Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ravikumar Aalinkeel | -- | 2501 | 2023-07-05 13:59:02 | | | |
| 2 | Jessie Wu | + 2 word(s) | 2503 | 2023-07-06 05:28:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sharma, S.; Mohler, J.L.; Mahajan, S.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Biofilms on Medical Devices. Encyclopedia. Available online: https://encyclopedia.pub/entry/46459 (accessed on 07 February 2026).
Sharma S, Mohler JL, Mahajan S, Schwartz SA, Bruggemann L, Aalinkeel R. Biofilms on Medical Devices. Encyclopedia. Available at: https://encyclopedia.pub/entry/46459. Accessed February 07, 2026.
Sharma, Satish, James L. Mohler, Supriya Mahajan, Stanley A Schwartz, Liana Bruggemann, Ravikumar Aalinkeel. "Biofilms on Medical Devices" Encyclopedia, https://encyclopedia.pub/entry/46459 (accessed February 07, 2026).
Sharma, S., Mohler, J.L., Mahajan, S., Schwartz, S.A., Bruggemann, L., & Aalinkeel, R. (2023, July 05). Biofilms on Medical Devices. In Encyclopedia. https://encyclopedia.pub/entry/46459
Sharma, Satish, et al. "Biofilms on Medical Devices." Encyclopedia. Web. 05 July, 2023.
Copy Citation
Biofilm is complex and consists of bacterial colonies that reside in an exopolysaccharide matrix that attaches to foreign surfaces in a living organism. Biofilm frequently leads to nosocomial, chronic infections in clinical settings. Since the bacteria in the biofilm have developed antibiotic resistance, using antibiotics alone to treat infections brought on by biofilm is ineffective.
biofilm
extracellular polysaccharides
healthcare-associated infection
medical device infections
antibiotic resistance
biofilm control
1. Introduction
Costerton et al. was the first to demonstrate an association between biofilm and medical devices [1]. Subsequent studies have demonstrated urinary catheters, central venous catheters, indwelling stents, contact lenses, intrauterine devices, and dental chair water lines all to be susceptible to bacterial adhesion and biofilm formation [2][3][4]. Catheters are inserted to administer liquids, blood and blood products, drugs, nourishment, and for hemodynamic monitoring [5]. The outer or inner lumen of the catheters can have biofilms. Routes for bacterial transmission include ascent up the catheter’s outside surface or transmission through the main channel. Platelets and other tissue proteins contribute to the films on the surface of catheters [5][6]. Adhesins including polysaccharide intercellular adhesin and hemagglutinin also form part of the biofilm architecture [7].
2. Prosthetic Heart Valves
Karchmer and Gibbons demonstrated biofilm’s association with prosthetic heart valves [8]. Prosthetic heart valves may be of two types: mechanical valves and bio-prostheses or tissue valves. However, the infection rates are similar in both [9]. Adjacent tissue damage during surgical implantation may lead to accumulation of platelets and fibrin with the potential for microbial colonization. Infection associated with prosthetic heart valves is known as prosthetic valve endocarditis (PVE) [10][11][12][13]. PVE is classified as early (≤12 months) or late (>12 months) after operation. The microbial organisms responsible for PVEs are predictable based on the time after valve implantation. Time of infection may reflect the pathogenic mechanism [13][14][15][16][17][18][19][20]. In the first 2 months after valve implantation, the most common pathogens are coagulase-negative staphylococci (CoNS) and S. aureus, followed by members of the Candida species and by Gram-negative bacilli. This range of bacteria reflects the usual nosocomial origin of these infections. From 2 to 12 months after valve implantation, the extremely common pathogens are coagulase-negative Staphylococci, S. aureus, and Streptococci, followed by Enterococci. After a year following the installation of a valve, the typical bacteria are CoNS, S. aureus Streptococci and Enterococci.
