Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nathupakorn Dechsupa | -- | 2437 | 2023-07-05 11:38:23 | | | |
| 2 | Alfred Zheng | Meta information modification | 2437 | 2023-07-06 05:44:56 | | | | |
| 3 | Alfred Zheng | Meta information modification | 2437 | 2023-07-07 10:37:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wen, C.; Dechsupa, N.; Yu, Z.; Zhang, X.; Liang, S.; Lei, X.; Xu, T.; Gao, X.; Hu, Q.; Innuan, P.; et al. Anticancer Activity of Pentagalloyl Glucose. Encyclopedia. Available online: https://encyclopedia.pub/entry/46447 (accessed on 15 January 2026).
Wen C, Dechsupa N, Yu Z, Zhang X, Liang S, Lei X, et al. Anticancer Activity of Pentagalloyl Glucose. Encyclopedia. Available at: https://encyclopedia.pub/entry/46447. Accessed January 15, 2026.
Wen, Chengli, Nathupakorn Dechsupa, Zehui Yu, Xu Zhang, Sicheng Liang, Xianying Lei, Tao Xu, Xiaolan Gao, Qinxue Hu, Phattarawadee Innuan, et al. "Anticancer Activity of Pentagalloyl Glucose" Encyclopedia, https://encyclopedia.pub/entry/46447 (accessed January 15, 2026).
Wen, C., Dechsupa, N., Yu, Z., Zhang, X., Liang, S., Lei, X., Xu, T., Gao, X., Hu, Q., Innuan, P., Kantapan, J., & Lü, M. (2023, July 05). Anticancer Activity of Pentagalloyl Glucose. In Encyclopedia. https://encyclopedia.pub/entry/46447
Wen, Chengli, et al. "Anticancer Activity of Pentagalloyl Glucose." Encyclopedia. Web. 05 July, 2023.
Copy Citation
Pentagalloyl glucose (PGG) is a natural hydrolyzable gallotannin abundant in various plants and herbs. It has a broad range of biological activities, specifically anticancer activities, and numerous molecular targets. PGG has a cytotoxic effect on many cancers, including prostate, breast, lung, head and neck, liver, leukemia, cervical, colorectal, and pancreatic cancers. PGG can affect different cancer stages and inhibit tumor growth through multiple mechanisms depending on cell origin, with minimal toxicity against normal cells. PGG targets several aberrant signal-transduction pathways that control cell growth and division, apoptosis, angiogenesis, and metastasis.
pentagalloyl glucose
anticancer activity
1. Introduction
Cancer is a significant public health concern worldwide. Despite the advances in early diagnostic strategies and targeted therapies, the incidence of cancer continues to increase with high mortality [1]. Currently, numerous cancer treatments are available, including surgery, chemotherapy, hormonal therapy, radiation, immunotherapy, and targeted therapies. Chemotherapy is the most common systemic cancer treatment, and chemotherapeutic drugs damage DNA to kill the cells or inhibit their growth. However, the treatment has several adverse effects, including hematological toxicity, disrupted gastrointestinal activity, alopecia, altered neurological activity, anaphylaxis, hepatotoxicity, and nephrotoxicity [2]. Therefore, plant-derived natural compounds with excellent safety profiles and minimal toxicity are ideal alternatives for safe and effective single or combined cancer therapies [3].
Natural products are the sources of complex molecules for discovering new drugs or lead compounds [4]. Natural compounds and their derivatives have long been used for treating many diseases [5][6], and their anticancer properties are an active research area. Apart from their antioxidant and anti-inflammatory activities, natural products also modulate multiple signal transduction pathways associated with cell survival, proliferation, differentiation, migration, angiogenesis, hormone activities, detoxification, and immune responses. In addition, several authors have documented the anticancer effects of natural polyphenols [3][7][8][9].
Penta-O-galloyl-D-glucose, also known as pentagalloyl glucose (PGG), is classified as a gallotannin; it is a hydrolyzable tannin abundant in many plants, including Rhus chinensis, Paeonia suffruticosa, Bouea macrophylla, and Toona sinensis [10][11][12][13]. The naturally occurring polyphenolic compound PGG exists in beta-PGG form, whereas an anomeric alpha-PGG is rarely found in nature [14]. However, both compounds can be chemically synthesized, and a highly purified material is obtained after crystallization [15]. Gallotannins are composed of gallic acid molecules bound to a central D-glucose via ester bonds (Figure 1). It can exist either in free form or as a core structure of tannic acid. PGG is mainly found in plants as a precursor of high molecular weight compounds, namely galloyl glucose, gallotannins, and ellagitannins [16], which impart astringency to the plant [17]. Notably, PGG has higher hydrophobicity than other polyphenols (octanol/water partition ratio of PGG: 129, gallic acid: 7.76, and hexagalloyl glucose: 1.51) [18]. Compared with rigid 1,2-di-O-galloyl-4,6-valoneoyl-β-d-glucose molecules, PGG is flexible with a lower surface area and higher dipole moment [19]. Moreover, PGG has potent biological activities, including antioxidative, anti-inflammatory, antiviral, antimicrobial, antidiabetic, and anticancer. In addition, PGG can reduce abdominal aortic aneurysms [20][21].
