrusions and filaments.
In nature, the most brilliant example of blue coloration stems from a helical arrangement of cellulose fibers in tropical fruits such as Pollia condensata and Margaritaria nobilis [15][43][44][45]. Mimicking these natural-colored photonic structures and getting inspiration for material design and sustainable processing using natural materials is an interesting area of research [46] and cellulosic materials have gained considerable prominence due to their renewable nature, compatibility with living organisms, and ability to degrade naturally, making them an eco-conscious alternative [47].
Revol et al. made a surprising finding when they stumbled upon the fact that a solid film could retain the chiral nematic liquid crystalline arrangement observed in a suspension of cellulose nanocrystals [48][49]. The researchers accomplished this by evaporating the water from a cellulose nanocrystal suspension with a concentration of approximately 3.5% by weight, resulting in a film with preserved chiral nematic order. Upon examination using polarized optical microscopy (POM), they observed that the films exhibited birefringence, and their structures were susceptible to disruption by shear forces. Films made from CNCs have unique optical properties, including iridescence and the ability to selectively reflect left circularly polarized light while transmitting right circularly polarized light [50][51][52]. Similar to a suspension of cellulose nanocrystals, the chiral nematic orientation within the solid films yielded a positive signal when subjected to CD spectroscopy [53]. The helical twist of the cellulose nanocrystals consistently displayed a left-handed nature, as confirmed by the positive CD signal for transmitted light and the generation of left-handed circularly polarized light upon reflection.
Films formed through the self-assembly of cellulose nanocrystals often exhibit captivating colors due to the helical arrangements of cholesteric liquid crystals. The orientation and pitch of these structures determine the photonic bandgap, with the ability to modify the pitch allowing for alterations in perceived color. When the helix’s pitch approximated the wavelengths of visible light, the CNC films exhibited striking iridescent structural colors. These chiral nematic CNC films can be considered as one-dimensional photonic crystals and this manipulation relies on Bragg-like reflections that generate various colors [54][55][56][57][58].
The precise optical properties that can be developed in CNC films depends on many parameters, which leads to the development of a variety of tunable photonic CNC materials and technologies. The effects of water evaporation (evaporation at different relative humidity) and initial CNC concentration have been investigated by several groups [52][54][59] and it was possible to obtain CNC films covering most the visible spectrum [60]. Later, Tran et al. [52][61] modified the evaporation time of CNC suspensions and obtained CNC films in the suspensions´ slow evaporation resulted in blue-shifted films. Utilizing differential evaporation, CNC films with gradients could be designed. They also discovered that the application of a cellulose acetate mask on top of a drying CNC suspension led to patterns with higher resolutions [52]. They also showed that the obtained colored patterns could be tuned, from red to blue, depending on the stage of self-assembly when the masks were applied.
Despite their capacity to generate films with vibrant colors, cellulose nanocrystals (CNCs) possess certain constraints that result in limited productivity. The process of self-assembly is highly susceptible to disruptions and may necessitate an extended period of several days for the complete evaporation of water. Chen et al. [62] developed a protocol consisted in a preliminary treatment of CNC suspensions through ultrasonication. They discovered that the duration of ultrasonication, volume of the suspension, and the application of vacuum were determinant in the preparation of iridescent CNC films. The resulting films exhibit striking and vibrant colors, when compared with those obtained with slower water evaporation techniques. Another influent parameter that was investigated was the surface upon which the CNC suspension is cast on [63][64][65]. Several surfaces were tested (including aluminum, silicon wafers, mica and polystyrene) and different optical properties were obtained, meaning that substrate surface properties, such as wettability and hydrophobicity, influence the self-assembly behavior of the CNCs.
Not only altering CNC suspensions can have impact on the characteristics of chiral nematic CNC films. Also, external factors, including temperature and additives, can have influence on the properties of CNC films. The introduction of energy through methods such as heating or sonication gave rise to CNC films with an enhanced helical pitch, allowing the production of films with adjustable chiral photonic properties [66][67][68][69][70].
As previously mentioned, cellulose possesses attractive qualities for optical and photonic applications, thanks to its refractive index, transparency, dielectric properties, and birefringence [71][72][73]. These combined characteristics enable the development of relevant technologies in the field of photonics. The crystal structure of cellulose plays a crucial role in modulating its optical properties, resulting in vibrant colors and establishing it as a valuable contender for sustainable bio-based optical materials. The self-assembly of cellulose nanocrystals offers a promising and cost-effective approach to producing optical materials on a large scale [74]. Given that sustainability and the circular economy are crucial concerns today, several scientists are exploring the potential use of cellulose derived from biomass and waste materials in photonic and its capacity to form chiral nematic structures upon drying, which exhibit fascinating optical and photonic properties [75][76][77][78][79]. Recent investigations in this field have primarily concentrated on understanding the self-assembly dynamics of helicoidal structures and optimizing them to achieve desired polarization responses.
Cellulose nanocrystals in water suspensions behave as lyotropic liquid crystals forming a chiral nematic phase above a critical concentration. It is well known that such an organization can be retained in solid films and give rise to an intense colored appearance. In several studies, researchers have characterized their optical response via optical and scanning electron microscopy, imaging scatterometry, and angle-resolved reflectance measurements [80][81][82]. Wilts et al. go further by showing that the experimental results can be well explained by computational modeling using the finite-difference time-domain method [82]. They performed 3-D finite-difference time-domain (FDTD) calculations, using a commercial-grade Maxwell equation solver, Lumerical FDTD Solutions 8.16, to simulate the polarization-dependent light scattering from a liquid-crystalline, helicoidal stack of cellulose in the wavelength range 350–700 nm. They examined the variation in reflectance with changing angles of a cellulose film formed through self-assembly and their findings demonstrate that the significant disparity in circular polarization is maintained across a wide range of incident light angles. This reflectance behavior can be effectively explained through the use of finite-difference time-domain modeling. Previous research has indicated that the color characteristics of these films can be manipulated by adjusting the self-assembly conditions of CNCs. Together, these studies contribute to a more holistic understanding of the angle-dependent color appearance in helicoidal layers, which holds potential for the development of sustainable colored materials, such as responsive dyes or food colorants.