3. Central Venous Catheters
Maki et al. was the first to demonstrate that central venous catheters (CVCs) are more vulnerable to device-related infections than any other indwelling medical device [5]. Three days after catheterization is the normal timeframe for colonization and biofilm growth on CVC. Raad et al. showed that catheters left in place for less than 10 days tended to generate biofilm more on the outside of the catheter, whereas catheters left in place for 30 days or more tended to form biofilm more extensively and frequently on the inside of the catheter [21]. Pathogens colonizing CVCs include S. aureus, P. aeruginosa, Klebsiella pneumoniae, Enterococcus faecalis, and Candida albicans [22]. The distal tip of the catheter is withdrawn aseptically and rolled over the surface of a nonselective media to identify CVC biofilms. The amount of organisms recovered upon contact with the agar surface determines the size of the biofilm on the catheter tip [6]. The roll-plate method for diagnosing catheter-related bacteremia has poor detection accuracy and poor predictive value, according to Slobbe et al. [23]. They observed that even a threshold of 104 CFU/tip indicated catheter-related septicemia by sonicating and vortexing catheter tips to improve biofilm quantification.
4. Contact Lenses
Contact lenses are hard or soft depending on the manufacturing material. Soft contact lenses composed of hydrogel or silicone allow oxygen diffusion through the lens material to the cornea. Hard contact lenses made of polymethylmethacrylate allow oxygen-containing tears to flow underneath the lens through movement with every blink. Both types of lenses are easily colonized by bacteria [24]. P. aeruginosa, S. aureus, S. epidermidis, Serratia spp., E. coli, Proteus spp., and Candida spp. are bacteria that have been reported to cling to contact lenses [25]. Miller and Ahearn concluded that the rate of adherence of P. aeruginosa to hydrophilic contact lenses varied based on water content [26]. Type of bacteria, pH, and substrate can affect the level of pathogen attachment. Moreover, biofilms develop on contact lens storage cases, and it was found that 80% of lens users without symptoms had contaminated storage cases [27].
5. Intrauterine Devices (IUDs)
intrauterine devices (IUDs) are made of polyethylene, a nonabsorbable polymer impregnated with barium sulfate. There are also varieties that release chemicals, including copper or a pro-gestational agent. IUD can cause pelvic inflammatory disease [28]. It has been shown that S. aureus, beta-hemolytic Streptococci, E. coli, and other anaerobic bacteria can be found in IUDs that have been removed from women with pelvic inflammatory disease. On the other hand, it has been observed that IUDs removed from asymptomatic women were highly infected with S. epidermidis, enterococci, and anaerobic lactobacilli [29]. Other pathogens that have been identified include Lactobacillus plantarum, S. epidermidis, Corynebacterium spp., Micrococcus spp., Candida albicans, S. aureus, and Enterococcus spp. In IUD-associated biofilms, Marrie and Costerton demonstrated the existence of human leukocytes and cellular detritus [30]. A potential major source of contamination could be the IUD’s tail. Studies have shown that microcolony production was most prevalent in the distal parts of the tail, which are exposed to the vaginal microbiota [31]. Tatum et al. proposed that the tail of the IUD provides the surface that allows microorganisms to reach the endometrial cavity through capillary action [29][32].
6. Dental Unit Water Lines
Pathogenic organisms in dental unit water lines can infect patients and dentists [33]. The water for various hand pieces, including the air-water syringe, the ultrasonic scaler, and the high-speed hand piece, is delivered to dental units via small-bore flexible plastic tubing. Water sources could be metropolitan, distilled, or sterile water reservoirs. Furuhashi and Miyamae demonstrated that the bacterial counts had increased from the typical municipal water supply of less than 40 cfu/mL to between 103 and 105 cfu/mL in water samples gathered from the three-way syringe [34]. They also observed that the cup water filler and air turbine hand piece both had high numbers. Whitehouse et al. showed a polysaccharide matrix embedded with a variety of bacteria [35]. Water counts and biofilm were shown to be positively correlated. They reported that even after 180 days of exposure, a dense, multi-layered extracellular polymeric material had completely covered the surface of the dental unit water line. It has also been demonstrated that dental suction devices such as saliva ejectors support biofilms containing both mixed skin microbiota and aquatic microorganisms.
