Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrey Khodonov | -- | 4955 | 2023-07-04 12:48:21 | | | |
| 2 | Camila Xu | Meta information modification | 4955 | 2023-07-05 03:28:09 | | | | |
| 3 | Andrey Khodonov | -42 word(s) | 4913 | 2023-07-11 12:55:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khodonov, A.A.; Belikov, N.E.; Lukin, A.Y.; Laptev, A.V.; Barachevsky, V.A.; Varfolomeev, S.D.; Demina, O.V. Structure, Spectral Properties and Chemistry of Spiropyrans. Encyclopedia. Available online: https://encyclopedia.pub/entry/46397 (accessed on 07 March 2026).
Khodonov AA, Belikov NE, Lukin AY, Laptev AV, Barachevsky VA, Varfolomeev SD, et al. Structure, Spectral Properties and Chemistry of Spiropyrans. Encyclopedia. Available at: https://encyclopedia.pub/entry/46397. Accessed March 07, 2026.
Khodonov, Andrey A., Nikolay E. Belikov, Alexey Yu. Lukin, Alexey V. Laptev, Valery A. Barachevsky, Sergey D. Varfolomeev, Olga V. Demina. "Structure, Spectral Properties and Chemistry of Spiropyrans" Encyclopedia, https://encyclopedia.pub/entry/46397 (accessed March 07, 2026).
Khodonov, A.A., Belikov, N.E., Lukin, A.Y., Laptev, A.V., Barachevsky, V.A., Varfolomeev, S.D., & Demina, O.V. (2023, July 04). Structure, Spectral Properties and Chemistry of Spiropyrans. In Encyclopedia. https://encyclopedia.pub/entry/46397
Khodonov, Andrey A., et al. "Structure, Spectral Properties and Chemistry of Spiropyrans." Encyclopedia. Web. 04 July, 2023.
Copy Citation
Spiropyrans (SP) are a well-studied class of photochromic compounds. These compounds are usually named in conformity with the IUPAC rules for nomenclature of heterocyclic spirocompounds, as derivatives of 1′,3′,3′-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2′-indoline] or 1′,3′-dihydro-1′,3′,3′-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2′-indole].
5′-substituted spiropyrans
photochromism
photochromic labels and probes
1. Structure and Spectral Properties of Spiropyrans
Spiropyrans (SP) are a well-studied class of photochromic compounds. These compounds are usually named in conformity with the IUPAC rules for nomenclature of heterocyclic spirocompounds, as derivatives of 1′,3′,3′-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2′-indoline] or 1′,3′-dihydro-1′,3′,3′-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2′-indole] (Figure 1). However, other naming variations of these compounds, such as 2H-chromene derivatives, were often used in early works.
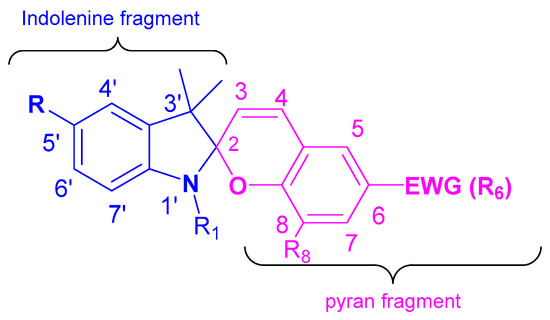
Figure 1. Spiropyran structure.
Spiropyrans typically can exist in an equilibrium mixture between spiroform (A) and colored forms of merocyanines (MC, B) which themselves can comprise mixtures of geometric isomers with a range of relative stabilities and reactivities. In darkness, spiroform (A) spiropyran molecule consists of two heterocyclic fragments in orthogonal orientation, that have a common central sp3-hybridized carbon atom, which is connected to the C2-position of 2H-pyran ring without conjugation between two parts of molecule. Photochromism of spiropyrans involves photodissociation of the C-O-bond in the initial cyclic spiroform (A) by action of UV light and subsequent thermal cis→trans isomerization into the deep colored merocyanine isomers (B, MC) with zwitterionic and/or quinoid structures. The reason for the UV-light-induced color change in this system is formation of a conjugation chain during the transition from spiroform (A) (colorless or pale yellow) to MC with the appearance of a deep color. Then it is transformed back into the initial spiroform (A) by the action of visible light absorbed by the photoinduced form B or spontaneously in the dark. Despite suffering from thermoinstability and low fatigue resistance, spiropyrans still offer a unique feature of significantly increased dipole moment after photoisomerization from spiroform (A) into MC charge-separated zwitterionic form. The open MC form has a larger dipole moment (14–20 D) than the closed spiroform (A) (4–6 D), and therefore the stability of the MC form strongly depends on the electronic effects of substituent(s) in indoline and 2H-pyran moieties, solvent polarity, and presence of metal complexation.
In most cases, the MC (B) form absorbs at longer wavelengths than spiroform (A) (positive photochromism), but the alternative case is also possible (negative or inverse photochromism). Negative photochromism is very much less common than the normal or positive variant and is exhibited by only a few spiropyran derivatives, especially those bearing free hydroxy, carboxy, or amino groups [1][2].
Under external action, spiropyrans, as a rule, undergo isomerization as a result of which the spiroform (A) is transformed into the merocyanine (MC) isomers (Figure 2). The MC form is characterized by the presence of a number of structural isomers of the quinoid and betaine types. This interconversion between two states is accompanied by change of color, but additional changes in refractive index, dielectric constant, redox potentials, solubility, viscosity, surface wettability, magnetism, luminescence, or mechanical effects are also possible.
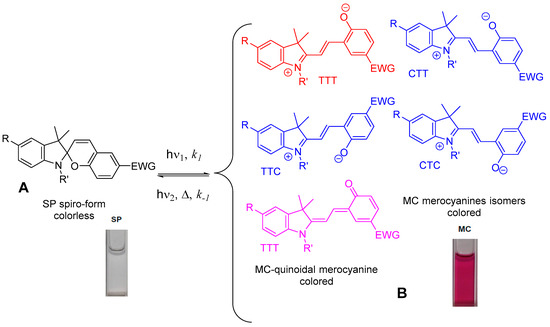
Figure 2. Phototransformations of the spiropyran molecule.
One of the unique features of spiropyrans is that the MC form is able to coordinate with metal ions and that the spiroform form (A) does not show such a property [3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19].
The structures of colorless (A) and colored MC forms of spiropyran molecule were approved unambiguously by modern spectral methods.
IR spectra of the spiroform (A) contain characteristic stretching vibrations of a spiro-C-O bond, an intense band at 940–960 cm−1, and a band of the double bond of the pyran ring at 1640 cm−1, which is not found in the IR spectra of the colored MC isomers [20][21].
1.1. UV-VIS-Spectra and Fluorescence Spectra of the 5′-Substituted Spiropyran Derivatives
Since the lifetime of a colored MC form often ranges from fractions of a second to several seconds, study of composition and structure of its photostationary mixture intermediates is greatly complicated. To solve this complex problem, modern spectral-kinetic methods were proposed, which make it possible to record spectral changes in time range from femtoseconds to minutes in UV- and visible spectral regions by pulsed absorption spectroscopy and laser flash photolysis. The UV-VIS spectra of spiropyran derivatives were discussed in many experimental and theoretical works devoted to study of photochromic compounds [9][10][22][23][24][25][26][27][28].