3. Cellulose-Based Composite Materials with Structural Color
As mentioned before, the self-assembly of CNCs into a chiral nematic structure is tolerant to additives, which has allowed incorporation of additives and enabled the creation of a range of interesting materials, such as thin-films [83][84], hydrogels [85][86][87][88][89][90], and organosilicas [91][92][93]. Incorporating additives provides a means to fine-tune the optical and mechanical properties of the resulting CNC-based materials. For instance, pure CNC thin films are known for their toughness but lack flexibility, besides by introducing hydroxypropyl cellulose (HPC) or chitosan/chitin into the matrix, the cellulose-based composite material’s flexibility can be significantly improved [94][95][96][97][98][99][100]. Previous research in this field has also explored the incorporation of inorganic materials like metallic nanoparticles [101][102][103][104], infiltration of proteins or amino acids [105][106][107][108] or the addition of organic units through careful surface modification [95][109], resulting in materials that exhibit unique chiroptical properties and are capable of changing color under applied pressure [94][96][97][110][111][112][113][114][115].
The optical properties of films are also dependent on surface roughness, as no surface of a biobased material is completely flat and, as shown previously, its surface roughness will directly impact its interaction with light [116]. In fact, surface patterning and successful production of highly precise structures in a predeterminate configuration can be employed to create light interactive-structures, where nanoscale and microscale patterns are generated to control diffraction, scattering, or light outcoupling. Various lithographic techniques, involving similar steps but differ in processing and curing specifics, can be applied. Initially, the material to be modified is uniformly distributed across a surface, followed by the application of a mask or mold, and finally, through chemical and/or physical treatments, the modified surface is obtained [117][118]. Several protocols process of obtaining modified surfaces on structural colored cellulosic-based materials using photolithography [116][119], soft lithography [120][121][122], and nanoimprint lithography [123][124][125][126] were documented.
Wolfberger et al. [119] described an easy and versatile efficient patterning method for cellulose thin films by means of photolithography and enzymatic digestion. Depending on the conditions of development, either negative and positive type cellulose structures can be obtained, offering lateral resolutions down to the single-digit micro meter range by means of contact photolithography. These photochemically structured cellulose thin films are successfully implemented as dielectric layers in prototype organic thin film transistors.
The research findings of Mihi et al. [120] introduced a groundbreaking approach to fabricating photonic crystals and plasmonic structures using a derivative of cellulose through the nanostructuring method known as soft lithography. Through the periodic nanostructuring of the cellulose film, its transparency is effectively eliminated, leading to the emergence of vibrant colors in its reflective properties, contingent upon the specific pattern employed during the molding process. By leveraging this innovative technique, which is both highly scalable and cost-effective, as an alternative to the conventional self-assembly of cellulose nanocrystals, a superior nanostructure is rapidly and reproducibly generated on the polymer substrate [127]. This process offers a wide spectrum of iridescent colors solely reliant on the size and morphology of the resultant structures. The resulting photonic crystals can be nanoimprinted onto diverse substrates to confer photonic capabilities on surfaces lacking this characteristic, such as paper. This technology exhibits immense potential as photonic ink and finds practical applications in domains like anti-counterfeiting technology, packaging, decorative paper, labels, and sensors, among others [116]. When these structures are coated with a thin layer of metal, they acquire plasmonic properties while retaining their flexibility, thereby intensifying the colors displayed. Additionally, the biodegradability and water solubility of the cellulose derivative can be adjusted based on the specific type employed. These plasmonic structures are ideal for disposable sensors, enabling Raman emission, or enhancing the light emitted by a dye [120].
In industrial settings, thermal imprinting has been implemented using roll-to-roll processing, which can also be utilized in thermal nanoimprinting lithography. Mäkelä et al. conducted experiments using a laboratory-scale roll-to-roll imprinting system to create cellulose acetate films, CNF films, and TEMPO-CNF films with pillar structures imprinted using a Ni-mold [123][124][126]. The formation of these structures was heavily influenced by the temperature, speed, and pressure applied during the process. The resulting films exhibited varying levels of surface roughness, leading to different levels of transparency. When white light passed through the microstructures, diffraction colors such as blue, red, and green were observed, showcasing the potential for applications in optics and electronics [123][124][125].
Considering the cost of production and application in lithography, high-throughput techniques like soft lithography and roll-to-roll lithography tend to be more cost-effective, while photo and e-beam lithography are often more expensive due to their limited scalability [128]. However, it should be noted that different surface patterning methods present varying restrictions on the achievable feature size of the fabricated surface structures.
References
- Fu, Y.; Tippets, C.A.; Donev, E.U.; Lopez, R. Structural Colors: From Natural to Artificial Systems. WIREs Nanomed. Nanobiotechnol. 2016, 8, 758–775.
- Burg, S.L.; Parnell, A.J. Self-Assembling Structural Colour in Nature. J. Phys. Condens. Matter 2018, 30, 413001.