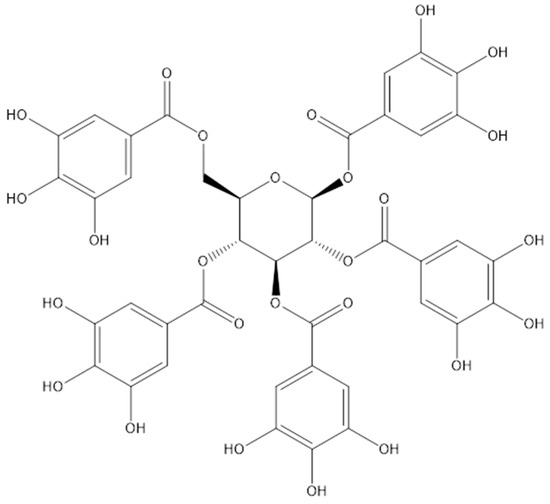
Figure 1. Chemical structure of the pentagalloyl glucose.
2. Anticancer Activity of PGG
PGG has a cytotoxic effect on many cancers, including prostate, breast, lung, head and neck, liver, leukemia, cervical, colorectal, and pancreatic cancers. The research summarized the studies on the anticancer effects of PGG on various cancer cell lines and animal models in the following sections (Table 1 and Table 2). PGG can affect different cancer stages and inhibit tumor growth through multiple mechanisms depending on cell origin, with minimal toxicity against normal cells. PGG targets several aberrant signal-transduction pathways that control cell growth and division, apoptosis, angiogenesis, and metastasis.
2.1. Breast Cancer
PGG has exhibited anticancer properties in various breast cancer cell lines, including the triple-negative breast cancer cell lines (such as MDA-MB-231 and MDA-MB-468) and the estrogen receptor-positive breast cancer MCF-7 cell line. Mendonca et al. [22] found that PGG inhibited tumor necrosis factor (TNF)-α-activated CXCL1/GRO-α expression by inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways in triple-negative breast cancer cell lines. The compound inhibited cell proliferation and induced apoptosis. Chai et al. demonstrated that PGG induced G1- and S-phase arrest by decreasing cyclin D1 concentration in vitro [23]. In a mouse animal model, gavage administration of PGG inhibited the MDA-MB-231 xenograft growth by >60%. Chai et al. reported that PGG suppressed triple-negative breast cancer xenograft growth and metastasis by inhibiting the JAK1–signal transducer and activator of the transcription (STAT)3 signaling pathway [24]. PGG suppressed the growth of MDA-MB-231 cells by downregulating fatty acid synthase (FAS); this enzyme activates caspase-3 and is highly expressed in some cancers [25]. Deiab et al. found that PGG inhibited the proliferation of MDA-MB-231 cells by inhibiting human lactic acid dehydrogenase-A [26]. Kantapan et al. revealed that PGG induced apoptosis in MCF-7 breast cancer cells by increasing reactive oxygen species (ROS) production, promoting mitochondrial membrane depolarization, and increasing the Bax/Bcl-2 ratio, indicating that PGG induced apoptosis in cancer cells by activating mitochondria-mediated pathway [27].
2.2. Prostate Cancer
Prostate cancer is the second leading cause of cancer-related deaths among men. Several authors have suggested the potential use of PGG as a chemopreventive agent against prostate cancer. PGG inhibits the growth and proliferation of prostate cancer cells by targeting multiple molecular pathways. For instance, PGG hindered the growth of prostate cancer cells by inhibiting epidermal growth factor (EGF)-induced nuclear translocation of NF-κB and subsequent activation of c-Jun N-terminal kinase (JNK), an upstream modulator of NF-κB [28]. Further, the authors intratibially injected PC-3 prostate cancer cells into nude mice, followed by an intraperitoneal injection of PGG, and found that PGG suppressed tumorigenesis in these mice. Hu and colleagues suggested that PGG activated the caspase-mediated apoptosis in DU145 and LNCaP prostate cancer cells to exert its anticancer effect. Notably, these two cell lines differed in p53 functionality. The apoptotic effects induced by PGG in the p53-mutant prostate cancer DU145 cells were linked to the inhibition of STAT3 phosphorylation followed by the downregulation of STAT3 transcriptional target genes Bcl-XL and Mcl-1. In contrast, the apoptosis of p53 wild-type LNCaP cells was mediated by PGG-induced ROS production that activated p53 [29]. PGG also induced autophagic cell death in prostate cancer cells with an aggressive phenotype (PC-3 cells with caspase-resistant properties) [30]. PGG induced cell cycle arrest by affecting DNA replication and reducing the expression of cyclin D1 [31]. Taken together, PGG acts on multiple targets and can be further developed as a potential candidate for prostate cancer therapy.