To determine the degree of influence of the nature and position of substituents in the photochromic molecule on its spectral parameters and optical characteristics, at first the properties of two reference compounds, unsubstituted spiropyran and its 6-nitroderivative, were studied.
According to these studies [11][22], the main absorption band of original spiroform (A) of the unsubstituted spiropyran SP1 in ethanol has an λAmax at 295 nm. Under UV irradiation, formation of photoinduced MC form was recorded, which manifests itself in appearance of an absorption band in the visible region of the spectrum (λBmax = 550 nm, ε550 = 35.0 103 M−1 cm−1), which immediately spontaneously disappears after turning off the light (kBAdb = 0.48 s−1) with regeneration of the original SP1 cyclic form. However, in the dark, an equilibrium occurs between two forms, and the solution acquires a deep violet color, due to a small amount of the MC form is present in it. The reverse reaction that produces the colorless spiroform (A) is induced by visible light or heat or even occurs spontaneously. The rate of these reactions depends on the reaction media (i.e., solvent polarity causing stabilization/destabilization of the zwitterionic MC form in polar/nonpolar solvents). Stabilization of the zwitterionic MC form in polar solvents leads to a larger energy activation and a slower MC→A transition compared to non-polar solvents.
The results of a complete standard study set are presented for the podand SP288 as an example, (see Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7 [29]). Figure 3 shows the photochemical properties of the podand SP288 in ethanol (A) and in toluene (B). This compound can be observed to exhibit photochromic properties which are typical for 6-nitro-substituted spiropyrans with various substitutients at the 5′-position. After UV irradiation, a structured absorption band of the MC form appears in the visible region of the spectrum (Figure 3A,B, crv. 2). After turning off the activating irradiation, it spontaneously disappears (Figure 3A,B, crv. 3, 4) accompanied by restoration of the absorption spectrum of the initial cyclic spiroform A (Figure 3A,B, crv. 1). Similar photoinduced spectral changes were observed for this compound in acetonitrile and in toluene.
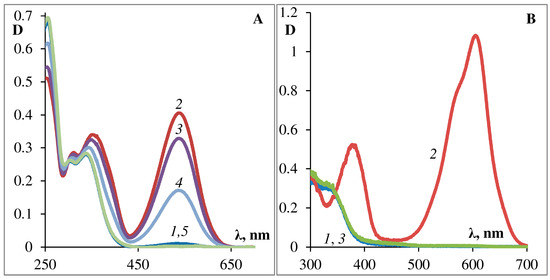
Figure 3. Photochemical properties of podand SP288 in ethanol (A) and in toluene (B). The curves on (A): 1—before illumination; 2—after 30 s of illumination with UFS-2 filter, 3, 4—after 10, 45 min in the dark, respectively, 5—after 30 s of illumination with visible light. The curves on (B): 1—before illumination; 2—after 10 s of illumination with UFS-2 filter, 3—after 30 s in the dark.
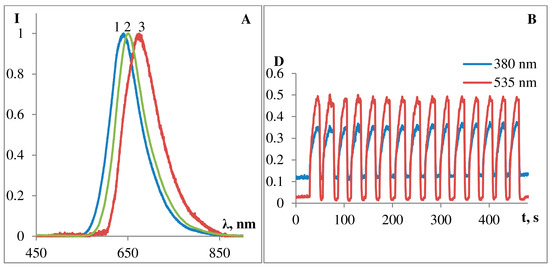
Figure 4. (A): Fluorescence spectra of photoinduced MC form of podand SP288 in ethanol (1), acetonitrile (2) and toluene (3) under light excitation with λex = 280–370 nm. (B): Reproductibility of photocoloring/photobleaching kinetics of podand SP288 in ethanol, 15 cycles, 25 °C.
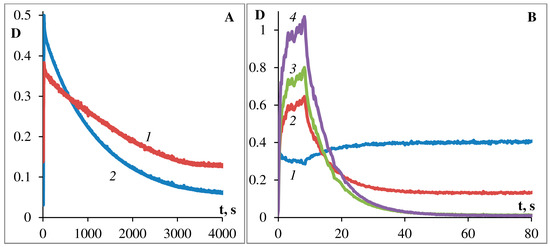
Figure 5. Kinetics of dark bleaching of podand SP288 in ethanol (A) and in toluene (B). The curves for (A): 1—at 380 nm, 2—at 535 nm. The curves for (B): 1—at 330 nm, 2—at 377 nm, 3—at 572 nm, 4—at 605 nm.
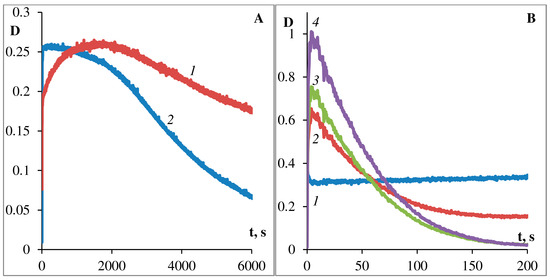
Figure 6. Kinetics of photodegradation of podand SP288 in ethanol (A) and in toluene (B). Sample was examined by exposure to unfiltered light illumination of Hamamatsu-LC8 lamp. The curves for (A): 1—at 380 nm, 2—at 535 nm. The curves for (B): 1—at 330 nm, 2—at 377 nm, 3—at 572 nm, 4—at 605 nm.
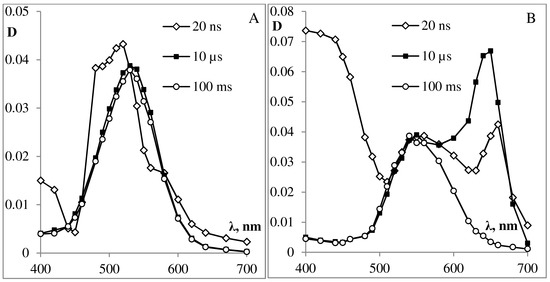
Figure 7. Absorption spectra of laser flash photolysis intermediates of photochromic podand SP288 in solution of ethanol (A) and toluene (B) (the value of the optical absorption density at 337 nm is 0.4) after 20 ns, 10 μs, 100 ms after the laser pulse.
Figure 4A shows fluorescence spectra of photoinduced MC form of the podand SP288 in ethanol (1), acetonitrile (2), and toluene (3). The emission band maxima are at λ 636 nm (in ethanol), at λ 666 nm (in toluene), and at λ 650 nm (in acetonitrile). Therefore, substituted spiroforms of 5′-substituted spiropyran derivatives were demonstrated to not exhibit pronounced fluorescence; however, they are able to reversibly switch from a nonfluorescent spiroform A to highly fluorescent MC form. These structures form the basis for creation of chemical sensors, when coupled with a suitable ionophore (e.g., iminodiacetate fragment in podand SP288). Switching is reversed on exposure to visible light or heat. Importantly, two isomers have a high switching reliability and fatigue resistance, which maximizes the number of switching cycles. Photochromic podand SP288 is characterized by rather high count of photochromic transformation cycles (Figure 4B). As it can be seen from Figure 4B, upon successive alternation of irradiation of the sample with visible and UV light, the intensity of the absorption band of the MC form in ethanol changes insignificantly.