- Datta, B.; Spero, E.F.; Martin-Martinez, F.J.; Ortiz, C. Socially-Directed Development of Materials for Structural Color. Adv. Mater. 2022, 34, 2100939.
- Tan, A.; Ahmad, Z.; Vukusic, P.; Cabral, J.T. Multifaceted Structurally Coloured Materials: Diffraction and Total Internal Reflection (TIR) from Nanoscale Surface Wrinkling. Molecules 2023, 28, 1710.
- Whitney, H.M.; Kolle, M.; Andrew, P.; Chittka, L.; Steiner, U.; Glover, B.J. Floral Iridescence, Produced by Diffractive Optics, Acts As a Cue for Animal Pollinators. Science 2009, 323, 130–133.
- Barthlott, W.; Mail, M.; Bhushan, B.; Koch, K. Plant Surfaces: Structures and Functions for Biomimetic Innovations. Nanomicro Lett. 2017, 9, 23.
- de Premorel, G.; Giurfa, M.; Andraud, C.; Gomez, D. Higher Iridescent-to-Pigment Optical Effect in Flowers Facilitates Learning, Memory and Generalization in Foraging Bumblebees. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171097.
- Gegear, R.J.; Burns, J.G. The Birds, the Bees, and the Virtual Flowers: Can Pollinator Behavior Drive Ecological Speciation in Flowering Plants? Am. Nat. 2007, 170, 551–566.
- Vignolini, S.; Moyroud, E.; Glover, B.J.; Steiner, U. Analysing Photonic Structures in Plants. J. R. Soc. Interface 2013, 10, 20130394.
- Antoniou Kourounioti, R.L.; Band, L.R.; Fozard, J.A.; Hampstead, A.; Lovrics, A.; Moyroud, E.; Vignolini, S.; King, J.R.; Jensen, O.E.; Glover, B.J. Buckling as an Origin of Ordered Cuticular Patterns in Flower Petals. J. R. Soc. Interface 2013, 10, 20120847.
- Kevan, P.G.; Lane, M.A. Flower Petal Microtexture Is a Tactile Cue for Bees. Proc. Natl. Acad. Sci. USA 1985, 82, 4750–4752.
- Huang, X.; Hai, Y.; Xie, W.-H. Anisotropic Cell Growth-Regulated Surface Micropatterns in Flower Petals. Theor. Appl. Mech. Lett. 2017, 7, 169–174.
- Vukusic, P. Evolutionary Photonics with a Twist. Science 2009, 325, 398–399.
- Chang, Y.; Middleton, R.; Ogawa, Y.; Gregory, T.; Steiner, L.M.; Kovalev, A.; Karanja, R.H.N.; Rudall, P.J.; Glover, B.J.; Gorb, S.N. Cell Wall Composition Determines Handedness Reversal in Helicoidal Cellulose Architectures of Pollia condensata Fruits. Proc. Natl. Acad. Sci. USA 2021, 118.
- Vignolini, S.; Rudall, P.J.; Rowland, A.V.; Reed, A.; Moyroud, E.; Faden, R.B.; Baumberg, J.J.; Glover, B.J.; Steiner, U. Pointillist Structural Color in Pollia Fruit. Proc. Natl. Acad. Sci. USA 2012, 109, 15712–15715.
- Tan, Y.; Hu, B.; Song, J.; Chu, Z.; Wu, W. Bioinspired Multiscale Wrinkling Patterns on Curved Substrates: An Overview. Nanomicro Lett. 2020, 12, 101.
- Mason, C.W. Structural Colors in Insects. I. J. Phys. Chem. 1926, 30, 383–395.
- Mason, C.W. Structural Colors in Insects. II. J. Phys. Chem. 1927, 31, 321–354.
- Jacucci, G.; Vignolini, S.; Schertel, L. The Limitations of Extending Nature’s Color Palette in Correlated, Disordered Systems. Proc. Natl. Acad. Sci. USA 2020, 117, 23345–23349.
- Roberts, N.W.; Marshall, N.J.; Cronin, T.W. High Levels of Reflectivity and Pointillist Structural Color in Fish, Cephalopods, and Beetles. Proc. Natl. Acad. Sci. USA 2012, 109.
- Fan, X.; Zheng, X.; An, T.; Li, X.; Leung, N.; Zhu, B.; Sui, T.; Shi, N.; Fan, T.; Zhao, Q. Light Diffraction by Sarcomeres Produces Iridescence in Transmission in the Transparent Ghost Catfish. Proc. Natl. Acad. Sci. USA 2023, 120, e2219300120.
- Medina, J.M.; Díaz, J.A.; Vukusic, P. Classification of Peacock Feather Reflectance Using Principal Component Analysis Similarity Factors from Multispectral Imaging Data. Opt. Express 2015, 23, 10198.
- Okazaki, T. Ultraviolet Reflectance Structures of Peacock Feathers. Zoolog. Sci. 2018, 35, 421–426.
- Eliason, C.M.; Shawkey, M.D. Rapid, Reversible Response of Iridescent Feather Color to Ambient Humidity. Opt. Express 2010, 18, 21284.
- Wang, Y.; Ren, Y.; Wang, Z.; Xu, Q.; Zhang, L. Study on the Microstructure and Its Coloration Mechanism of Peacock Feather by the FDTD Method. J. Phys. Conf. Ser. 2020, 1549, 032036.
- Okazaki, T. Structural Color Expression Due to Specular Reflection from Bird Feathers. FORMA 2022, 37, 5–12.