7. Urinary Catheters
Urinary catheters are of two types, either latex or silicone devices. They are inserted via the urethra into the bladder to determine urine yield, collect urine during surgery, control urinary incontinence, or relieve urinary retention. The devices are either open or closed. The catheter empties into an open collection receptacle in open devices, which are primarily utilized only in developing nations, as opposed to a plastic collection bag in closed systems used elsewhere. Urinary catheters in the initial stages are colonized by a single species, such as Enterococcus faecalis, E. coli, S.epidermidis, or Proteus mirabilis. Subsequently, mixed communities develop, containing organisms such as Providencia stuartii, P. aeruginosa, Proteus mirabilis, and Klebsiella pneumoniae [36][37]. Since certain constituent organisms in biofilms on urinary catheters have the potential to change the local pH by producing urease, which hydrolyzes urea in urine to produce free ammonia, these biofilms are distinctive. As a result of the ammonia raising local pH, minerals including calcium phosphate (hydroxyapatite) and magnesium ammonium phosphate might precipitate (struvite) (Figure 1). In the catheter biofilms, these minerals will be deposited and form a mineral encrustation [36][37].
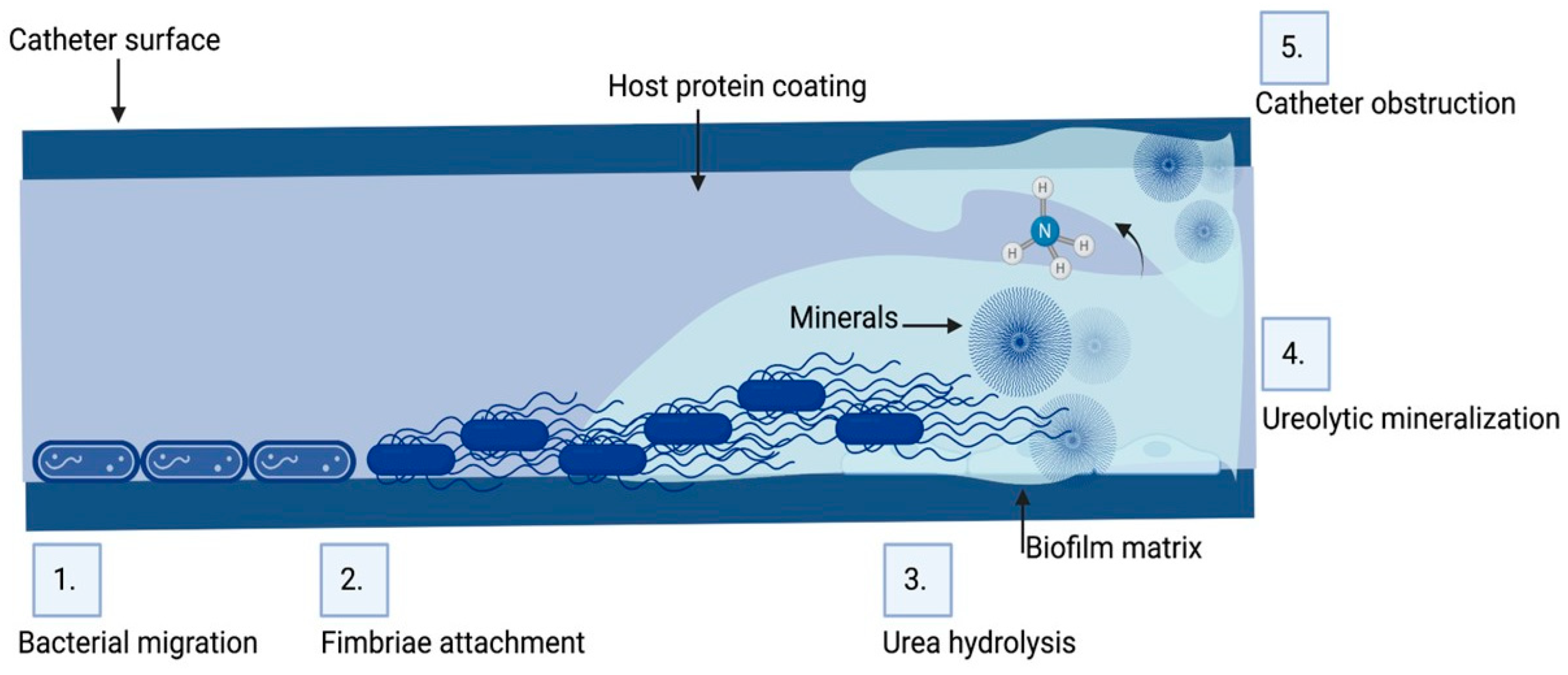
Figure 1. Biofilm formation and pathogenesis mechanism of CAUTI: The environmental conditions created on the catheter surface make it an ideal site for bacterial attachment and formation of biofilm structures. (1) Bacteria migrates through the periurethral area along the catheter surface. (2) Fimbriae attach to the body-fluid-derived catheter surface or directly to the catheter material inducing EPS production and biofilm formation. (3) Some bacteria such as P. mirabilis produce enzymes involved in the hydrolysis of urea in urine into ammonia, increasing the local pH leading to the production of minerals in urine which results in struvite crystals. (4) Struvite formed is incorporated into the developing biofilm—a process called ureolytic mineralization, which is also facilitated by the capsule polysaccharides. (5) Fully developed crystalline biofilm eventually causes catheter obstruction. Created on Biorender.com (31 March 2023).