2.3. Lung Cancer
Angiogenesis is the formation of new capillaries from existing blood vessels in tumors for growth, invasion, and metastasis. Huh et al. revealed that PGG inhibited the growth of MRC5-SV2 lung cancer cells by inhibiting cyclooxygenase-2 and MAPK-dependent signaling pathways, which, in turn, inhibited angiogenesis [32]. The result showed that PGG treatment notably decreased tumor volume over time, and tumor weight decreased to 43% and 9% of that in the control group in low- and high-dose groups, respectively.
2.4. Liver Cancer
PGG showed promising therapeutic potential in hepatocellular carcinoma (HCC). Oh et al. determined that PGG inhibited the growth of SK-HEP-1 cells (an HCC cell line) by arresting the cell cycle in the G0/G1 phase and inhibiting the activation of NF-κB [33]. Yin et al. demonstrated that PGG induces senescence-like S-phase arrest in hepatoma cell lines (HepG2 and Huh-7) by increasing intracellular ROS production [34]. Moreover, PGG induced autophagy-mediated senescence-like arrest in liver cancer cells [35]. Recently, Kant et al. screened a natural compound, PGG, which worked as a glycine N-methyltransferase (GNMT)-inducer in hepatocellular carcinoma (HCC) therapy [36]. GNMT is a tumor suppressor for HCC because it protects the cells from the cytotoxicity induced by carcinogens. Notably, GNMT was downregulated in the tumor tissues collected from patients with HCC. The authors also evaluated the antiproliferative effect of PGG on multiple HCC cell lines, including Huh7, Hep 3B, SK-HEP-1, Mahlavu, and HepG2. They found that PGG inhibited the proliferation of HCC cells in a dose-dependent manner. Further, PGG inhibits the growth of Huh7 xenograft tumors in a mouse model by inducing the expression of GNMT. In another study, the same group of authors revealed that PGG induced GNMT through proteasome-independent MYC downregulation [37]. These findings indicated that PGG induced GNMT to exert its antiproliferative effect on HCC cells. Therefore, PGG shows notable therapeutic potential for liver cancers.
2.5. Pancreatic Cancer
PGG acted as an insulin-mimetic compound that damaged pancreatic cancer cells (MiaPaCa2 and Panic-1) and alleviated cachexia in tumor-bearing mice by inhibiting insulin receptor/insulin-like growth factor receptor-1 activity and decreasing glycolytic enzymes in pancreatic cancer cells [38]. The cluster of differentiation (CD)44 is a critical cancer stem cell (CSC) marker associated with pancreatic cancer, and pancreatic CSCs are vital in sustaining continuous tumor growth and chemoresistance [39][40]. Patients with CD44-positive pancreatic cancer have shorter median survival than those with CD44-negative disease [41]. Kim et al. revealed that PGG inhibited the expression of pancreatic CSCs, CD44, and CD44v3 by inducing the phosphorylation of p53 and suppressing NF-κB and fork-head box O3. This resulted in the downregulation of CSC regulatory factors, namely Nanog, Oct-4, and Sox-2, which act downstream of CD44v3 signaling. These findings suggested that PGG can inhibit CSC markers and may have a therapeutic effect on pancreatic cancer [42].
2.6. Head and Neck Cancer
Kantapan et al. found that PGG extracted from the Bouea macrophylla seeds inhibited the growth of human head and neck squamous cell carcinoma CAL27 and FaDu cell lines [12]. Recently, Fan and colleagues tested the anticancer effect of PGG on nasopharyngeal cancer cells (CNE1 and CNE2) and found that it regulated the cell cycle by affecting the expression of p53, cyclin D1, cyclin-dependent kinase (CDK)2, and cyclin E1 proteins. Moreover, PGG induced apoptosis and autophagy in these cell lines. In addition, PGG decreased NPC cell migration by increasing E-cadherin and decreasing N-cadherin, vimentin, and CD44 protein concentrations, thereby downregulating the p-mTOR and β-catenin expression. Overall, PGG inhibited nasopharyngeal cancer cell growth and lung metastasis [43].
2.7. Colorectal Cancer
Colorectal cancer is the third most common cancer and ranks second in cancer-related mortalities [1]. PGG suppressed the growth and proliferation of colorectal cancer cells. The researchers treated HCT116 and HT29 colorectal cancer cells with different concentrations of PGG for 48 h, and the corresponding IC50 values were displayed in Table 2 [44]. PGG induced the expression of p53 while increasing the expression of p21. PGG affected the expression of cell cycle-related proteins (such as cyclin E and CDK2) and inhibited apoptosis-related proteins (Bcl-2 and cleaved caspase-3).
2.8. Glioma Cancer
Glioma cancer is a common intracranial tumor. PGG inhibits glioma cancer cells by suppressing fatty acid synthase and activating caspase-3 [25]. The IC50 of PGG for glioma cancer cells treated for 24 h was 25 µM.