- Liao, S.-F.; Yao, C.-Y.; Lee, C.-C. Measuring and Modeling the Inconspicuous Iridescence of Formosan Blue Magpie’s Feather (Urocissacaerulea). Appl. Opt. 2015, 54, 4979.
- Jeon, D.-J.; Ji, S.; Lee, E.; Kang, J.; Kim, J.; D’Alba, L.; Manceau, M.; Shawkey, M.D.; Yeo, J.-S. How Keratin Cortex Thickness Affects Iridescent Feather Colours. R. Soc. Open. Sci. 2023, 10.
- Doucet, S.M.; Shawkey, M.D.; Hill, G.E.; Montgomerie, R. Iridescent Plumage in Satin Bowerbirds: Structure, Mechanisms and Nanostructural Predictors of Individual Variation in Colour. J. Exp. Biol. 2006, 209, 380–390.
- Kertész, K.; Bálint, Z.; Piszter, G.; Horváth, Z.E.; Biró, L.P. Multi-Instrumental Techniques for Evaluating Butterfly Structural Colors: A Case Study on Polyommatus Bellargus (Rottemburg, 1775) (Lepidoptera: Lycaenidae: Polyommatinae). Arthropod. Struct. Dev. 2021, 61, 101010.
- Ghiradella, H. Light and Color on the Wing: Structural Colors in Butterflies and Moths. Appl. Opt. 1991, 30, 3492.
- Kinoshita, S.; Yoshioka, S.; Kawagoe, K. Mechanisms of Structural Colour in the Morpho Butterfly: Cooperation of Regularity and Irregularity in an Iridescent Scale. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 1417–1421.
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Cuticle Formation and Pigmentation in Beetles. Curr. Opin. Insect Sci. 2016, 17, 1–9.
- Barrows, F.P.; Bartl, M.H. Photonic Structures in Biology: A Possible Blueprint for Nanotechnology. Nanomater. Nanotechnol. 2014, 4, 1.
- Scalet, J.M.; Sprouse, P.A.; Schroeder, J.D.; Dittmer, N.; Kramer, K.J.; Kanost, M.R.; Gehrke, S.H. Temporal Changes in the Physical and Mechanical Properties of Beetle Elytra during Maturation. Acta Biomater. 2022, 151, 457–467.
- Vincent, J.F.V.; Wegst, U.G.K. Design and Mechanical Properties of Insect Cuticle. Arthropod. Struct. Dev. 2004, 33, 187–199.
- Hernández-Jiménez, M.; Azofeifa, D.E.; Libby, E.; Barboza-Aguilar, C.; Solís, Á.; Arce-Marenco, L.; García-Aguilar, I.; Hernández, A.; Vargas, W.E. Qualitative Correlation between Structural Chirality through the Cuticle of Chrysina Aurigans Scarabs and Left-Handed Circular Polarization of the Reflected Light. Opt. Mater. Express 2014, 4, 2632.
- Available online: https://en.wikipedia.org/wiki/Compton_scattering (accessed on 26 May 2023).
- Available online: https://bmet.fandom.com/wiki/Diffraction (accessed on 26 May 2023).
- Available online: https://en.wikipedia.org/wiki/Polarization_%28physics%29 (accessed on 26 May 2023).
- Available online: https://www.e-education.psu.edu/mcl-optpro/book/export/html/858 (accessed on 26 May 2023).
- Malshe, A.; Bapat, S.; Rajurkar, K.; Melkote, S. Biological Strategies from Natural Structures for Resilience in Manufacturing. CIRP J. Manuf. Sci. Technol. 2021, 34, 146–156.
- McDougal, A.; Miller, B.; Singh, M.; Kolle, M. Biological Growth and Synthetic Fabrication of Structurally Colored Materials. J. Opt. 2019, 21, 073001.
- Shatkin, J.A.; Wegner, T.H.; Bilek, E.M.; Cowie, J. Market Projections of Cellulose Nanomaterial-Enabled Products—Part 1: Applications. TAPPI J. 2014, 13, 9–16.
- Vignolini, S.; Gregory, T.; Kolle, M.; Lethbridge, A.; Moyroud, E.; Steiner, U.; Glover, B.J.; Vukusic, P.; Rudall, P.J. Structural Colour from Helicoidal Cell-Wall Architecture in Fruits of Margaritaria nobilis. J. R. Soc. Interface 2016, 13, 20160645.
- Parker, R.M.; Guidetti, G.; Williams, C.A.; Zhao, T.; Narkevicius, A.; Vignolini, S.; Frka-Petesic, B. The Self-Assembly of Cellulose Nanocrystals: Hierarchical Design of Visual Appearance. Adv. Mater. 2018, 30, 1704477.
- Tan, K.; Heo, S.; Foo, M.; Chew, I.M.; Yoo, C. An Insight into Nanocellulose as Soft Condensed Matter: Challenge and Future Prospective toward Environmental Sustainability. Sci. Total Environ. 2019, 650, 1309–1326.
- Revol, J.-F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal Self-Ordering of Cellulose Microfibrils in Aqueous Suspension. Int. J. Biol. Macromol. 1992, 14, 170–172.
- Revol, J.-F.; Godbout, L.; Gray, D.G. Solid Self-Assembled Films of Cellulose with Chiral Nematic Order and Optically Variable Properties. J. Pulp Pap. Sci. 1998, 24, 146–149.
- Fernandes, S.N.; Almeida, P.L.; Monge, N.; Aguirre, L.E.; Reis, D.; de Oliveira, C.L.P.; Neto, A.M.F.; Pieranski, P.; Godinho, M.H. Mind the Microgap in Iridescent Cellulose Nanocrystal Films. Adv. Mater. 2017, 29, 1603560.