The consequence of biofilms on urinary catheters is that patients develop UTI within 4 days of insertion [38]. Virtually all healthcare-associated UTI are due to insertion of a urinary catheter and these UTIs are referred to as catheter-associated urinary tract infections (CAUTIs) [39]. The frequency of CAUTIs are lower in closed systems and urine can remain sterile for 10–14 days in approximately half the patients [40]. Stickler et al. in their study demonstrated that 10–50% of patients undergoing short-term (<7 days) catheterization develop CAUTI, whereas essentially all patients undergoing long-term (>28 days) catheterization develop CAUTI [37]. According to McLean et al., bacterial climbing from the catheter to the bladder is the main cause of the 10% rise in CAUTI risk for each day the catheter stays in place [41]. Nickel et al. stated that biofilms formed in CAUTI have multiple species of bacteria within them resulting in a thick coherent biofilm that confers significant resistance to antibiotics even though individual bacteria in the biofilm remain sensitive, thus accounting for the failure of antibiotic therapy [42]. Moreover, they noted that there was no connection between the length of catheter use and the degree of biofilm growth. The attributable cost of CAUTI exceeds USD 1000 per patient, but the total economic loss annually is about USD 1.7 billion [43]. In 2008, Center for Medicare Services (CMS) listed CAUTI as one of the 14 hospital-acquired and preventable conditions and therefore stopped reimbursing hospitals as part of the Hospital Acquired Conditions Reduction Program (HACRP), creating a financial incentive for CAUTI prevention efforts.
Common symptoms of CAUTI include increased urinary frequency and urgency, dysuria, abdominal pain, and tachycardia. Catheter obstruction, hematuria, and cloudy urine are signs of CAUTI. The most common species responsible for CAUTI is the Gram-negative bacteria E. coli, but Pseudomonas, Klebsiella, Proteus genus, and Gram-positive bacteria such as Staphylococcus aureus, Enterococcus faecalis have been reported. When indwelling catheters are inserted or the collection system is handled improperly, bacteria from the patient’s colonic or perineal microbiota, from the hands of medical professionals, or from the hands of the patient can enter the urinary tract. Bactria are protected inside the biofilm since it acts as a reservoir of infection and promotes anti-microbial resistance.