2.9. Cervical Cancer
Vaccinia H1-related phosphatase (VHR) dephosphorylates MAPKs, such as extracellular signal-regulated kinase (ERK) and JNK [45]. VHR is upregulated in various cervical cancer cell lines [46]. PGG inhibited the catalytic activity of VHR in vitro. The incubation of HeLa cervical cancer cells with PGG markedly decreased their viability, reduced the concentration of cyclin D1 and Bcl-2, and inhibited STAT3 phosphorylation [47].
2.10. Leukemia
Leukemia is a systemic cancer commonly treated using chemotherapy. Acquired drug resistance is a common complication of the available therapeutic options, and patients eventually develop relapsed or refractory disease [48]. Moreover, chemotherapeutic drugs used for leukemia treatment have high costs and severe side effects [49]. Several authors have reported the antileukemic effects of PGG. Pan et al. showed that PGG effectively inhibited the growth of human promyelocytic leukemia HL-60 cells and induced apoptosis in them [50]. In addition, Kwon et al. demonstrated that PGG enhanced the anticancer activity of imatinib in chronic myelogenous leukemia K562 cells in mice through the ROS-dependent JNK and down-regulated domain-associated protein (DAXX) signaling pathway [51]. Recently, Tseeleesuren and coworkers reported that PGG has therapeutic potential for multiple myeloma. In this study, PGG inhibited MYC transcription and promoted MYC degradation through a proteasome-independent pathway, thereby inducing G1-phase cycle arrest and apoptosis in multiple myeloma cell lines [52].
Table 1. In vitro studies on the anticancer activity of PGG.
| Cancer Type and Cell Lines | Plant Source of PGG | IC50 (Exposure Time) | Effect | References |
|---|---|---|---|---|
| Breast | ||||
| MDA-MB-231 | 47.25 ± 2.03 µg/mL (24 h) | Inhibit tumor cell proliferation and induce cell apoptosis | [22] | |
| <11.76 µg/mL (72 h) | Induced cell S-phase arrest | [23] | ||
| 23.52 µg/mL (24 h) | Inhibit tumor cell growth | [25] | ||
| Gallnut of Rhus chinensis Mill |
1.13 µg/mL (72 h) | Attenuate cell proliferation | [26] | |
| Bouea macrophylla seeds | 26.46 ± 6.53 µg/mL (72 h) | Induce cell apoptosis | [27] | |
| MDA-MB-468 | 33.60 ± 0.70 µg/mL (24 h) | Inhibit tumor cell proliferation and induce cell apoptosis | [22] | |
| MCF-7 | <11.76 µg/mL (72 h) | Induced cell S-phase arrest | [23] | |
| Bouea macrophylla seeds | >100 µg/mL (72 h) | Induce apoptosis | [27] | |
| Lung | ||||
| MRC5-SV2 | Anacardium occidentale L. | 52.24 µg/mL (48 h) | Induce cell oxidative stress, cytotoxicity |
[53] |
| LLC | Gallnut of Rhus chinensis Mill | >70.55 µg/mL(48 h) | Induce cell apoptosis | [32] |
| Liver | ||||
| Huh7 | Paeonia lactiflora | 30 µg/mL (72 h) | Induce cell apoptosis, reduced the colony formation | [36] |
| Hep G2 | Paeonia lactiflora | 160 µg/mL (72 h) | Inhibit tumor cell proliferation | [36] |
| Hep3B | Paeonia lactiflora | 70 µg/mL (72 h) | Inhibit tumor cell proliferation | [36] |
| SK-HEP-1 | Paeonia lactiflora | 100 µg/mL (72 h) | Inhibit tumor cell proliferation | [36] |
| HepG2 | 27.94 µg/mL (48 h) | inhibit the proliferation, migration and invasion, induce G1 arrest and apoptosis | [54] | |
| Prostate | ||||
| LNCaP | Gallnut of Rhus chinensis Mill | ≤23.52 µg/mL (96 h) | Induce G1-cell cycle arrests | [31] |
| DU145 | ≤23.52 µg/mL (96 h) | Induce S-cell cycle arrests | ||
| Head and Neck | ||||
| CAL27 | Bouea macrophylla seed | 16.68 ± 1.20 µg/mL (48 h) | suppress the tumer cells stemness trait | [12] |
| FaDu | Bouea macrophylla seed | 26.50 ± 1.46 µg/mL (48 h) | suppress the tumer cells stemness trait | [12] |
| Colorectal | ||||
| HCT116 | 0.65 ± 0.34 µg/mL (48 h) | Induce cell apoptosis | [44] | |
| HT29 | 4.19 ± 1.09 µg/mL (48 h) | Induce cell apoptosis | [44] | |
| Glioma cancer | ||||
| U87 | 23.52 µg/mL | Inhibit tumor cell growth | [25] |
LLC, Lewis lung carcinoma.