- Wang, C.; Tang, C.; Wang, Y.; Shen, Y.; Qi, W.; Zhang, T.; Su, R.; He, Z. Chiral Photonic Materials Self-Assembled by Cellulose Nanocrystals. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101017.
- Tran, A.; Hamad, W.Y.; MacLachlan, M.J. Tactoid Annealing Improves Order in Self-Assembled Cellulose Nanocrystal Films with Chiral Nematic Structures. Langmuir 2018, 34, 646–652.
- Dionne, G.F.; Allen, G.A.; Haddad, P.R.; Ross, C.A.; Lax, B. Circular Polarization and Nonreciprocal Propagation in Magnetic Media. Linc. Lab. J. 2005, 15, 323–340.
- Moud, A.A.; Moud, A.A. Flow and assembly of cellulose nanocrystals (CNC): A bottom-up perspective—A review. Int. J. Biol. Macromol. 2023, 232, 123391.
- Frka-Petesic, B.; Vignolini, S. So Much More than Paper. Nat. Photonics 2019, 13, 365–367.
- Liang, H.-L.; Bay, M.M.; Vadrucci, R.; Barty-King, C.H.; Peng, J.; Baumberg, J.J.; De Volder, M.F.L.; Vignolini, S. Roll-to-Roll Fabrication of Touch-Responsive Cellulose Photonic Laminates. Nat. Commun. 2018, 9, 4632.
- Nasseri, R.; Deutschman, C.P.; Han, L.; Pope, M.A.; Tam, K.C. Cellulose Nanocrystals in Smart and Stimuli-Responsive Materials: A Review. Mater. Today Adv. 2020, 5, 100055.
- Lagerwall, J.P.F.; Schütz, C.; Salajkova, M.; Noh, J.; Hyun Park, J.; Scalia, G.; Bergström, L. Cellulose Nanocrystal-Based Materials: From Liquid Crystal Self-Assembly and Glass Formation to Multifunctional Thin Films. NPG Asia Mater. 2014, 6, e80.
- Tran, A.; Boott, C.E.; MacLachlan, M.J. Understanding the Self-Assembly of Cellulose Nanocrystals—Toward Chiral Photonic Materials. Adv. Mater. 2020, 32, 1905876.
- Dumanli, A.G.; Kamita, G.; Landman, J.; van der Kooij, H.; Glover, B.J.; Baumberg, J.J.; Steiner, U.; Vignolini, S. Controlled, Bio-inspired Self-Assembly of Cellulose-Based Chiral Reflectors. Adv. Opt. Mater. 2014, 2, 646–650.
- Tran, A.; Hamad, W.Y.; MacLachlan, M.J. Fabrication of Cellulose Nanocrystal Films through Differential Evaporation for Patterned Coatings. ACS Appl. Nano Mater. 2018, 1, 3098–3104.
- Chen, Q.; Liu, P.; Nan, F.; Zhou, L.; Zhang, J. Tuning the Iridescence of Chiral Nematic Cellulose Nanocrystal Films with a Vacuum-Assisted Self-Assembly Technique. Biomacromolecules 2014, 15, 4343–4350.
- Nguyen, T.-D.; Hamad, W.Y.; MacLachlan, M.J. Tuning the Iridescence of Chiral Nematic Cellulose Nanocrystals and Mesoporous Silica Films by Substrate Variation. Chem. Commun. 2013, 49, 11296.
- O’Keeffe, O.; Wang, P.-X.; Hamad, W.Y.; MacLachlan, M.J. Boundary Geometry Effects on the Coalescence of Liquid Crystalline Tactoids and Formation of Topological Defects. J. Phys. Chem. Lett. 2019, 10, 278–282.
- Tardy, B.L.; Mattos, B.D.; Greca, L.G.; Kämäräinen, T.; Klockars, K.W.; Rojas, O.J. Tessellation of Chiral-Nematic Cellulose Nanocrystal Films by Microtemplating. Adv. Funct. Mater. 2019, 29, 1808518.
- Beck, S.; Bouchard, J.; Chauve, G.; Berry, R. Controlled Production of Patterns in Iridescent Solid Films of Cellulose Nanocrystals. Cellulose 2013, 20, 1401–1411.
- Dumanli, A.G.; van der Kooij, H.M.; Kamita, G.; Reisner, E.; Baumberg, J.J.; Steiner, U.; Vignolini, S. Digital Color in Cellulose Nanocrystal Films. ACS Appl. Mater. Interfaces 2014, 6, 12302–12306.
- Liu, D.; Wang, S.; Ma, Z.; Tian, D.; Gu, M.; Lin, F. Structure–Color Mechanism of Iridescent Cellulose Nanocrystal Films. RSC Adv. 2014, 4, 39322–39331.
- Jativa, F.; Schütz, C.; Bergström, L.; Zhang, X.; Wicklein, B. Confined Self-Assembly of Cellulose Nanocrystals in a Shrinking Droplet. Soft Matter 2015, 11, 5374–5380.
- Beck, S.; Bouchard, J.; Berry, R. Controlling the Reflection Wavelength of Iridescent Solid Films of Nanocrystalline Cellulose. Biomacromolecules 2011, 12, 167–172.
- De La Cruz, J.A.; Liu, Q.; Senyuk, B.; Frazier, A.W.; Peddireddy, K.; Smalyukh, I.I. Cellulose-Based Reflective Liquid Crystal Films as Optical Filters and Solar Gain Regulators. ACS Photonics 2018, 5, 2468–2477.