Comparison of the data obtained for solutions of photochromic compound shows that upon going from ethanol to toluene, the photostability significantly decreases and the process of dark photobleaching significantly accelerates (Figure 3A,B, Figure 5A,B and Figure 6A,B). Figure 5 and Figure 6 show the kinetics of photocoloring/photobleaching/photo-degradation processes of podand SP288 samples in ethanol (A) and toluene (B) solutions. The photo-degradation parameter was characterized by how long the irradiation took to decrease photoinduced optical density for the photostationary state at the absorption maximum of the MC form by one half (τ1/2, s).
The bands of the photoinduced MC form of photochromic podand SP288 (538 nm ethanol, 605 nm toluene) spontaneously slowly disappear when the sample is kept in the dark or quickly when irradiated with visible light, and in toluene the process of bleaching of the photoinduced merocyanine form occurs 100 times faster than in ethanol. Moreover, comparison of the data obtained for solutions of SP288 in ethanol and in toluene shows that their fatigue light resistance is sharply increased when changing toluene to ethanol. The absorption band maximum of the photoinduced MC form undergoes a hypsochromic shift when polarity of the solvent increases, which is consistent with the behavior of spiropyrans in solvents of different polarity. In this case, the rate of spontaneous dark discoloration of the MC form slows down (Figure 5A,B) and the resistance of the compound to irreversible photoconversion increases (Figure 6A,B). The smallest value of the dark relaxation rate constant and high resistance to photodegradation of podand SP288 in ethanol can be explained by formation of a hydrogen bond between the phenolate oxygen of MC forms of spiropyran podand and solvent molecules.
Laser excitation of spiropyran SP288 solution in ethanol (A) or in toluene (B) leads to the formation of a triplet state (3MC) of the MC form (see Figure 7), similar to what was well-documented for the 6-nitro-substituted derivatives without substituents in the indoline ring [9][10][28][30][31].
In 1982, Krysanov and Alfimov were the first to examine the transient absorption spectra in the photocoloration and photobleaching of the 6-nitro-spiropyran derivatives, which have been investigated with transient picosecond spectroscopy. Their results demonstrated cleavage of the C-O bond between the spiro carbon and oxygen, which is believed to occur in picosecond to subpicosecond time region, and is assumed to lead to the formation of a primary photoproduct (nonplanar cis-cisoid intermediate X at 440 nm) with an orthogonal parent geometry, which is produced in less than 10 ps, followed by a geometrical change to the planar MC forms [27].
This data suggest that for 6-nitro-substituted spiropyrans, MCs are formed via both singlet and triplet routes. In contrast to 6-nitro-substituted derivatives, unsubstituted indolino spiropyrans demonstrate photochromism only through the excited singlet intermediate, which was confirmed by analyzing the oxygen effect on transient absorption [25][32][33][34].
Photochromic parameters and spectral properties data for 5′-substituted spiropyrans and their photointermediates were determined by spectral-kinetic methods (stationary and pulsed absorption spectroscopy and laser flash photolysis in the UV- and visible spectral regions). For the series of substituted dyads (SP263–SP265, SP270, SP271, SP273) containing a fragment of the stilbene fluorophore at the 5′-position of the indoline part of the photochromic molecule, the nature of the substituents in the aryl ring of the fluorophore was found to have the strongest influence mainly on the spectra of the spiroform (A): λAmax 335–409 nm (ΔλAmax 74 nm) in ethanol and λAmax 325–407 nm (ΔλAmax 82 nm) in toluene solutions. The difference in the λmax for the Z- and E-isomers in the series of these derivatives, containing a substituted aryl ring in the stilbene fluorophore fragment for spiroform (A) was small (ΔλAmax 7–9 nm) and it was almost absent for the MC form [35][36].
1.2. NMR Spectroscopy
Since the lifetime of a colored MC form often ranges from fractions of a second, several seconds to minute time scale, it greatly complicates the study of composition and structure of its photostationary mixture intermediates. As in the case of study of absorption spectra, the problem of a short lifetime of a colored MC form arose, and was not completely solved; therefore, the use of the NMR method was limited only to recording the spectra of a stable spiroforms A, and wide application of the NMR spectroscopy in study of photochromic compounds’ properties is limited due to the short lifetime of photointermediates of the spiropyran MC form. However this problem is solvable with the help of novel equipment for NMR spectroscopy, in which NMR spectra are recorded simultaneously with irradiation of a sample with light of given wavelength [37]. The difference in the spectra of A and B isomers makes it possible to identify them when both are present. An alternative decision to this problem lies in the development of new approaches to the stabilization of the spiropyran MC form lifetime by using more viscous or polar solvents [38], as well as introducing additional functional substituents or fragments of heterocycles into the pyran part of the photochrome molecule. At the same time, a novel way was proposed to stabilize the MC form lifetime by complexation with certain cations.
In case of cyclic form A of SP1, the upfield initial signals of protons and carbons of two nonequivalent 3′,3′-methyl groups in the form of 2 singlets at δ 1.18 ppm/1.31 ppm (20.8/26.6 ppm) become equivalent due to the quasiplanar structure of complex of MC form SP1/Al(NO3)3 and transform with a downfield shift into a single singlet (6H) at δ 1.87 ppm/(27.2 ppm). Similarly, the signal N-1′−CH3 in the SP1 cyclic form at δ 2.74 ppm/(29.5 ppm) and in the complex of MC form SP1/Al(NO3)3 has downfield shift at δ 4.16 ppm/(34.8 ppm) due to the presence of a positively charged N-atom.
In cyclic form SP1, the signals of the AB nuclei in the C3−C4 position of the pyran ring in the form of 2 doublets at δ 5.77 ppm/6.96 ppm with J 10.2 Hz are also shifted downfield in the spectrum of complex of MC form SP1/Al(NO3)3 (δ 8.72 ppm/7.77 ppm, J 16.4 Hz), which makes it possible to unambiguously attribute the C=C bond configuration in complex MC form SP1/Al(NO3)3 as the trans-isomer (TTT) (see Figure 8).
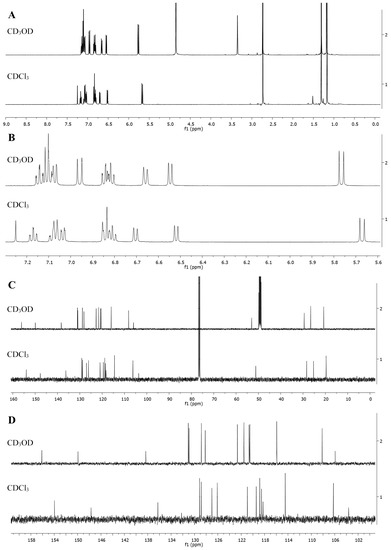
Figure 8. 1H (A,B) and 13C (C,D) NMR spectra of the spiroform SP1 in CD3OD and in CDCl3, where (A,C)—spectra of the full range of chemical shifts of signals, (B,D)—spectra fragments of signals from nuclei of aromatic rings (indoline and benzopyran), Bruker Avance III-500 NMR spectrometer.