CAUTIs are most frequently caused by rectal microbiota contaminating the urethra. Then, the bacteria migrate to the bladder, adhere, and colonize there [44]. Furthermore, bacterial proteases and toxins cause damage to the epithelium. Bacteria then grow and create biofilms (Figure 1). Both with and without a urinary catheter, the basic stages of infection proceed in the same way. With urinary catheters, the bladder can be directly connected to the outside world. Although in certain people this conduit is crucial for urine evacuation, it also serves as a pathway for rectal and periurethral microbe ascent to the bladder, where they can build a base for infection. The risk of UTI is raised by catheters because they bypass the urethral sphincters, lessen the turbulence that normally occurs during spontaneous urination, and act as an infection nidus. Moreover, catheters could irritate and traumatize the uroepithelium, rupturing the mucopolysaccharide layer that normally protects it, and making it vulnerable to bacterial adhesion and invasion. An ideal environment for adhesion by uropathogens that produce fibrinogen-binding proteins is created by the robust immunological response to catheterization, which causes fibrinogen to accumulate on the catheter [44][45]. For instance, Enterococcus faecalis does not grow in urine or bind to catheter material in a culture setting, but it does grow in urine that has been supplemented with fibrinogen and clings to a catheter coated with fibrinogen [46]. One important first step in UTI is adherence. Two species of bacteria may stick to the bladder’s uroepithelium in cases of uncomplicated UTI, giving the infection time to take hold. The production of a biofilm is thus enabled by bacterial adhesion to a suprapubic tube or urethral catheter [47].
Using indwelling urinary catheters only when necessary is the single most crucial step that can lower the prevalence of CAUTI [48]. Catheterization should only be used in acute circumstances, should be avoided, or used sparingly for the management of urine incontinence and chronic conditions, and alternatives to urethral catheterization should be researched. Several studies stress the significance of standardizing the insertion criteria for indwelling urinary catheters and, when necessary, reducing their usage entirely in favor of alternatives such as intermittent catheterization. Indwelling urinary catheters should be inserted using sterile procedures.
Enclosed drainage systems may lower the infection risk, but there is no concrete evidence that they reduce the frequency of CAUTI [49]. The drainage system must be emptied with the utmost care using aseptic methods, and the same collecting unit should never be used in any other patient.
Flores-Mireles et al. recommend utilizing the CDC “prevention guidelines” in bundles to prevent CAUTIs [50]. These guidelines and recommendations state that urinary catheterization should not be used to treat incontinence in patients or nursing home residents, that urinary catheters should only be used in surgical patients when absolutely necessary, and that the Foley catheter should be removed as soon as possible after surgery, preferably within 24 h [50]. This bundle provides effective ways to decrease CAUTI cases. Gray et al. reviewed the utilization of external urinary collection devices for males as a substitute to indwelling urinary catheters to reduce CAUTI [51]. The types of external collection devices include condom catheters, reusable body-worn urinals, and non-sheath, glans-adherent external collection devices [51]. Patients who are deemed to be particularly high risk for developing CAUTI can use anti-infective catheters [52].
Current research has demonstrated the efficacy of multimodal UTI prevention techniques and combinations in critical care units [53]. A specialized set of interventions for CAUTI prevention, education, outcome and process surveillance, feedback on CAUTI rates, and performance indices for infection control procedures are a few examples of such approaches. These techniques have been successfully used in critical care settings for both adults and children. Critical care units (CCUs) have seen a decrease in CAUTI rates thanks to these multifaceted infection control regimens, which were also linked to better hand cleanliness [52][54][55]. Hence, improvements in care practices can lower the likelihood of CAUTI and its negative effects, especially in CCUs in resource-constrained nations. Sustained, continuous improvements also must be made in community practices and extended care facilities.
CAUTIs are the most commonly acquired infection in hospitals probably because 15–25% of patients are catheterized during hospitalization [56]. The Institute for Health Care Improvement reports that 80% of all UTIs are caused by indwelling urethral catheters [57]. Increased costs, longer hospital stays, and increased mortality rates are some of the adverse impacts of CAUTI [56]. Hospitals are aware of the need to prevent CAUTIs due to their cost and the lack of reimbursement from CMS. Extended care facilities can prevent CAUTIs by implementing evidence-based practices, such as insertion criteria, providing proper perineal care, and timely removal of urinary catheters [56]. Use of silicone, instead of latex, catheters is shown to reduce the incidence of CAUTI [58].
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322.
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067.
- VanEpps, J.S.; Younger, J.G. Implantable Device-Related Infection. Shock 2016, 46, 597–608.
- Pandey, V.K.; Srivastava, K.R.; Ajmal, G.; Thakur, V.K.; Gupta, V.K.; Upadhyay, S.N.; Mishra, P.K. Differential Susceptibility of Catheter Biomaterials to Biofilm-Associated Infections and Their Remedy by Drug-Encapsulated Eudragit RL100 Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5110.