- Caligiuri, V.; Tedeschi, G.; Palei, M.; Miscuglio, M.; Martin-Garcia, B.; Guzman-Puyol, S.; Hedayati, M.K.; Kristensen, A.; Athanassiou, A.; Cingolani, R. Biodegradable and Insoluble Cellulose Photonic Crystals and Metasurfaces. ACS Nano 2020, 14, 9502–9511.
- Davis, C.S.; Grolman, D.L.; Karim, A.; Gilman, J.W. What Do We Still Need to Understand to Commercialize Cellulose Nanomaterials. Green Mater. 2015, 3, 53–58.
- Sun, J.; Bhushan, B. Structure and Mechanical Properties of Beetle Wings: A Review. RSC Adv. 2012, 2, 12606.
- Pan, J.; Hamad, W.; Straus, S.K. Parameters Affecting the Chiral Nematic Phase of Nanocrystalline Cellulose Films. Macromolecules 2010, 43, 3851–3858.
- Meda, R.S.; Jain, S.; Singh, S.; Verma, C.; Nandi, U.; Maji, P.K. Novel Lagenaria Siceraria Peel Waste Based Cellulose Nanocrystals: Isolation and Rationalizing H-Bonding Interactions. Ind. Crops Prod. 2022, 186, 115197.
- Bhardwaj, S.; Singh, S.; Meda, R.S.; Jain, S.; Maji, P.K. Structural and Morphological Exploration of Cellulose Nanocrystals Extracted from Lignocellulosic Waste Biomass of Brassica Nigra (Mustard Straw). Biomass Convers. Biorefin. 2023.
- Rani, A.; Kumari, A.; Thakur, M.; Mandhan, K.; Chandel, M.; Sharma, A. Bionanocomposite Synthesized from Nanocellulose Obtained from Agricultural Biomass as Raw Material. In Biorenewable Nanocomposite Materials, Vol. 1: Electrocatalysts and Energy Storage; American Chemical Society: Washington, DC, USA, 2022; pp. 47–74.
- Raza, M.; Abu-Jdayil, B.; Banat, F.; Al-Marzouqi, A.H. Isolation and Characterization of Cellulose Nanocrystals from Date Palm Waste. ACS Omega 2022, 7, 25366–25379.
- Gray, D. Recent Advances in Chiral Nematic Structure and Iridescent Color of Cellulose Nanocrystal Films. Nanomaterials 2016, 6, 213.
- Silva, P.E.S.; Chagas, R.; Fernandes, S.N.; Pieranski, P.; Selinger, R.L.B.; Godinho, M.H. Travelling Colourful Patterns in Self-Organized Cellulose-Based Liquid Crystalline Structures. Commun. Mater. 2021, 2, 79.
- Wilts, B.D.; Dumanli, A.G.; Middleton, R.; Vukusic, P.; Vignolini, S. Invited Article: Chiral Optics of Helicoidal Cellulose Nanocrystal Films. APL Photonics 2017, 2, 040801.
- Trindade, A.C.; Carreto, M.; Helgesen, G.; Knudsen, K.D.; Puchtler, F.; Breu, J.; Fernandes, S.; Godinho, M.H.; Fossum, J.O. Photonic Composite Materials from Cellulose Nanorods and Clay Nanolayers. Eur. Phys. J. Spec. Top. 2020, 229, 2741–2755.
- Kumar, A.; Cruz, C.; Figueirinhas, J.L.; Sebastião, P.J.; Trindade, A.C.; Fernandes, S.N.; Godinho, M.H.; Fossum, J.O. Water Dynamics in Composite Aqueous Suspensions of Cellulose Nanocrystals and a Clay Mineral Studied through Magnetic Resonance Relaxometry. J. Phys. Chem. B 2021, 125, 12787–12796.
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.Y.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive Photonic Hydrogels Based on Nanocrystalline Cellulose. Angew. Chem. Int. Ed. 2013, 52, 8912–8916.
- Yurtsever, A.; Wang, P.-X.; Priante, F.; Morais Jaques, Y.; Miyazawa, K.; MacLachlan, M.J.; Foster, A.S.; Fukuma, T. Molecular Insights on the Crystalline Cellulose-Water Interfaces via Three-Dimensional Atomic Force Microscopy. Sci. Adv. 2022, 8.
- Yan, D.; Lu, W.; Qiu, L.; Meng, Z.; Qiao, Y. Thermal and Stress Tension Dual-Responsive Photonic Crystal Nanocomposite Hydrogels. RSC Adv. 2019, 9, 21202–21205.
- Chakrabarty, A.; Teramoto, Y. Recent Advances in Nanocellulose Composites with Polymers: A Guide for Choosing Partners and How to Incorporate Them. Polymers 2018, 10, 517.
- Zhou, S.; Han, C.; Ni, Z.; Yang, C.; Ni, Y.; Lv, Y. Gelatin-Oxidized Nanocellulose Hydrogels Suitable for Extrusion-Based 3D Bioprinting. Processes 2022, 10, 2216.
- Han, C.; Wang, X.; Ni, Z.; Ni, Y.; Huan, W.; Lv, Y.; Bai, S. Effects of Nanocellulose on Alginate/Gelatin Bio-Inks for Extrusion-Based 3D Printing. Bioresources 2020, 15, 7357–7373.
- Szymkowiak, J.K.; Walters, C.M.; Hamad, W.Y.; MacLachlan, M.J. Tuning the Properties of Chiral Nematic Mesoporous (Organo)Silica Through Thiol-Ene Click Chemistry. Eur. J. Inorg. Chem. 2022, 2022, e202200218.