Table 2. In vivo studies on the anticancer activity of PGG.
| Cancer Type and Cell Lines | Plant Source of PGG | Model Treatment Dose | (Administration Route) | Eeffect | References |
|---|---|---|---|---|---|
| Breast | |||||
| MDA-MB-231 | MDA-MB-231 injected subcutaneously into the right flank of each 6-week-old female athymic nude mouse | 20 mg/kg (O.G.) | Inhibition of breast cancer cell growth | [23] | |
| Gallnut of Rhus chinensis Mill | MDA-MB-231 injected subcutaneously into the right flank of a 6-week-old female BALB/c athymic nude mice | 10 mg/kg (O.G.) | Inhibition of MDA-MB-231 xenograft growth and lung metastasis | [24] | |
| Lung | |||||
| LLC | Gallnut of Rhus chinensis Mill | tumor inoculation in C57BL/6 mice | 4 or 20 mg/kg (I.P.) alternate days for 17 days from day 3 | Decrease in tumor volume over time, suppression of the weight of the tumor, and inhibite tumor angiogenesis | [32] |
| Liver | |||||
| Huh7 | Paeonia lactiflora | Huh7 cells subcutaneously implanted subcutaneously into Balb/c nude mice | 300 mg/kg (O.G.) | Suppression of the tumor growth by inhibiting the expression of MYC | [36][37] |
| Prostate | |||||
| PC-3 | Intratibial injection of PC-3 in nude mice | 25 mg/kg (I.P.) | Suppression of Tumorigenesis in nude mice | [28] | |
| Pancreatic | |||||
| MiaPaCa2 | tumor cells were transplanted subcutaneously in male athymic Balb/c mice | 20 mg/kg (O.G.) | Alleviates cancer cachexia |
[38] | |
| A piece of tumor tissue made of MiaPaCa2 cells was embedded orthotopically in athymic mice | 20 mg/kg (O.G.) | Intra-pancreatic insulin normally combated the pharmacologic effects of PGG | [55] |
O.G., Oral gavage; I.P., Intraperitoneal injection.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Phillips, M.C.; Mousa, S.A. Clinical application of nano-targeting for enhancing chemotherapeutic efficacy and safety in cancer management. Nanomedicine 2022, 17, 405–421.
- Nasir, A.; Bullo, M.M.H.; Ahmed, Z.; Imtiaz, A.; Yaqoob, E.; Jadoon, M.; Ahmed, H.; Afreen, A.; Yaqoob, S. Nutrigenomics: Epigenetics and cancer prevention: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1375–1387.
- Li, G.; Lin, P.; Wang, K.; Gu, C.-C.; Kusari, S. Artificial intelligence-guided discovery of anticancer lead compounds from plants and associated microorganisms. Trends Cancer 2022, 8, 65–80.
- Kimura, I.; Kagawa, S.; Tsuneki, H.; Tanaka, K.; Nagashima, F. Multitasking bamboo leaf-derived compounds in prevention of infectious, inflammatory, atherosclerotic, metabolic, and neuropsychiatric diseases. Pharmacol. Ther. 2022, 235, 108159.
- Bailly, C. The traditional and modern uses of Selaginella tamariscina (P.Beauv.) Spring, in medicine and cosmetic: Applications and bioactive ingredients. J. Ethnopharmacol. 2021, 280, 114444.
- Long, J.; Guan, P.; Hu, X.; Yang, L.; He, L.; Lin, Q.; Luo, F.; Li, J.; He, X.; Du, Z.; et al. Natural Polyphenols as Targeted Modulators in Colon Cancer: Molecular Mechanisms and Applications. Front. Immunol. 2021, 12, 635484.
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385.
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447.
- Zeng, J.; Han, J.; Liu, Z.; Yu, M.; Li, H.; Yu, J. Pentagalloylglucose disrupts the PALB2-BRCA2 interaction and potentiates tumor sensitivity to PARP inhibitor and radiotherapy. Cancer Lett. 2022, 546, 215851.
- Park, K.-Y.; Lee, H.-J.; Jeong, S.-J.; Kim, H.-S.; Kim, S.-H.; Lim, S.; Kim, H.-C.; Lü, J.; Kim, S.-H. 1,2,3,4,6-Penta-O-galloly-beta-D-glucose Suppresses Hypoxia-Induced Accumulation of Hypoxia-Inducible Factor-1α and Signaling in LNCaP Prostate Cancer Cells. Biol. Pharm. Bull. 2010, 33, 1835–1840.
- Kantapan, J.; Dechsupa, N.; Tippanya, D.; Nobnop, W.; Chitapanarux, I. Gallotannin from Bouea macrophylla Seed Extract Suppresses Cancer Stem-like Cells and Radiosensitizes Head and Neck Cancer. Int. J. Mol. Sci. 2021, 22, 9253.
- Meng, J.; Li, Q.; Cao, Z.; Gu, D.; Wang, Y.; Zhang, Y.; Wang, Y.; Yang, Y.; He, F. Rapid screening and separation of active compounds against α-amylase from Toona sinensis by ligand fishing and high-speed counter-current chromatography. Int. J. Biol. Macromol. 2021, 174, 270–277.