- Maki, D.G.; Cobb, L.; Garman, J.K.; Shapiro, J.M.; Ringer, M.; Helgerson, R.B. An attachable silver-impregnated cuff for prevention of infection with central venous catheters: A prospective randomized multicenter trial. Am. J. Med. 1988, 85, 307–314.
- Maki, D.G.; Weise, C.E.; Sarafin, H.W. A semiquantitative culture method for identifying intravenous-catheter-related infection. N. Engl. J. Med. 1977, 296, 1305–1309.
- Rupp, M.E.; Ulphani, J.S.; Fey, P.D.; Mack, D. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 1999, 67, 2656–2659.
- Karchmer, A.; Gibbons, G.W. Infections of prosthetic heart valves and vascular grafts. In Infections Associated with Indwelling Medical Devices, 2nd ed.; Bisno, A.L., Waldvogel, F.A., Eds.; American Society for Microbiology: Washington, DC, USA, 2000; pp. 213–249.
- Pibarot, P.; Dumesnil, J.G. Prosthetic heart valves: Selection of the optimal prosthesis and long-term management. Circulation 2009, 119, 1034–1048.
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486.
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128.
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893.
- Wang, A.; Athan, E.; Pappas, P.A.; Fowler, V.G., Jr.; Olaison, L.; Pare, C.; Almirante, B.; Munoz, P.; Rizzi, M.; Naber, C.; et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007, 297, 1354–1361.
- Regueiro, A.; Linke, A.; Latib, A.; Ihlemann, N.; Urena, M.; Walther, T.; Husser, O.; Herrmann, H.C.; Nombela-Franco, L.; Cheema, A.N.; et al. Association Between Transcatheter Aortic Valve Replacement and Subsequent Infective Endocarditis and In-Hospital Death. JAMA 2016, 316, 1083–1092.
- Amat-Santos, I.J.; Messika-Zeitoun, D.; Eltchaninoff, H.; Kapadia, S.; Lerakis, S.; Cheema, A.N.; Gutierrez-Ibanes, E.; Munoz-Garcia, A.J.; Pan, M.; Webb, J.G.; et al. Infective endocarditis after transcatheter aortic valve implantation: Results from a large multicenter registry. Circulation 2015, 131, 1566–1574.
- Chu, V.H.; Miro, J.M.; Hoen, B.; Cabell, C.H.; Pappas, P.A.; Jones, P.; Stryjewski, M.E.; Anguera, I.; Braun, S.; Munoz, P.; et al. Coagulase-negative staphylococcal prosthetic valve endocarditis—A contemporary update based on the International Collaboration on Endocarditis: Prospective cohort study. Heart 2009, 95, 570–576.
- Rivas, P.; Alonso, J.; Moya, J.; de Gorgolas, M.; Martinell, J.; Fernandez Guerrero, M.L. The impact of hospital-acquired infections on the microbial etiology and prognosis of late-onset prosthetic valve endocarditis. Chest 2005, 128, 764–771.
- Karchmer, A.W.; Longworth, D.L. Infections of intracardiac devices. Infect. Dis. Clin. N. Am. 2002, 16, 477–505, xii.
- Hill, E.E.; Herregods, M.C.; Vanderschueren, S.; Claus, P.; Peetermans, W.E.; Herijgers, P. Management of prosthetic valve infective endocarditis. Am. J. Cardiol. 2008, 101, 1174–1178.
- Lee, J.H.; Burner, K.D.; Fealey, M.E.; Edwards, W.D.; Tazelaar, H.D.; Orszulak, T.A.; Wright, A.J.; Baddour, L.M. Prosthetic valve endocarditis: Clinicopathological correlates in 122 surgical specimens from 116 patients (1985–2004). Cardiovasc. Pathol. 2011, 20, 26–35.
- Raad, I.; Costerton, W.; Sabharwal, U.; Sacilowski, M.; Anaissie, E.; Bodey, G.P. Ultrastructural analysis of indwelling vascular catheters: A quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis. 1993, 168, 400–407.