1.3. Solvatochromism
Most of the spiropyrans known today in spiroform (A) are colorless or slightly colored crystalline substances that are essentially insoluble in water, only slightly soluble in alcohols and aliphatic hydrocarbons, and quite soluble in aromatic hydrocarbons and haloalkanes.
UV irradiation of solutions containing spiropyran results in the formation of colored MC form, which is a highly polar isomer and hence the nature of the microenvironment influences the properties of spiropyrans. This causes hypsochromic (blue shift; decrease in λmax) or bathochromic (red shift; increase in λmax) shifts in their absorption spectra depending on the solvent polarities. The effect of solvent polarity and hydrogen bonding on λmax of MC form has been investigated. Their solutions in nonpolar solvents are usually colorless, whereas in polar solvents they may be more or less intensely colored, depending on the structure and the nature of the substituents because of thermal equilibrium between A and MC isomers.
It was found that by dissolving the spiropyran dye in different solvents, a mixture of spiroform (A) and MC form may be observed due to the occurrence of different interactions between the solvents and the solute, and subsequent effects on ground state and excited state energy levels of the conjugated π electrons in MC. The MC form is occasionally further stabilized under certain conditions, such as hydrogen bonding, combination with crown ether or β-cyclodextrin and complexation with metal cations.
It can be seen from data in Figure 9A,B, that like other merocyanines, the open MC form of 5′-substituted spiropyrans is a negative solvatochromic dye, i.e., with increasing solvent polarity, the absorption band undergoes a hypsochromic (or blue) shift.
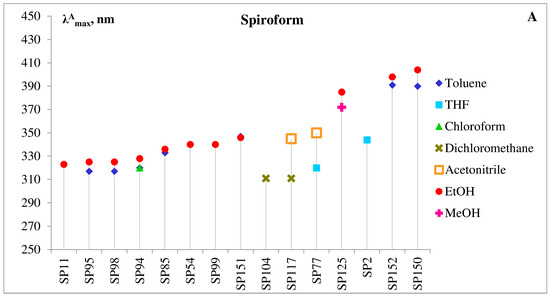
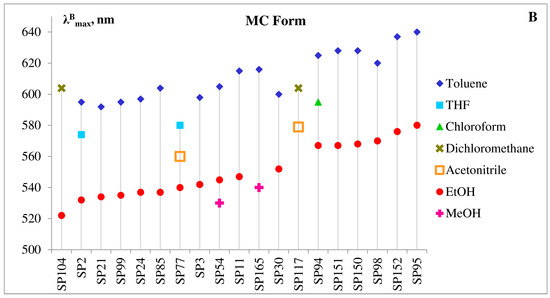
Figure 9. Correlation of molecular structure and absorption maxima for the selected 5′-substituted spiropyrans in various solvents. (A)—Absorption maxima of spiroform (A,B)—absorption maxima of MC form. Legends list solvents in order of increasing their relative polarity.
However, the electronic structure of the MCs derivatives was found to be extremely sensitive to influence of substituents in both indoline and pyran rings in these photochromes. By varying the acceptor or donor properties, steric hidrances and positions of the substituents, it is possible to polarize the π-system of the MC form sufficiently to reach a zwitterionic-like structure and observe negative solvatochromism in the push-pull-type dyes, containing electron-donating groups in the indoline and electron-withdrawing ones in the pyran moieties [7][21][22][24][25][39][40][41][42][43].
Series of works on spiropyran derivatives demonstrate the possibility of a hydrolytic decomposition of MC isomer in aqueous media. MC form was also shown to become more stable than spiroform (A), and a reverse (negative) photochromic behavior is detected in aqueous solvents.
In recent years, increased interest has been observed in the theoretical and experimental study of isomerization processes of organic photochromes in solid state. Many spiropyran derivatives exhibit photochromic properties in powders or even in single crystals [44].
1.4. Acidochromism
Transformation of spiropyran molecules from the cyclic spiroform (A) to the opened MC form can be initiated by light and other reasons: changes in temperature, pH, redox potential, polarity of a medium, and even by mechanical stress. For these dyes, the effect on the spectral properties caused by changes in temperature (thermochromism), pH (acidochromism), solvent polarity (solvatochromism), redox potential (electrochromism), interactions with metal ions (ionochromism), and mechanical stress (mechanochromism) has been well studied. Moreover, presence of many metal cations, several nucleophilic anions, and some organic species can also induce their isomerization. Thus, spiropyran-like systems meet the basic requirements for multi-functionality and sensitivity that make them promising building blocks for creation of various dynamic stimulus-responsive materials and systems [8][24][35][36][39].
The two isomeric states of spiropyran have different properties. The MC isomer is significantly more basic than spiroform (A) (upon the transformation of closed spiroform to MC, pKa value changes by more than six units).
The action of acids on spiropyrans’ solutions may be accompanied by: (1) cleavage of the [2H]-pyran ring, (2) protonation the phenolate anion of the MC form [45]. The protonation causes a significant shift of the absorption band maximum toward the shorter wavelength compared to that of the MC form. In case of SP2, two salts with HCl have been isolated, differing in physical characteristics. In toluene at −78 °C a yellowish salt is formed, and it is converted completely into the MCH+ upon boiling sample in alcohol for 10 min (see Figure 10 and Figure 11).
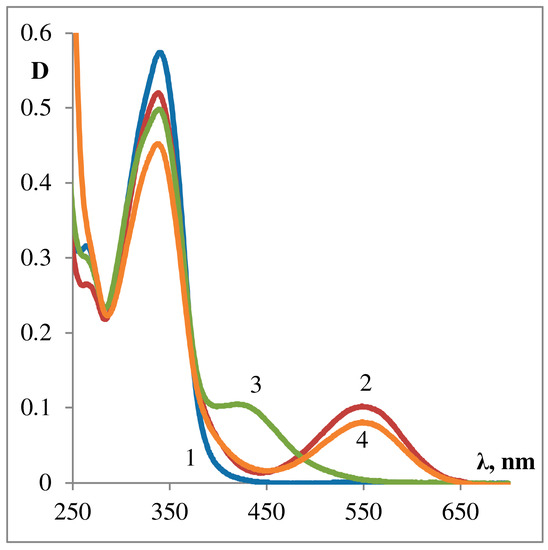
Figure 10. Protonation and deprotonation of MC form of SP263 in ethanol. 1—Spiroform (A); 2—MC form, after 2 min of sample illumination with UFS-2 filter; 3—MCH+ form protonated with CF3COOH; 4—MCH+ form deprotonated with Et3N.
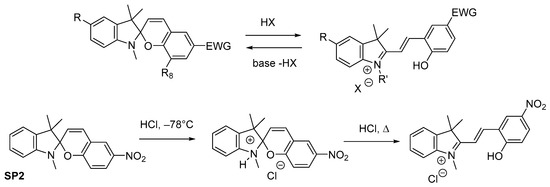
Figure 11. Protonation and deprotonation of the spiropyran molecule by acids.
Protonation and deprotonation of MC form of the dyad SP263 (see λmaxMCH+ of SP263 426 nm) in ethanol is shown on Figure 10. It should also be noted that dyads of this series (SP263–SP273) have a very low threshold of sensitivity to the traces of acids of various strengths, which allows to consider their possible use as pH sensitive elements of sensors [8][36].