- Chen, X.; Daniels, N.A.; Cottrill, D.; Cao, Y.; Wang, X.; Li, Y.; Shriwas, P.; Qian, Y.; Archer, M.W.; Whitticar, N.B.; et al. Natural Compound α-PGG and Its Synthetic Derivative 6Cl-TGQ Alter Insulin Secretion: Evidence for Diminishing Glucose Uptake as a Mechanism. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 759–772.
- Sylla, T.; Pouységu, L.; Da Costa, G.; Deffieux, D.; Monti, J.-P.; Quideau, S. Gallotannins and Tannic Acid: First Chemical Syntheses and In Vitro Inhibitory Activity on Alzheimer’s Amyloid β-Peptide Aggregation. Angew. Chem. Int. Ed. 2015, 54, 8217–8221.
- Bai, Z.; Yu, R.; Zheng, T.; Sun, D.; Zhou, Y.; Tang, J.; Zhu, H.; Li, G.; Niu, L.; Cui, L.; et al. A Novel Strategy for Unveiling Spatial Distribution Pattern of Gallotannins in Paeonia rockii and Paeonia ostii Based on LC–QTRAP–MS. Metabolites 2022, 12, 326.
- Reis, A.; Soares, S.; Sousa, C.F.; Dias, R.; Gameiro, P.; Soares, S.; de Freitas, V. Interaction of polyphenols with model membranes: Putative implications to mouthfeel perception. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183133.
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-Cancer, Anti-Diabetic and Other Pharmacologic and Biological Activities of Penta-Galloyl-Glucose. Pharm. Res. 2009, 26, 2066–2080.
- Sekowski, S.; Veiko, A.; Olchowik-Grabarek, E.; Dubis, A.; Wilczewska, A.Z.; Markiewicz, K.H.; Zavodnik, I.B.; Lapshina, E.; Dobrzynska, I.; Abdulladjanova, N.; et al. Hydrolysable tannins change physicochemical parameters of lipid nano-vesicles and reduce DPPH radical—Experimental studies and quantum chemical analysis. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183778.
- Chen, R.H.; Yang, L.J.; Hamdoun, S.; Chung, S.K.; Lam, C.W.-K.; Zhang, K.X.; Guo, X.; Xia, C.; Law, B.Y.K.; Wong, V.K.W. 1,2,3,4,6-Pentagalloyl Glucose, a RBD-ACE2 Binding Inhibitor to Prevent SARS-CoV-2 Infection. Front. Pharmacol. 2021, 12, 634176.
- Golledge, J.; Thanigaimani, S.; Phie, J. A Systematic Review and Meta-Analysis of the Effect of Pentagalloyl Glucose Administration on Aortic Expansion in Animal Models. Biomedicines 2021, 9, 1442.
- Mendonca, P.; Alghamdi, S.; Messeha, S.; Soliman, K.F.A. Pentagalloyl glucose inhibits TNF-α-activated CXCL1/GRO-α expression and induces apoptosis-related genes in triple-negative breast cancer cells. Sci. Rep. 2021, 11, 5649.
- Chai, Y.; Lee, H.-J.; Shaik, A.A.; Nkhata, K.; Xing, C.; Zhang, J.; Jeong, S.-J.; Kim, S.-H.; Lü, J. Penta-O-galloyl-β-D-glucose induces G1arrest and DNA replicative S-phase arrest independently of P21 cyclin-dependent kinase inhibitor 1A, P27 cyclin-dependent kinase inhibitor 1B and P53 in human breast cancer cells and is orally active against triple-negative xenograft growth. Breast Cancer Res. 2010, 12, R67.
- Lee, H.-J.; Seo, N.-J.; Jeong, S.-J.; Park, Y.; Jung, D.-B.; Koh, W.; Lee, E.-O.; Ahn, K.S.; Lü, J.; Kim, S.-H. Oral administration of penta-O-galloyl-D-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with JAK1-STAT3 inhibition. Carcinogenesis 2011, 32, 804–811.
- Zhao, W.; Wang, Y.; Hao, W.; Zhao, M.; Peng, S. In vitro inhibition of fatty acid synthase by 1,2,3,4,6-penta-O-galloyl-β-d-glucose plays a vital role in anti-tumour activity. Biochem. Biophys. Res. Commun. 2014, 445, 346–351.
- Deiab, S.; Mazzio, E.; Eyunni, S.; McTier, O.; Mateeva, N.; Elshami, F.; Soliman, K.F.A. 1,2,3,4,6-Penta-O-galloylglucose within Galla Chinensis Inhibits Human LDH-A and Attenuates Cell Proliferation in MDA-MB-231 Breast Cancer Cells. Evid.-Based Complement. Altern. Med. 2015, 2015, 276946.
- Kantapan, J.; Paksee, S.; Chawapun, P.; Sangthong, P.; Dechsupa, N. Pentagalloyl Glucose- and Ethyl Gallate-Rich Extract from Maprang Seeds Induce Apoptosis in MCF-7 Breast Cancer Cells through Mitochondria-Mediated Pathway. Evid.-Based Complement. Altern. Med. 2020, 2020, 5686029.
