
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chen-Kuei Chung | -- | 2428 | 2023-07-04 11:39:27 | | | |
| 2 | Lindsay Dong | -11 word(s) | 2417 | 2023-07-05 03:03:56 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2417 | 2023-07-10 03:34:02 | | |
Video Upload Options
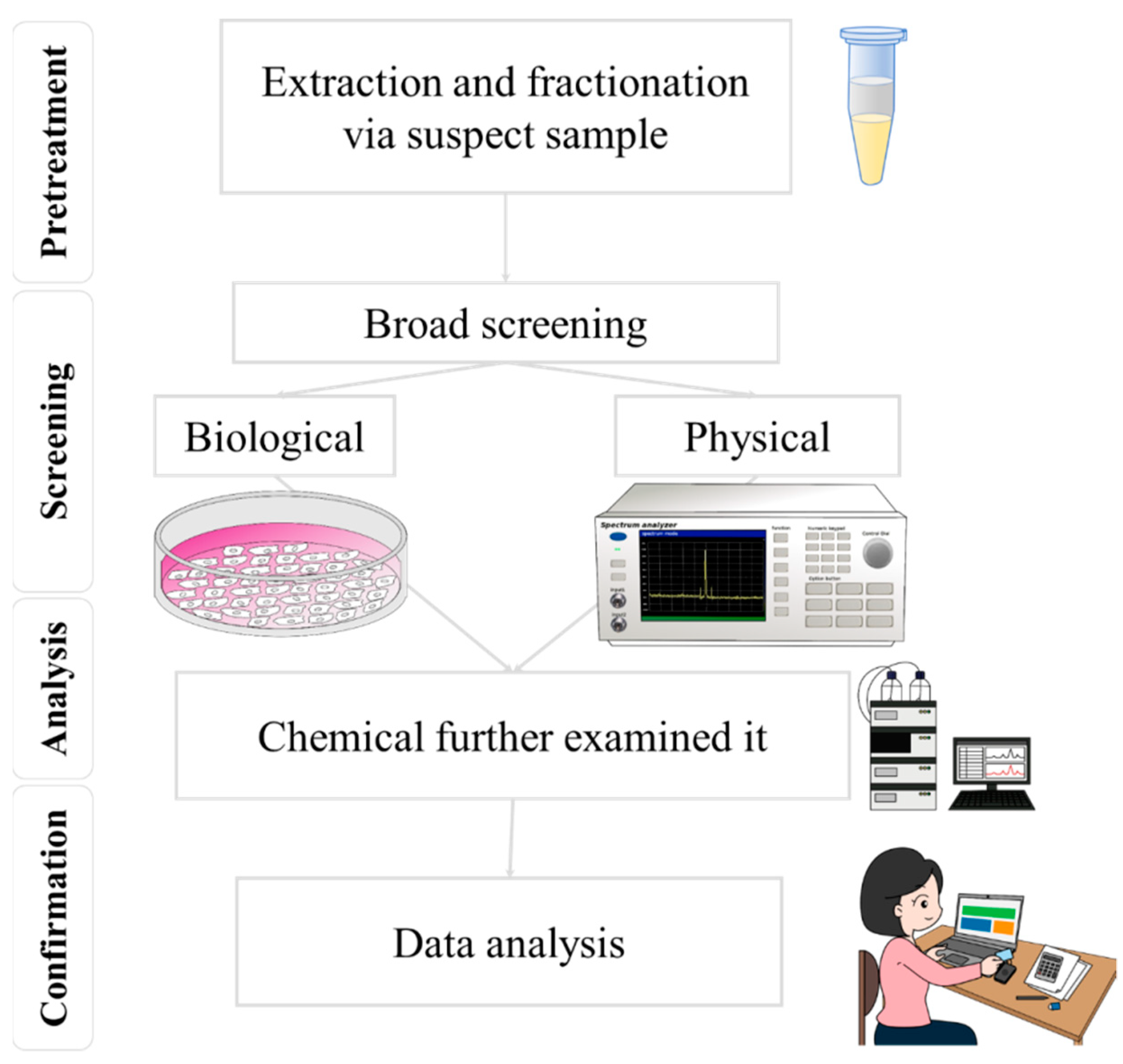
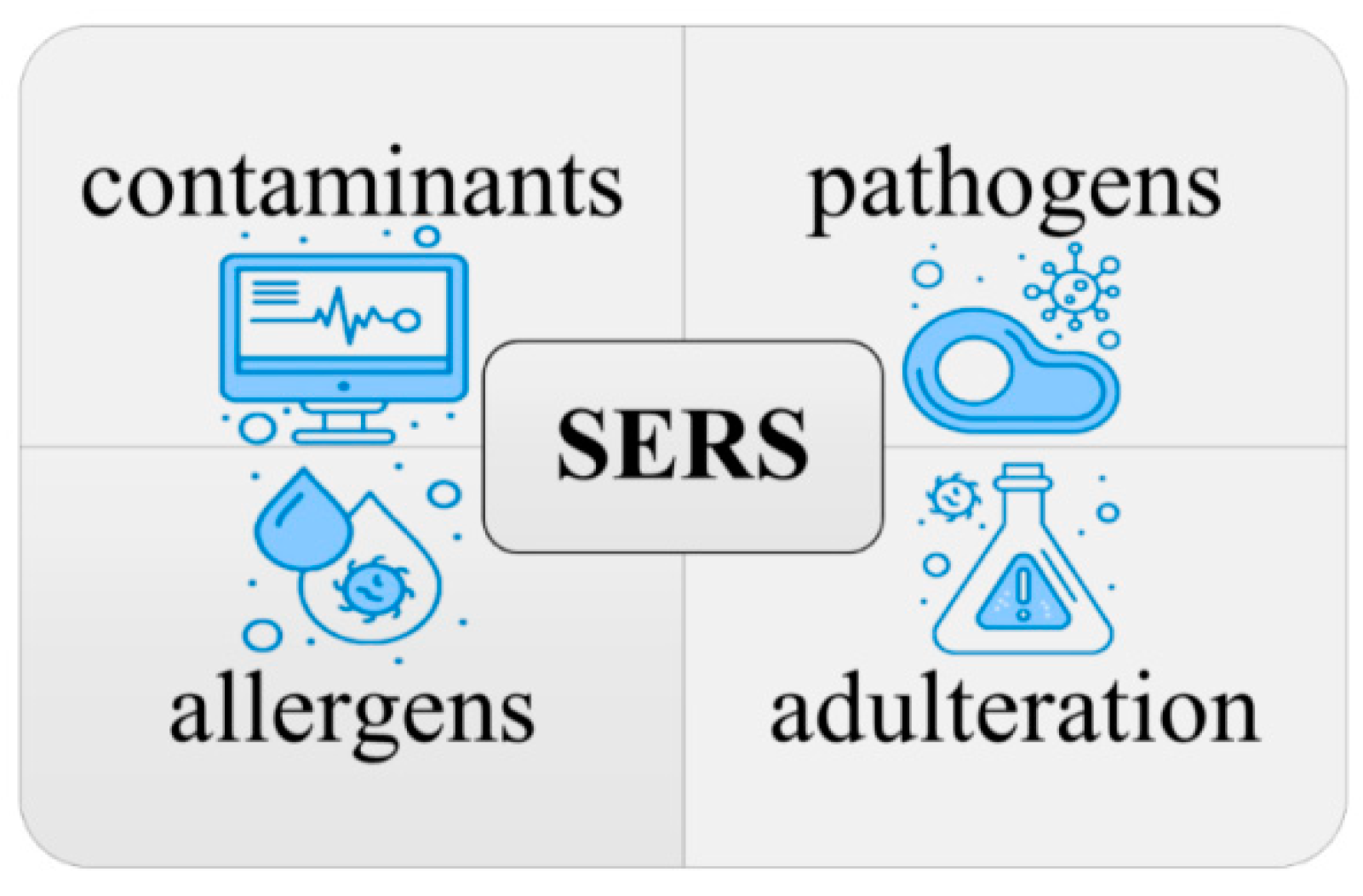
Food safety analysis currently relies on the enzyme-linked immunosorbent assay (ELISA), polymerase chain reaction (PCR), high-performance liquid chromatography (HPLC), gas chromatography (GC), UV-visible spectrophotometry, and tandem mass spectrometry (MS), all of which require significant time to train qualified food safety testing laboratory operators. These factors have hindered the development of rapid food safety monitoring systems, especially in remote areas or areas with a relative lack of testing resources. Surface-enhanced Raman spectroscopy (SERS) has emerged as one of the tools of choice for food safety testing that can overcome these dilemmas. SERS offers advantages over chromatographic mass spectrometry analysis due to its portability, non-destructive nature, and lower cost implications. However, as it currently stands, Raman spectroscopy is a supplemental tool in chemical analysis, reinforcing and enhancing the completeness and coverage of the food safety analysis system. SERS combines portability with non-destructive and cheaper detection costs to gain an advantage over chromatographic mass spectrometry analysis.