- Cangui-Panchi, S.P.; Nacato-Toapanta, A.L.; Enriquez-Martinez, L.J.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Biofilm-forming microorganisms causing hospital-acquired infections from intravenous catheter: A systematic review. Curr. Res. Microb. Sci. 2022, 3, 100175.
- Slobbe, L.; El Barzouhi, A.; Boersma, E.; Rijnders, B.J. Comparison of the roll plate method to the sonication method to diagnose catheter colonization and bacteremia in patients with long-term tunnelled catheters: A randomized prospective study. J. Clin. Microbiol. 2009, 47, 885–888.
- Dart, J.K.G. Contact lens and prosthesis infections. In Duane’s Foundations of Clinical Ophthalmology; Tasman, W., Jaeger, E.A., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1996; pp. 1–30.
- Konduri, R.; Saiabhilash, C.R.; Shivaji, S. Biofilm-Forming Potential of Ocular Fluid Staphylococcus aureus and Staphylococcus epidermidis on Ex Vivo Human Corneas from Attachment to Dispersal Phase. Microorganisms 2021, 9, 1124.
- Miller, M.J.; Ahearn, D.G. Adherence of Pseudomonas aeruginosa to hydrophilic contact lenses and other substrata. J. Clin. Microbiol. 1987, 25, 1392–1397.
- McLaughlin-Borlace, L.; Stapleton, F.; Matheson, M.; Dart, J.K. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J. Appl. Microbiol. 1998, 84, 827–838.
- Toivonen, J. Intrauterine contraceptive device and pelvic inflammatory disease. Ann. Med. 1993, 25, 171–173.
- Wolf, A.S.; Krieger, D. Bacterial colonization of intrauterine devices (IUDs). Arch. Gynecol. 1986, 239, 31–37.
- Marrie, T.J.; Costerton, J.W. A scanning and transmission electron microscopic study of the surfaces of intrauterine contraceptive devices. Am. J. Obstet. Gynecol. 1983, 146, 384–394.
- Pruthi, V.; Al-Janabi, A.; Pereira, B.M. Characterization of biofilm formed on intrauterine devices. Indian J. Med. Microbiol. 2003, 21, 161–165.
- Tatum, H.J.; Schmidt, F.H.; Phillips, D.; McCarty, M.; O’Leary, W.M. The Dalkon Shield controversy. Structural and bacteriological studies of IUD tails. JAMA 1975, 231, 711–717.
- Dahlen, G. Biofilms in Dental Unit Water Lines. Monogr. Oral. Sci. 2021, 29, 12–18.
- Furuhashi, M.; Miyamae, T. Prevention of bacterial contamination of water in dental units. J. Hosp. Infect. 1985, 6, 81–88.
- Whitehouse, R.L.; Peters, E.; Lizotte, J.; Lilge, C. Influence of biofilms on microbial contamination in dental unit water. J. Dent. 1991, 19, 290–295.
- Stickler, D.; Morris, N.; Moreno, M.C.; Sabbuba, N. Studies on the formation of crystalline bacterial biofilms on urethral catheters. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 649–652.
- Stickler, D.J.; King, J.B.; Winters, C.; Morris, S.L. Blockage of urethral catheters by bacterial biofilms. J. Infect. 1993, 27, 133–135.
- Saint, S.; Lipsky, B.A.; Goold, S.D. Indwelling urinary catheters: A one-point restraint? Ann. Intern. Med. 2002, 137, 125–127.
- Rubi, H.; Mudey, G.; Kunjalwar, R. Catheter-Associated Urinary Tract Infection (CAUTI). Cureus 2022, 14, e30385.
- Werneburg, G.T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res. Rep. Urol. 2022, 14, 109–133.
- McLean, R.J.C.; Nickel, J.C.; Olson, M.E. Biofilm Associated Urinary Tract Infections; Lappin-Scott, H.M., Costerton, J.W., Eds.; Cambridge University Press: Cambridge, UK, 1995.
- Nickel, J.C.; Downey, J.A.; Costerton, J.W. Ultrastructural study of microbiologic colonization of urinary catheters. Urology 1989, 34, 284–291.