- Kuo, P.-T.; Lin, T.-P.; Liu, L.-C.; Huang, C.-H.; Lin, J.-K.; Kao, J.-Y.; Way, T.-D. Penta-O-galloyl-β-d-glucose Suppresses Prostate Cancer Bone Metastasis by Transcriptionally Repressing EGF-Induced MMP-9 Expression. J. Agric. Food Chem. 2009, 57, 3331–3339.
- Hu, H.; Lee, H.-J.; Jiang, C.; Zhang, J.; Wang, L.; Zhao, Y.; Xiang, Q.; Lee, E.-O.; Kim, S.-H.; Lü, J. Penta-1,2,3,4,6-O-galloyl-β-d-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol. Cancer Ther. 2008, 7, 2681–2691.
- Hu, H.; Chai, Y.; Wang, L.; Zhang, J.; Lee, H.J.; Kim, S.-H.; Lü, J. Pentagalloylglucose induces autophagy and caspase-independent programmed deaths in human PC-3 and mouse TRAMP-C2 prostate cancer cells. Mol. Cancer Ther. 2009, 8, 2833–2843.
- Hu, H.; Zhang, J.; Lee, H.J.; Kim, S.-H.; Lü, J. Penta-O-galloyl-beta-D-glucose induces S- and G1-cell cycle arrests in prostate cancer cells targeting DNA replication and cyclin D1. Carcinogenesis 2009, 30, 818–823.
- Huh, J.-E.; Lee, E.-O.; Kim, M.-S.; Kang, K.-S.; Kim, C.-H.; Cha, B.-C.; Surh, Y.-J.; Kim, S.-H. Penta-O-galloyl-beta-d-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: Roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis 2005, 26, 1436–1445.
- Oh, G.-S.; Pae, H.-O.; Oh, H.; Hong, S.-G.; Kim, I.-K.; Chai, K.-Y.; Yun, Y.-G.; Kwon, T.-O.; Chung, H.-T. In vitro anti-proliferative effect of 1,2,3,4,6-penta-O-galloyl-beta-d-glucose on human hepatocellular carcinoma cell line, SK-HEP-1 cells. Cancer Lett. 2001, 174, 17–24.
- Yin, S.; Dong, Y.; Li, J.; Lü, J.; Hu, H. Penta-1,2,3,4,6-O-galloyl-beta-D-glucose induces senescence-like terminal S-phase arrest in human hepatoma and breast cancer cells. Mol. Carcinog. 2011, 50, 592–600.
- Dong, Y.; Yin, S.; Jiang, C.; Luo, X.; Guo, X.; Zhao, C.; Fan, L.; Meng, Y.; Lu, J.; Song, X.; et al. Involvement of autophagy induction in penta-1,2,3,4,6-O-galloyl-β-D-glucose-induced senescence-like growth arrest in human cancer cells. Autophagy 2014, 10, 296–310.
- Kant, R.; Yen, C.-H.; Lu, C.-K.; Lin, Y.-C.; Li, J.-H.; Chen, Y.-M.A. Identification of 1,2,3,4,6-Penta-O-galloyl-β-d-glucopyranoside as a Glycine N-Methyltransferase Enhancer by High-Throughput Screening of Natural Products Inhibits Hepatocellular Carcinoma. Int. J. Mol. Sci. 2016, 17, 669.
- Kant, R.; Yen, C.-H.; Hung, J.-H.; Lu, C.-K.; Tung, C.-Y.; Chang, P.-C.; Chen, Y.-H.; Tyan, Y.-C.; Chen, Y.-M.A. Induction of GNMT by 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranoside through proteasome-independent MYC downregulation in hepatocellular carcinoma. Sci. Rep. 2019, 9, 1968.
- Yang, J.; Wang, F.; Chen, X.; Qiu, S.; Cui, L.; Hu, L. β-Pentagalloyl-Glucose Sabotages Pancreatic Cancer Cells and Ameliorates Ca-chexia in Tumor-Bearing Mice. Am. J. Chin. Med. 2019, 47, 675–689.
- Palamaris, K.; Felekouras, E.; Sakellariou, S. Epithelial to Mesenchymal Transition: Key Regulator of Pancreatic Ductal Adenocarcinoma Progression and Chemoresistance. Cancers 2021, 13, 5532.
- Patil, K.; Khan, F.B.; Akhtar, S.; Ahmad, A.; Uddin, S. The plasticity of pancreatic cancer stem cells: Implications in therapeutic resistance. Cancer Metastasis Rev. 2021, 40, 691–720.
- Gzil, A.; Zarębska, I.; Bursiewicz, W.; Antosik, P.; Grzanka, D.; Szylberg, Ł. Markers of pancreatic cancer stem cells and their clinical and therapeutic implications. Mol. Biol. Rep. 2019, 46, 6629–6645.