- Terpstra, A.S.; Arnett, L.P.; Manning, A.P.; Michal, C.A.; Hamad, W.Y.; MacLachlan, M.J. Iridescent Chiral Nematic Mesoporous Organosilicas with Alkylene Spacers. Adv. Opt. Mater. 2018, 6, 1800163.
- Zhang, Z.; Wang, C.; Wang, Q.; Zhao, Y.; Shang, L. Cholesteric Cellulose Liquid Crystal Ink for Three-Dimensional Structural Coloration. Proc. Natl. Acad. Sci. USA 2022, 119.
- Giese, M.; Khan, M.K.; Hamad, W.Y.; MacLachlan, M.J. Imprinting of Photonic Patterns with Thermosetting Amino-Formaldehyde-Cellulose Composites. ACS Macro. Lett. 2013, 2, 818–821.
- Lizundia, E.; Nguyen, T.-D.; Vilas, J.L.; Hamad, W.Y.; MacLachlan, M.J. Chiroptical, Morphological and Conducting Properties of Chiral Nematic Mesoporous Cellulose/Polypyrrole Composite Films. J. Mater. Chem. A Mater. 2017, 5, 19184–19194.
- Sun, C.; Zhu, D.; Jia, H.; Lei, K.; Zheng, Z.; Wang, X. Humidity and Heat Dual Response Cellulose Nanocrystals/Poly(N-Isopropylacrylamide) Composite Films with Cyclic Performance. ACS Appl. Mater. Interfaces 2019, 11, 39192–39200.
- Walters, C.M.; Boott, C.E.; Nguyen, T.-D.; Hamad, W.Y.; MacLachlan, M.J. Iridescent Cellulose Nanocrystal Films Modified with Hydroxypropyl Cellulose. Biomacromolecules 2020, 21, 1295–1302.
- Chen, J.; Ling, Z.; Wang, X.; Ping, X.; Xie, Y.; Ma, H.; Guo, J.; Yong, Q. All Bio-Based Chiral Nematic Cellulose Nanocrystals Films under Supramolecular Tuning by Chitosan/Deacetylated Chitin Nanofibers for Reversible Multi-Response and Sensor Application. Chem. Eng. J. 2023, 466, 143148.
- Chen, J.; Zhu, Z.; Chen, J.; Luo, Y.; Li, L.; Liu, K.; Ding, S.; Li, H.; Liu, M.; Zhou, C. Photocurable Liquid Crystal Hydrogels with Different Chargeability and Tunable Viscoelasticity Based on Chitin Whiskers. Carbohydr. Polym. 2023, 301, 120299.
- Basta, A.A.H.; Lotfy, V.; Micky, J.; Salem, A.M. Selective Route for Enhancing Liquid Crystal-Based Hydroxylpropyl Cellulose by Esterification. Pigment. Resin. Technol. 2023, 52, 285–298.
- Querejeta-Fernández, A.; Chauve, G.; Methot, M.; Bouchard, J.; Kumacheva, E. Chiral Plasmonic Films Formed by Gold Nanorods and Cellulose Nanocrystals. J. Am. Chem. Soc. 2014, 136, 4788–4793.
- Xia, K.; Zheng, X.; Wang, Y.; Zhong, W.; Dong, Z.; Ye, Z.; Zhang, Z. Biomimetic Chiral Photonic Materials with Tunable Metallic Colorations Prepared from Chiral Melanin-like Nanorods for UV Shielding, Humidity Sensing, and Cosmetics. Langmuir 2022, 38, 8114–8124.
- Qi, F.; Jeong, K.-J.; Gong, J.; Tang, Z. Modulation of Nano-Superstructures and Their Optical Properties. Acc. Chem. Res. 2022, 55, 2425–2438.
- Schlesinger, M.; Giese, M.; Blusch, L.K.; Hamad, W.Y.; MacLachlan, M.J. Chiral Nematic Cellulose–Gold Nanoparticle Composites from Mesoporous Photonic Cellulose. Chem. Commun. 2015, 51, 530–533.
- Bast, L.K.; Klockars, K.W.; Greca, L.G.; Rojas, O.J.; Tardy, B.L.; Bruns, N. Infiltration of Proteins in Cholesteric Cellulose Structures. Biomacromolecules 2021, 22, 2067–2080.
- Mehranfar, A.; Khavani, M.; Mofrad, M.R.K. Adsorption Process of Various Antimicrobial Peptides on Different Surfaces of Cellulose. ACS Appl. Bio Mater. 2023, 6, 1041–1053.
- Mohammadi, P.; Gandier, J.; Nonappa; Wagermaier, W.; Miserez, A.; Penttilä, M. Bioinspired Functionally Graded Composite Assembled Using Cellulose Nanocrystals and Genetically Engineered Proteins with Controlled Biomineralization. Adv. Mater. 2021, 33, 2102658.
- Xiao, X.; Chen, J.; Ling, Z.; Guo, J.; Huang, J.; Ma, J.; Jin, Z. Chiral Nematic Cellulose Nanocrystal Films Cooperated with Amino Acids for Tunable Optical Properties. Polymers 2021, 13, 4389.
- Aalbers, G.J.W.; Boott, C.E.; D’Acierno, F.; Lewis, L.; Ho, J.; Michal, C.A.; Hamad, W.Y.; MacLachlan, M.J. Post-Modification of Cellulose Nanocrystal Aerogels with Thiol–Ene Click Chemistry. Biomacromolecules 2019, 20, 2779–2785.