- Hollenbeak, C.S.; Schilling, A.L. The attributable cost of catheter-associated urinary tract infections in the United States: A systematic review. Am. J. Infect. Control. 2018, 46, 751–757.
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284.
- Parsons, C.L. Pathogenesis of urinary tract infections. Bacterial adherence, bladder defense mechanisms. Urol. Clin. N. Am. 1986, 13, 563–568.
- Gaston, J.R.; Andersen, M.J.; Johnson, A.O.; Bair, K.L.; Sullivan, C.M.; Guterman, L.B.; White, A.N.; Brauer, A.L.; Learman, B.S.; Flores-Mireles, A.L.; et al. Enterococcus faecalis Polymicrobial Interactions Facilitate Biofilm Formation, Antibiotic Recalcitrance, and Persistent Colonization of the Catheterized Urinary Tract. Pathogens 2020, 9, 835.
- Werneburg, G.T.; Nguyen, A.; Henderson, N.S.; Rackley, R.R.; Shoskes, D.A.; Le Sueur, A.L.; Corcoran, A.T.; Katz, A.E.; Kim, J.; Rohan, A.J.; et al. The Natural History and Composition of Urinary Catheter Biofilms: Early Uropathogen Colonization with Intraluminal and Distal Predominance. J. Urol. 2020, 203, 357–364.
- Feneley, R.C.; Hopley, I.B.; Wells, P.N. Urinary catheters: History, current status, adverse events and research agenda. J. Med. Eng. Technol. 2015, 39, 459–470.
- Assadi, F. Strategies for Preventing Catheter-associated Urinary Tract Infections. Int. J. Prev. Med. 2018, 9, 50.
- Flores-Mireles, A.; Hreha, T.N.; Hunstad, D.A. Pathophysiology, Treatment, and Prevention of Catheter-Associated Urinary Tract Infection. Top. Spinal Cord. Inj. Rehabil. 2019, 25, 228–240.
- Gray, M.; Skinner, C.; Kaler, W. External Collection Devices as an Alternative to the Indwelling Urinary Catheter: Evidence-Based Review and Expert Clinical Panel Deliberations. J. Wound Ostomy Cont. Nurs. 2016, 43, 301–307.
- Parida, S.; Mishra, S.K. Urinary tract infections in the critical care unit: A brief review. Indian J. Crit. Care Med. 2013, 17, 370–374.
- Rosenthal, V.D.; Todi, S.K.; Alvarez-Moreno, C.; Pawar, M.; Karlekar, A.; Zeggwagh, A.A.; Mitrev, Z.; Udwadia, F.E.; Navoa-Ng, J.A.; Chakravarthy, M.; et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: Findings of the International Nosocomial Infection Control Consortium (INICC). Infection 2012, 40, 517–526.
- Kanj, S.S.; Zahreddine, N.; Rosenthal, V.D.; Alamuddin, L.; Kanafani, Z.; Molaeb, B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int. J. Infect. Dis. 2013, 17, e686–e690.
- Jaggi, N.; Sissodia, P. Multimodal supervision programme to reduce catheter associated urinary tract infections and its analysis to enable focus on labour and cost effective infection control measures in a tertiary care hospital in India. J. Clin. Diagn. Res. 2012, 6, 1372–1376.
- Van Decker, S.G.; Bosch, N.; Murphy, J. Catheter-associated urinary tract infection reduction in critical care units: A bundled care model. BMJ Open. Qual. 2021, 10, e001534.
- Letica-Kriegel, A.S.; Salmasian, H.; Vawdrey, D.K.; Youngerman, B.E.; Green, R.A.; Furuya, E.Y.; Calfee, D.P.; Perotte, R. Identifying the risk factors for catheter-associated urinary tract infections: A large cross-sectional study of six hospitals. BMJ Open. 2019, 9, e022137.
- Verma, A.; Bhani, D.; Tomar, V.; Bachhiwal, R.; Yadav, S. Differences in Bacterial Colonization and Biofilm Formation Property of Uropathogens between the Two most Commonly used Indwelling Urinary Catheters. J. Clin. Diagn. Res. 2016, 10, PC01–PC03.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
06 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