2. Chemistry of Spiropyrans
The possibility of directional and reversible change of the structure of spiropyrans and their unique properties generate continuously growing interest in the delopment of novel synthetic methods for producing photochromes of this class and study of their properties. The fine tunability of photochromes’ chemical structure and their optical properties provides opportunities for designing and developing smart materials for multidisciplinary applications. To make these tasks possible, simple and reliable synthesis methods for both well-known and recently developed photochrome series were needed.
Below, both already well-established methods and the latest approaches for 5′-substituted spiropyran derivatives synthesis are reviewed and critically analyzed.
Synthesis of the 5′-Substituted Spiropyran Derivatives. Side Reactions in the Synthesis
Synthetic approaches and methods to the 5′-substituted spiropyrans synthesis can be conventionally sorted into following groups:
(A) “Complete” synthesis of target derivatives by the condensation of two or more key intermediates: X + Y = SP;
(B) One-step direct modification of a precursor with gived structure: SP-precursor → SP;
(C) Production of a target molecule in several stages by progressive elaboration of the anchor group by introduction of the necessary fragments with a given set of functional groups: SP-precursor1 → SP-precursor2 → SP-precursorn → SP;
(D) Modification of the final targets by the photoactive ligands with reactive terminal functions by doping or by immobilization methods.
Researchers labeled them as pathways (A–D). The functional linker fragment and anchor group at the C5′-atom indoline part are located along the same axis as the EWG group at C6-postion pyran fragment (uniaxially).
The honorable first place in its popularity among known methods for synthesis of indoline spiropyrans is deservedly occupied by a group of condensations of two key components shown in Figure 12A–C (pathway A). Most often indolinospiropyrans are synthesized by condensation of Fischer’s base (2-methylene-1,3,3-trimethylindoline) and its analogs or their salts with salicylaldehyde derivatives at reflux (see Figure 12A,B). Organic solvents such as methanol, isopropyl alcohol, toluene, DMF are often used. Synthesis simply involves condensation of two key components through reflux in ethanol, then isolation of the precipitated spiropyran dye by filtration from the mother liquor after cooling. The yields of indolinospiropyrans tend to be good: typically at least 70% and sometimes being near quantitative. To improve quality and yield, the following are recommended: (1) Vacuum distillation of these starting materials prior to use; or (2) replacement of unstable free bases with solid hydrohalides or perchlorate salts of Fisher’s base, which are much more stable and easier to handle than liquid free bases. Rather than converting them back to free base form immediately before use, the salts may be employed directly (Figure 12B) [22]. The synthesis of spiropyrans using a wide range of organic or inorganic bases such as Et3N, piperidine, pyridine, and NaOH is reported. To reduce the yield of the “bis-condensed” by-product, it is recommended to use the corresponding quaternary indolenyl salts instead of the free methylene bases in a mixture with an equimolar amount of an organic base (most often piperidine) or to use a slight excess of the aldehyde component [20][21][45][46][47].
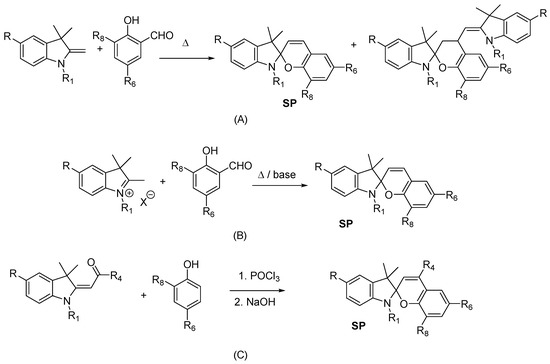
Figure 12. Classic 5′-substituted indoline spiropyran synthesis (pathway A; do not confuse with subfigures A–C explained below). Condensation of Fischer’s base (2-methylene-1,3,3-trimethylindoline) (A) or their salts (B) with salicylaldehyde derivatives; condensation of acyl- or formyl- Fischer’s base derivatives with substituted phenols (C).
The first 5′-substituted indoline spiropyrans were prepared by Wizinger [48] in high yields by condensation of 5-methoxy derivatives of 1,3,3-trimethyl-2-methyleneindoline (the Fischer base) with salicylaldehyde and 2-hydroxynaphthaldehyde by heating in methanol. The condensation of acyl- or formyl- Fischer’s base derivatives with substituted phenols was used much less frequently (Figure 12C).
A series of 5′-acetyl-substituted SP99–SP102 was synthesized by condensation of 5-acetyl-substituted Fischer base with 3-substituted-5-nitro-salicylaldehyde. The introduction of an acetyl group does not change the spectral characteristics of the merocyanine form but leads to a decrease in the efficiency of photocoloration [49].
Diversely halogenated, hydroxyl-, and triflat spiropyran derivatives series SP55, SP56, SP60, SP61, SP63, SP64, SP66, SP67, SP73–SP75, SP162–SP164, were synthesized from respectively 5-substituted indolium salts and salicylaldehydes, using a versatile piperidine promoted procedure in ethanol as the solvent. The base was required to induce the in situ formation of 2-methyleneindolines (Fischer’s bases) as reactive species from indolium salts. The starting 5-substituted indole species were prepared as indolium iodide salts, starting from 1,4-substituted phenylhydrazines. Overall yields: 5R = Br 73%; 5R = I 73%; 5R = OH 56%.
For the synthesis of spiropyrans, the use of indolium salts is advantageous compared to directly using the corresponding 5-Fischer’s bases as starting materials, because indolium salts are stable against air and moisture and as solids easy to handle. All products SP55, SP56, SP60, SP61, SP63, SP64, SP66, SP67, SP73–SP75, SP162–SP164, were isolated after crystallization from the reaction mixture. The spiropyrans bearing either bromide, iodide or hydroxy functions showed a negative photochromism on silica gel. This means that their rings have opened which leads to the zwitterionic MC isomer whose hydroxyl groups can interact with the silica surface by hydrogen bonding leading to severe yield losses in the purification via column chromatography. Furher functionalization of the hydroxy functions to give the corresponding trifluoromethanesulfonyl (triflat) groups was accomplished using trifluorosulfonyl anhydride as trifluorosulfonylating agent and pyridine as base in CH2Cl2 as the solvent [50].
To develop an organic–inorganic hybrid photomagnet, intercalation of spiropyran-5′-sulfonate anions into layered cobalt hydroxides was performed, yielding SP159-CoLHSP photoresponsive compound [51][52]. Target spiropyran-5′-sulfonate was synthesized from K salt SP160, which was prepared by condensation of Fischer’s base analog (2-methylene-1,3,3-trimethylindoline-5-sulfonato potassium salt) with salicylaldehyde derivatives at reflux. After UV irradiation (313 nm), the optical and magnetic properties of CoLHSP clearly changed. Some of them demonstrate increased solubility in water and negative photochromic properties.
In similar maner, SP161 spiropyran with isothiocyanate substituent at 5′-position was prepared from the 5-isothiocyanato-2-methylene-1,3,3-trimethylindoline [53][54].