- Kim, E.-Y.; Lee, S.-U.; Kim, Y.H. 1,2,3,4,6-Penta-O-galloyl-β-D-glucose Inhibits CD44v3, a cancer stem cell marker, by regulating its transcription factor, in human pancreatic cancer cell line. Anim. Cells Syst. 2022, 26, 328–337.
- Fan, C.-W.; Tang, J.; Jiang, J.-C.; Zhou, M.-M.; Li, M.-S.; Wang, H.-S. Pentagalloylglucose suppresses the growth and migration of human nasopharyngeal cancer cells via the GSK3β/β-catenin pathway in vitro and in vivo. Phytomedicine 2022, 102, 154192.
- Kawk, S.H.; Kang, Y.R.; Kim, Y.H. 1,2,3,4,6-Penta-O-galloyl-β-d-glucose suppresses colon cancer through induction of tumor suppressor. Bioorg. Med. Chem. Lett. 2018, 28, 2117–2123.
- Wang, J.-Y.; Yeh, C.-L.; Chou, H.-C.; Yang, C.-H.; Fu, Y.-N.; Chen, Y.-T.; Cheng, H.-W.; Huang, C.-Y.F.; Liu, H.-P.; Huang, S.-F.; et al. Vaccinia H1-related Phosphatase is a Phosphatase of ErbB Receptors and is Down-regulated in Non-small Cell Lung Cancer. J. Biol. Chem. 2011, 286, 10177–10184.
- Wu, S.; Vossius, S.; Rahmouni, S.; Miletic, A.V.; Vang, T.; Vazquez-Rodriguez, J.; Cerignoli, F.; Arimura, Y.; Williams, S.; Hayes, T.; et al. Multidentate Small-Molecule Inhibitors of Vaccinia H1-Related (VHR) Phosphatase Decrease Proliferation of Cervix Cancer Cells. J. Med. Chem. 2009, 52, 6716–6723.
- Yoon, S.; Kim, D.; Roh, K.M.; Ahn, D.; Kang, H.J.; Chung, S.J. Identification of Vaccinia-H1 Related Phosphatase as an Anticancer Target for 1,2,3,4,6-O-Pentagalloylglucose. Chem. Biodivers. 2020, 17, e1900414.
- Zhang, J.; Gu, Y.; Chen, B. Mechanisms of drug resistance in acute myeloid leukemia. OncoTargets Ther. 2019, 12, 1937–1945.
- Gay, F.; Palumbo, A. Management of disease- and treatment-related complications in patients with multiple myeloma. Med. Oncol. 2010, 27, 43–52.
- Pan, M.-H.; Lin, J.-H.; Lin-Shiau, S.-Y.; Lin, J.-K. Induction of apoptosis by penta-O-galloyl-β-d-glucose through activation of caspase-3 in human leukemia HL-60 cells. Eur. J. Pharmacol. 1999, 381, 171–183.
- Kwon, T.-R.; Jeong, S.-J.; Lee, H.-J.; Sohn, E.J.; Jung, J.H.; Kim, J.-H.; Jung, D.-B.; Lu, J.; Kim, S.-H. Reactive oxygen species-mediated activation of JNK and down-regulation of DAXX are critically involved in penta-O-galloyl-beta-d-glucose-induced apoptosis in chronic myeloid leukemia K562 cells. Biochem. Biophys. Res. Commun. 2012, 424, 530–537.
- Tseeleesuren, D.; Kant, R.; Yen, C.-H.; Hsiao, H.-H.; Chen, Y.-M.A. 1,2,3,4,6-Penta-O-Galloyl-Beta-D-Glucopyranoside Inhibits Proliferation of Multiple Myeloma Cells Accompanied with Suppression of MYC Expression. Front. Pharmacol. 2018, 9, 2066–2080.
- Taiwo, B.J.; Popoola, T.D.; Van Heerden, F.R.; Fatokun, A.A. Pentagalloylglucose, isolated from the leaf extract of Anacardium occidentale L., could elicit rapid and selective cytotoxicity in cancer cells. BMC Complement. Med. Ther. 2020, 20, 287.
- Ding, X.-Q.; Zhao, S.; Wang, J.-Y.; Zheng, H.-C.; Ma, C.-M. Inhibitory effects and molecular mechanisms of pentagalloyl glucose in combination with 5-FU on aggressive phenotypes of HepG2 cells. Nat. Prod. Res. 2021, 35, 815–818.
- Hu, L.; Chen, X.; Qiu, S.; Yang, J.; Liu, H.; Zhang, J.; Zhang, D.; Wang, F. Intra-Pancreatic Insulin Nourishes Cancer Cells: Do Insulin-Receptor Antagonists such as PGG and EGCG Play a Role? Am. J. Chin. Med. 2020, 48, 1005–1019.
More
Information
Subjects:
Oncology; Integrative & Complementary Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
903
Revisions:
3 times
(View History)
Update Date:
07 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