- Xu, Y.-T.; Walters, C.M.; D’Acierno, F.; Hamad, W.Y.; Michal, C.A.; MacLachlan, M.J. Cellulose Nanocrystal Chiral Nematic Composites with Wet Mechanical Adaptability. Chem. Mater. 2022, 34, 4311–4319.
- Andrew, L.J.; Walters, C.M.; Hamad, W.Y.; MacLachlan, M.J. Coassembly of Cellulose Nanocrystals and Neutral Polymers in Iridescent Chiral Nematic Films. Biomacromolecules 2023, 24, 896–908.
- Zhao, G.; Zhang, Y.; Zhai, S.; Sugiyama, J.; Pan, M.; Shi, J.; Lu, H. Dual Response of Photonic Films with Chiral Nematic Cellulose Nanocrystals: Humidity and Formaldehyde. ACS Appl. Mater. Interfaces 2020, 12, 17833–17844.
- Peng, N.; Huang, D.; Gong, C.; Wang, Y.; Zhou, J.; Chang, C. Controlled Arrangement of Nanocellulose in Polymeric Matrix: From Reinforcement to Functionality. ACS Nano 2020, 14, 16169–16179.
- Ling, Z.; Chen, J.; Wang, X.; Shao, L.; Wang, C.; Chen, S.; Guo, J.; Yong, Q. Nature-Inspired Construction of Iridescent CNC/Nano-Lignin Films for UV Resistance and Ultra-Fast Humidity Response. Carbohydr. Polym. 2022, 296, 119920.
- Duan, R.; Lu, M.; Tang, R.; Guo, Y.; Zhao, D. Structural Color Controllable Humidity Response Chiral Nematic Cellulose Nanocrystalline Film. Biosensors 2022, 12, 707.
- Kaschuk, J.J.; Al Haj, Y.; Rojas, O.J.; Miettunen, K.; Abitbol, T.; Vapaavuori, J. Plant-Based Structures as an Opportunity to Engineer Optical Functions in Next-Generation Light Management. Adv. Mater. 2022, 34, 2104473.
- Tran, K.T.M.; Nguyen, T.D. Lithography-Based Methods to Manufacture Biomaterials at Small Scales. J. Sci. Adv. Mater. Devices 2017, 2, 1–14.
- Kasani, S.; Curtin, K.; Wu, N. A Review of 2D and 3D Plasmonic Nanostructure Array Patterns: Fabrication, Light Management and Sensing Applications. Nanophotonics 2019, 8, 2065–2089.
- Wolfberger, A.; Petritz, A.; Fian, A.; Herka, J.; Schmidt, V.; Stadlober, B.; Kargl, R.; Spirk, S.; Griesser, T. Photolithographic Patterning of Cellulose: A Versatile Dual-Tone Photoresist for Advanced Applications. Cellulose 2015, 22, 717–727.
- Espinha, A.; Dore, C.; Matricardi, C.; Alonso, M.I.; Goñi, A.R.; Mihi, A. Hydroxypropyl Cellulose Photonic Architectures by Soft Nanoimprinting Lithography. Nat. Photonics 2018, 12, 343–348.
- Chu, G.; Camposeo, A.; Vilensky, R.; Vasilyev, G.; Martin, P.; Pisignano, D.; Zussman, E. Printing Flowers? Custom-Tailored Photonic Cellulose Films with Engineered Surface Topography. Matter 2019, 1, 988–1000.
- Chu, G.; Qu, D.; Camposeo, A.; Pisignano, D.; Zussman, E. When Nanocellulose Meets Diffraction Grating: Freestanding Photonic Paper with Programmable Optical Coupling. Mater. Horiz. 2020, 7, 511–519.
- Khakalo, A.; Mäkelä, T.; Johansson, L.-S.; Orelma, H.; Tammelin, T. High-Throughput Tailoring of Nanocellulose Films: From Complex Bio-Based Materials to Defined Multifunctional Architectures. ACS Appl. Bio Mater. 2020, 3, 7428–7438.
- Mäkelä, T.; Hokkanen, A.; Sneck, A.; Ruotsalainen, T.; Khakalo, A.; Tammelin, T. Vapour-Assisted Roll-to-Roll Nanoimprinting of Micropillars on Nanocellulose Films. Microelectron. Eng. 2020, 225, 111258.
- Mäkelä, T.; Kainlauri, M.; Willberg-Keyriläinen, P.; Tammelin, T.; Forsström, U. Fabrication of Micropillars on Nanocellulose Films Using a Roll-to-Roll Nanoimprinting Method. Microelectron. Eng. 2016, 163, 1–6.
- Mäkelä, T.; Haatainen, T.; Ahopelto, J. Roll-to-Roll Printed Gratings in Cellulose Acetate Web Using Novel Nanoimprinting Device. Microelectron. Eng. 2011, 88, 2045–2047.
- Daqiqeh Rezaei, S.; Dong, Z.; You En Chan, J.; Trisno, J.; Ng, R.J.H.; Ruan, Q.; Qiu, C.-W.; Mortensen, N.A.; Yang, J.K.W. Nanophotonic Structural Colors. ACS Photonics 2021, 8, 18–33.
- Zhu, S.; Tang, Y.; Lin, C.; Liu, X.Y.; Lin, Y. Recent Advances in Patterning Natural Polymers: From Nanofabrication Techniques to Applications. Small Methods 2021, 5, 2001060.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
802
Revisions:
2 times
(View History)
Update Date:
06 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