5′-Aryl-substituted SP20–SP27, SP263 were synthesized by standard method, but with very insignificant yields [55][56][57]. It is interesting to compare the efficienty of this route with direct Wittig olefination method of the precursor SP94 (3% vs. 62%) [36][55].
The Fischer’s base moiety is readily replaced with other heterocycles, producing considerable variation in kinetics and in optical parameters of photochromism. In addition, spiropyran scaffold is in some cases sufficiently resistant to functional group transformation to modify properties or to introduce linker motifs with terminal reactive groups or “molecular address” to allow incorporation of the photochromic unit into/on different targets. Overviews of such synthetic possibilities are given in [21][22][25][45][58][59].
For further details as well as excellent overview of the progress in spiropyran dye synthesis, the reader is referred to [46]—although published over twenty years ago, it features a perspective gained in industry and also discusses the preparation and quality of indoline- and salicylaldehyde-based intermediates in depth.
At the end of this section, it is also necessary to mention the latest achievements in the field of organic synthesis, which have been successfully used to produce substituted derivatives of spiropyrans.
The solid-phase synthesis of small organic molecules has emerged as an important tool. Its use can avoid extensive workup, recrystallization, and chromatographic purification of the targeted products. It also allows for easy automation of the synthesis process and convenient handling of polar molecules throughout the synthetic protocol. Moreover, difficult or slow reactions can be facilitated by use of excess of reagents without any added complications in the ultimate purification step. Zhao et al. reports a successful application of solid-phase synthesis methods to the preparation of photochomic materials, such as spiropyran dyes. Using this SPOS method, new library of 5′-succinimide spiropyran derivatives SP154 was synthesized on the Wang resin. Final products can be easily transformed into target 5′-succinylaminoderivatives SP155 in high yields; the opening of the succinimide ring in spiropyran could be realized under mild conditions [60].
Because microwave irradiation-promoted reactions are typically rapid and energy efficient, and employ environmentally benign solvents, in the research [61] synthesis of stereochemically biased spiropyrans by the microwave-promoted, two-steps one-pot procedure was explored. In other work, the spiropyran synthesis using ultrasound irradiation instead of high temperatures was proposed since this type of energy offers different advantages such as reaction acceleration and less drastic operational conditions [62].
Pargaonkar reported the “greener” route for the synthesis of photo- and thermochromic spiropyrans promoted by biocompatible choline hydroxide in the water. This procedure provides several advantages such as simple workup, mild reaction conditions, short reaction time, and high yields of the products because choline hydroxide is a suitable basic catalyst in organic transformations [63].
The reversibility of condensation of 2-methyleneindolines (Fischer bases) with the most substituted salicylaldehydes in alcohol is reported in the reviews [20][46] on the basis of unpublished data of Bertelson.
The reaction goes virtually to completion only with 3,5-dinitrosalicylaldehyde because of the very low solubility of the condensation product (it is isolated in the MC form). So, Bertelson used the Fischer base for protection of the o-hydroxyformyl grouping in the case of chemical transformations with other substituents. Moreover, in [64], an efficient protecting method of 2-hydroxybenzaldehydes using Fischer’s base has been reported; protection and deprotection of the hydroxyl and aldehyde group of 2-hydroxybenzaldehydes are also reported. The reaction of 2-hydroxybenzaldehydes with Fischer’s base in ethanol under reflux produced the corresponding spiropyrans with high yield in specific conditions. The treatment of spiropyran with reagents such as KMnO4 or NaIO4 produced initial 2-hydroxybenzaldehyde in various solvent systems in low yield (less than 20%) as well as unidentified side products. However, when spiropyran derivative was treated with ozone at −78 °C in methanol, starting 2-hydroxybenzaldehyde was obtained with 85% yield. As a result, the hydroxyl and aldehyde group of 2-hydroxybenzaldehydes were protected at the same time by their reaction with Fischer’s base in ethanol under reflux to give the corresponding spiropyrans, the protected form of 2-hydroxybenzaldehydes. The spiropyrans were efficiently cleaved by ozonolysis to produce the corresponding 2-hydroxybenzaldehydes with high yields.
References
- Barachevsky, V.A. Negative Photochromism in Organic Systems. Rev. J. Chem. 2017, 7, 334–371.
- Aiken, S.; Edgar, R.J.L.; Gabbutt, C.D.; Heron, B.M.; Hobson, P.A. Negatively Photochromic Organic Compounds: Exploring the Dark Side. Dyes Pigments 2018, 149, 92–121.
- Tian, H.; Zhang, J.; He, T. (Eds.) Photochromic Materials: Preparation, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2016; ISBN 978-3-527-68370-3.
- Coudret, C.; Chernyshev, A.V.; Metelitsa, A.V.; Micheau, J.C. New Trends in Spiro-Compounds Photochromic Metals Sensors: Quantitative Aspects. In Photon-Working Switches; Yokoyama, Y., Nakatani, K., Eds.; Springer: Tokyo, Japan, 2017; pp. 3–35. ISBN 978-4-431-56542-0.
- Barachevsky, V.A. Photochromic Spirocompounds and Chromenes for Sensing Metal Ions. Rev. J. Chem. 2013, 3, 52–94.
- Paramonov, S.V.; Lokshin, V.; Fedorova, O.A. Spiropyran, Chromene or Spirooxazine Ligands: Insights into Mutual Relations between Complexing and Photochromic Properties. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 209–236.
- Keyvan Rad, J.; Balzade, Z.; Mahdavian, A.R. Spiropyran-Based Advanced Photoswitchable Materials: A Fascinating Pathway to the Future Stimuli-Responsive Devices. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100487.
- Ali, A.A.; Kharbash, R.; Kim, Y. Chemo- and Biosensing Applications of Spiropyran and Its Derivatives—A Review. Anal. Chim. Acta 2020, 1110, 199–223.
- Chibisov, A.K.; Görner, H. Photochromism of Spirobenzopyranindolines and Spironaphthopyranindolines. Phys. Chem. Chem. Phys. 2001, 3, 424–431.
- Görner, H. Photochromism of Nitrospiropyrans: Effects of Structure, Solvent and Temperature. Phys. Chem. Chem. Phys. 2001, 3, 416–423.
- Belikov, N.E.; Lukin, A.Y.; Varfolomeev, S.D.; Levina, I.I.; Petrovskaya, L.E.; Demina, O.V.; Barachevsky, V.A.; Khodonov, A.A. Spectral Study of the Structure and Properties of Complexes of Unsubstituted Indoline Spiropyran with Aluminum Ions. Opt. Spectrosc. 2022, 130, 538–546.
- Kume, S.; Nishihara, H. Photochrome-Coupled Metal Complexes: Molecular Processing of Photon Stimuli. Dalton Trans. 2008, 25, 3260–3271.
- Guerchais, V.; Ordronneau, L.; Le Bozec, H. Recent Developments in the Field of Metal Complexes Containing Photochromic Ligands: Modulation of Linear and Nonlinear Optical Properties. Coord. Chem. Rev. 2010, 254, 2533–2545.
- Zvezdin, K.V.; Belikov, N.E.; Laptev, A.V.; Lukin, A.Y.; Demina, O.V.; Levin, P.P.; Brichkin, S.B.; Spirin, M.G.; Razumov, V.F.; Shvets, V.I.; et al. New Hybrid Photochromic Materials with Switchable Fluorescence. Nanotechnol. Russ. 2012, 7, 308–317.
- Kume, S.; Nishihara, H. Metal-Based Photoswitches Derived from Photoisomerization. In Photofunctional Transition Metal Complexes; Yam, V.W.W., Ed.; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2006; Volume 123, pp. 79–112. ISBN 978-3-540-36809-0.
- Chernyshev, A.V.; Voloshin, N.A.; Rostovtseva, I.A.; Demidov, O.P.; Shepelenko, K.E.; Solov’eva, E.V.; Gaeva, E.B.; Metelitsa, A.V. Benzothiazolyl Substituted Spiropyrans with Ion-Driven Photochromic Transformation. Dyes Pigments 2020, 178, 108337.
- Belser, P.; De Cola, L.; Hartl, F.; Adamo, V.; Bozic, B.; Chriqui, Y.; Iyer, V.M.; Jukes, R.T.F.; Kühni, J.; Querol, M.; et al. Photochromic Switches Incorporated in Bridging Ligands: A New Tool to Modulate Energy-Transfer Processes. Adv. Funct. Mater. 2006, 16, 195–208.
- Jukes, R.T.F.; Bozic, B.; Belser, P.; De Cola, L.; Hartl, F. Photophysical and Redox Properties of Dinuclear Ru and Os Polypyridyl Complexes with Incorporated Photostable Spiropyran Bridge. Inorg. Chem. 2009, 48, 1711–1721.
- Abdullah, A.; Roxburgh, C.J.; Sammes, P.G. Photochromic Crowned Spirobenzopyrans: Quantitative Metal-Ion Chelation by UV, Competitive Selective Ion-Extraction and Metal-Ion Transportation Demonstration Studies. Dyes Pigments 2008, 76, 319–326.
- Bertelson, R.C. Chapter 3 “Photochromic Process Involving Heterolytic Cleavage”. In Photochromism; Brown, G.H., Ed.; Techniques of Chemistry; Wiley: London, UK, 1971; Volume III, pp. 45–431.
- Zakhs, E.R.; Martynova, V.M.; Efros, L.S. Synthesis and Properties of Spiropyrans That Are Capable of Reversible Opening of the Pyran Ring (Review). Chem. Heterocycl. Compd. 1979, 15, 351–372.
- Towns, A. Spiropyran Dyes. Phys. Sci. Rev. 2021, 6, 341–368.
- Kortekaas, L.; Browne, W.R. Spiropyran—Multifaceted Chromic Compounds. In Molecular Photoswitches; Pianowski, Z.L., Ed.; Wiley: Hoboken, NJ, USA, 2022; pp. 131–149. ISBN 978-3-527-35104-6.
- Kortekaas, L.; Browne, W.R. The Evolution of Spiropyran: Fundamentals and Progress of an Extraordinarily Versatile Photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424.
- Minkin, V.I. Photo-, Thermo-, Solvato-, and Electrochromic Spiroheterocyclic Compounds. Chem. Rev. 2004, 104, 2751–2776.
- Kholmanskii, A.S.; Dyumaev, K.M. The Photochemistry and Photophysics of Spiropyrans. Russ. Chem. Rev. 1987, 56, 136–151.
- Krysanov, S.A.; Alfimov, M.V. Ultrafast Formation of Transients in Spiropyran Photochromism. Chem. Phys. Lett. 1982, 91, 77–80.
- Görner, H.; Atabekyan, L.S.; Chibisov, A.K. Photoprocesses in Spiropyran-Derived Merocyanines: Singlet versus Triplet Pathway. Chem. Phys. Lett. 1996, 260, 59–64.
- Melnikova, I.A.; Belikov, N.E.; Bakholdina, A.G.; Lukin, A.Y.; Varfolomeev, S.D.; Demina, O.V.; Khodonov, A.A. Study of the Interaction of a New Photochromic Podand with Metal Salts in Solutions of Organic Solvents. In Proceedings of the XIX Annual International Youth Conference IBCP RAS-Universities “Biochemical Physics”, Moscow, Russia, 28–30 October 2019; pp. 152–155.
- Demina, O.V.; Levin, P.P.; Belikov, N.E.; Laptev, A.V.; Lukin, A.Y.; Barachevsky, V.A.; Shvets, V.I.; Varfolomeev, S.D.; Khodonov, A.A. Synthesis and Photochromic Reaction Kinetics of Unsaturated Spiropyran Derivatives. J. Photochem. Photobiol. Chem. 2013, 270, 60–66.
- Levin, P.P.; Tatikolov, A.S.; Laptev, A.V.; Lukin, A.Y.; Belikov, N.E.; Demina, O.V.; Khodonov, A.A.; Shvets, V.I.; Varfolomeev, S.D. The Investigation of the Intermediates of Spiropyran Retinal Analogs by Laser Flash Photolysis Techniques with Different Excitation Wavelengths. J. Photochem. Photobiol. Chem. 2012, 231, 41–44.
- Tamai, N.; Miyasaka, H. Ultrafast Dynamics of Photochromic Systems. Chem. Rev. 2000, 100, 1875–1890.
- Murin, V.A.; Mandjikov, V.F.; Barachevskii, V.A. Study of Triplet-Triplet Absorption of Photochromic Spiropyran by Laser Photoexcitation. Opt. Spectrosc. 1976, 40, 1084–1086.
- Murin, V.A.; Mandjikov, V.F.; Barachevskii, V.A. Study of Photochromism of Methoxysubstituted Indolenine Spiropyrans by Laser Nanosecond Spectroscopy. Opt. Spectrosc. 1977, 41, 79–81.
- Demina, O.V.; Belikov, N.E.; Melnikova, I.A.; Lukin, A.Y.; Varfolomeev, S.D.; Khodonov, A.A. New Labels and Probes for the Application in Bionanophotonics. Russ. J. Phys. Chem. B 2019, 13, 938–941.
- Melnikova, I.A.; Lukin, A.Y.; Belikov, N.E.; Demina, O.V.; Levin, P.P.; Varfolomeev, S.D.; Khodonov, A.A. Synthesis and Study of the Spectral Properties of a Series of Stilbenes Containing, as One of the Aryl Fragments, a Molecule of a Photochromic Label of the Spiropyran Series. In Proceedings of the XIV Annual International Youth Conference IBCP RAS-Universities “Biochemical Physics”, Moscow, Russia, 28–30 October 2014; pp. 128–133.
- Feuerstein, T.J.; Müller, R.; Barner-Kowollik, C.; Roesky, P.W. Investigating the Photochemistry of Spiropyran Metal Complexes with Online LED-NMR. Inorg. Chem. 2019, 58, 15479–15486.
- Pugachev, A.D.; Ozhogin, I.V.; Lukyanova, M.B.; Lukyanov, B.S.; Kozlenko, A.S.; Rostovtseva, I.A.; Makarova, N.I.; Tkachev, V.V.; Aldoshin, S.M.; Metelitsa, A.V. Synthesis, Structure and Photochromic Properties of Indoline Spiropyrans with Electron-Withdrawing Substituents. J. Mol. Struct. 2021, 1229, 129615.
- Kozlenko, A.S.; Ozhogin, I.V.; Pugachev, A.D.; Lukyanova, M.B.; El-Sewify, I.M.; Lukyanov, B.S. A Modern Look at Spiropyrans: From Single Molecules to Smart Materials. Top. Curr. Chem. 2023, 381, 8.
- Kozlenko, A.S.; Pugachev, A.D.; Ozhogin, I.V.; El-Sewify, I.M.; Lukyanov, B.S. Spiropyrans: Molecules in Motion. Chem. Heterocycl. Compd. 2021, 57, 984–989.
- Tian, W.; Tian, J. An Insight into the Solvent Effect on Photo-, Solvato-Chromism of Spiropyran through the Perspective of Intermolecular Interactions. Dyes Pigments 2014, 105, 66–74.
- Zhou, J.; Li, Y.; Tang, Y.; Zhao, F.; Song, X.; Li, E. Detailed Investigation on a Negative Photochromic Spiropyran. J. Photochem. Photobiol. Chem. 1995, 90, 117–123.
- Song, X.; Zhou, J.; Li, Y.; Tang, Y. Correlations between Solvatochromism, Lewis Acid-Base Equilibrium and Photochromism of an Indoline Spiropyran. J. Photochem. Photobiol. Chem. 1995, 92, 99–103.
- Seiler, V.K.; Robeyns, K.; Tumanov, N.; Cinčić, D.; Wouters, J.; Champagne, B.; Leyssens, T. A Coloring Tool for Spiropyrans: Solid State Metal–Organic Complexation versus Salification. CrystEngComm 2019, 21, 4925–4933.
- Lukyanov, B.S.; Lukyanova, M.B. Spiropyrans: Synthesis, Properties, and Application. (Review). Chem. Heterocycl. Compd. 2005, 41, 281–311.
- Bertelson, R.C. Spiropyrans. In Organic Photochromic and Thermochromic Compounds; Crano, J.C., Guglielmetti, R., Eds.; Plenum Press: New York, NY, USA, 1999; Volume I, pp. 11–83.
- Keum, S.-R.; Ku, B.-S.; Shin, J.-T.; Ko, J.J.; Buncel, E. Stereoselective Formation of Dicondensed Spiropyran Product Obtained from the Reaction of Excess Fischer Base with Salicylaldehydes: First Full Characterization by X-Ray Crystal Structure Analysis of a DC·acetone Crystal. Tetrahedron 2005, 61, 6720–6725.
- Wizinger, R.; Wenning, H. Über Intramolekulare Ionisation. Helv. Chim. Acta 1940, 23, 247–271.
- Gal’bershtam, M.A.; Bondarenko, E.M.; Khrolova, O.R.; Bolyleva, G.K.; Pod’yachev, Y.B.; Przhiyalgovskaya, N.M.; Suvorov, N.N. Synthesis and Photochromic Properties of 5-Acetyl-Substituted Indolinospirochromenes. Chem. Heterocycl. Compd. 1979, 15, 1329–1333.
- Schulz-Senft, M.; Gates, P.J.; Sönnichsen, F.D.; Staubitz, A. Diversely Halogenated Spiropyrans—Useful Synthetic Building Blocks for a Versatile Class of Molecular Switches. Dyes Pigments 2017, 136, 292–301.
- Sugahara, A.; Tanaka, N.; Okazawa, A.; Matsushita, N.; Kojima, N. Photochromic Property of Anionic Spiropyran with Sulfonate-Substituted Indoline Moiety. Chem. Lett. 2014, 43, 281–283.
- Tanaka, N.; Okazawa, A.; Sugahara, A.; Kojima, N. Development of a Photoresponsive Organic–Inorganic Hybrid Magnet: Layered Cobalt Hydroxides Intercalated with Spiropyran Anions. Bull. Chem. Soc. Jpn. 2015, 88, 1150–1155.
- Zhang, P.; Meng, J.; Li, X.; Wang, Y.; Matsuura, T. Synthesis and Photochromism of Photochromic Spiro Compounds Having a Reactive Pendant Group. J. Heterocycl. Chem. 2002, 39, 179–184.
- Zhang, P.; Meng, J.B.; Matsuura, T.; Wang, Y.M. DNA Modification with Photochromic Spiro Compounds. Chin. Chem. Lett. 2002, 13, 299–302.
- Gal’bershtam, M.A.; Lazarenko, I.B.; Bobyleva, G.K.; Pod’yachev, Y.B.; Przhiyalgovskaya, N.M.; Suvorov, N.N. Photochromic 5-Styryl-Substituted Indolinospirochromenes. Chem. Heterocycl. Compd. 1984, 20, 1222–1225.
- Gal’bershtam, M.A.; Przhiyalgovskaya, N.M.; Lazarenko, I.B.; Kononova, V.S.; Suvorov, N.N. Synthesis and Spectral Characteristics of Photochromic 5′-Aryl-1′,3′,3′-trimethyl-6-nitro-2H-chromene-2-spiro-2′-indolines. Chem. Heterocycl. Compd. 1976, 12, 417–419.
- Gal’bershtam, M.A.; Przhiyalgovskaya, N.M.; Samoilova, N.P.; Braude, E.V.; Lazarenko, I.B.; Suvorov, N.N. Effect of Aryl Substituents on the Rate of Dark Decolorization of Photochromic Spirochromenes of the Indoline Series. Chem. Heterocycl. Compd. 1977, 13, 67–69.
- Crano, J.C.; Guglielmetti, R.J. (Eds.) Organic Photochromic and Thermochromic Compounds; Topics in Applied Chemistry; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; ISBN 978-0-306-45882-8.
- Samat, A.; De Keukeleire, D.; Guglielmetti, R. Synthesis and Spectrokinetic Properties of Photochromic Spiropyrans. Bull. Sociétés Chim. Belg. 2010, 100, 679–700.
- Zhao, W.; Carreira, E.M. Solid-Phase Synthesis of Photochromic Spiropyrans. Org. Lett. 2005, 7, 1609–1612.
- Perry, A.; Davis, K.; West, L. Synthesis of Stereochemically-Biased Spiropyrans by Microwave-Promoted, One-Pot Alkylation–Condensation. Org. Biomol. Chem. 2018, 16, 7245–7254.
- Silvia, T.R.; Ana, V.S.L.; González, E.A.S. Novel Syntheses of Spiropyran Photochromatic Compounds Using Ultrasound. Synth. Commun. 1995, 25, 105–110.
- Pargaonkar, J.G.; Patil, S.K.; Vajekar, S.N. Greener Route for the Synthesis of Photo- and Thermochromic Spiropyrans Using a Highly Efficient, Reusable, and Biocompatible Choline Hydroxide in an Aqueous Medium. Synth. Commun. 2018, 48, 208–215.
- Cho, Y.J.; Lee, S.H.; Bae, J.W.; Pyun, H.-J.; Yoon, C.M. Fischer’s Base as a Protecting Group: Protection and Deprotection of 2-Hydroxybenzaldehydes. Tetrahedron Lett. 2000, 41, 3915–3917.
More
Information
Subjects:
Chemistry, Organic; Chemistry, Physical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.9K
Revisions:
3 times
(View History)
Update Date:
11 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