1. Introduction
2. Design, Fabrication, and Applications of SERS
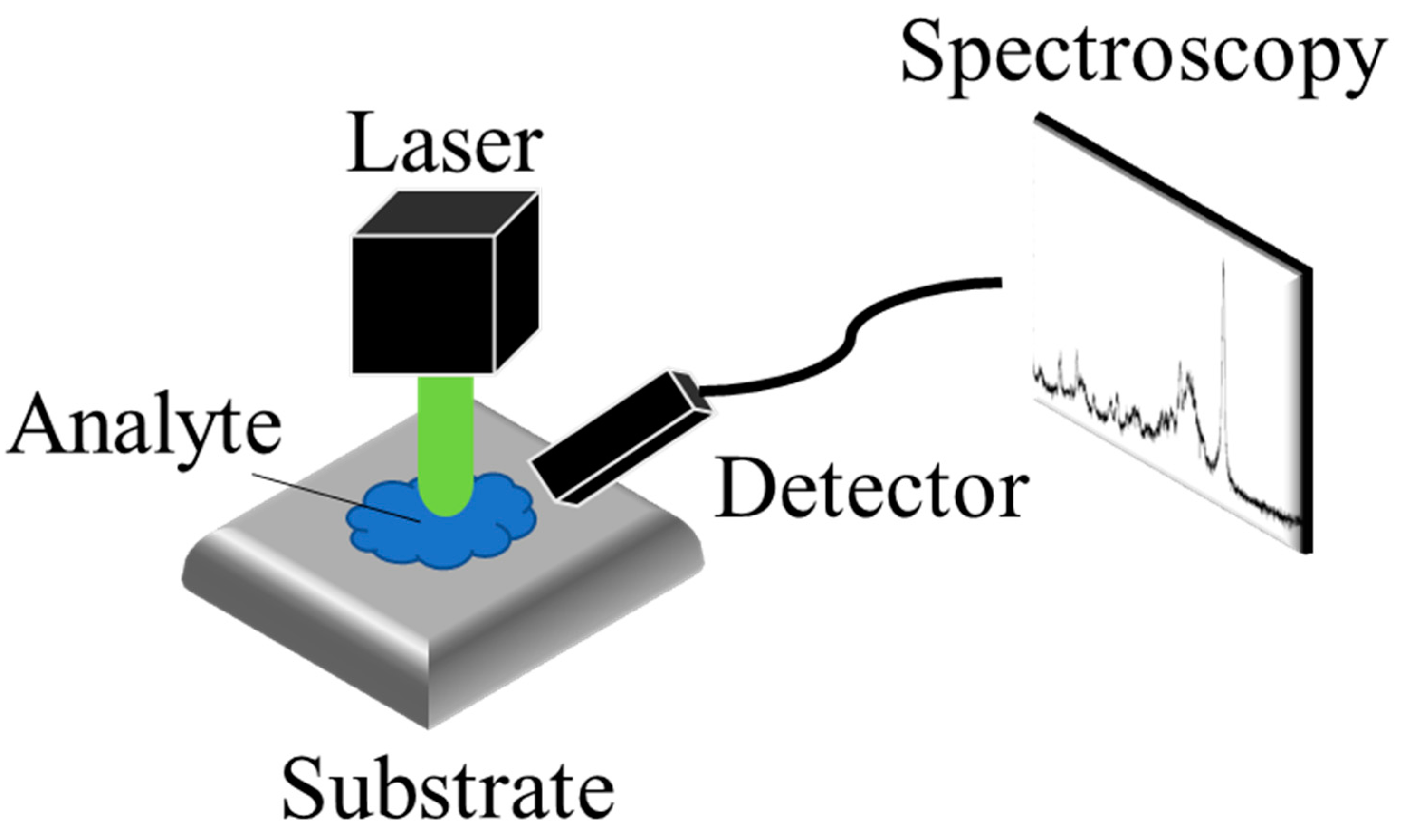
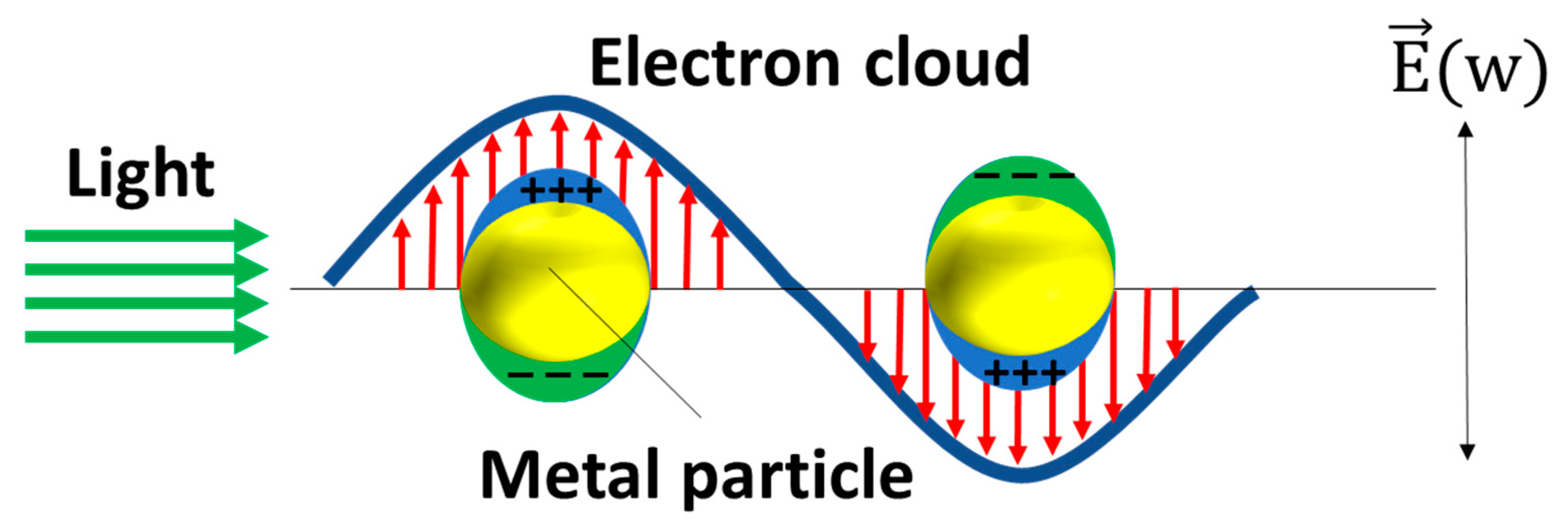
2.1. The Design of the SERS Substrates
2.2. The Fabrication of the SERS Substrates
2.3. The SERS Application on Food Safety
References
- Gallo, M.; Ferrara, L.; Calogero, A.; Montesano, D.; Naviglio, D. Relationships between food and diseases: What to know to ensure food safety. Food Res. Int. 2020, 137, 109414.
- Amelin, V.G.; Lavrukhina, O.I. Food safety assurance using methods of chemical analysis. J. Anal. Chem. 2017, 72, 1–46.
- Lebesi, D.; Dimakou, C.; Alldrick, A.J.; Oreopoulou, V. Rapid test methods: A versatile tool to assist food-safety management: Use and need of rapid test methods. Qual. Assur. Saf. Crop. Foods 2010, 2, 173–181.
- Tang, Y.; Lu, L.; Zhao, W.; Wang, J. Rapid Detection Techniques for Biological and Chemical Contamination in Food: A Review. Int. J. Food Eng. 2009, 5.
- Darwish, A.; Ricci, M.; Zidane, F.; Vasquez, J.A.T.; Casu, M.R.; Lanteri, J.; Migliaccio, C.; Vipiana, F. Physical Contamination Detection in Food Industry Using Microwave and Machine Learning. Electronics 2022, 11, 3115.
- Välitalo, P.; Massei, R.; Heiskanen, I.; Behnisch, P.; Brack, W.; Tindall, A.J.; Du Pasquier, D.; Küster, E.; Mikola, A.; Schulze, T.; et al. Effect-based assessment of toxicity removal during wastewater treatment. Water Res. 2017, 126, 153–163.
- Hilscherová, K.; Machala, M.; Kannan, K.; Blankenship, A.L.; Giesy, J.P. Cell bioassays for detection of aryl hydrocarbon (AhR) and estrogen receptor (ER) mediated activity in environmental samples. Environ. Sci. Pollut. Res. 2000, 7, 159–171.
- Hoogenboom, L.A.; Hamers, A.R.; Bovee, T.F. Bioassays for the detection of growth-promoting agents, veterinary drugs and environmental contaminants in food. Analyst 1999, 124, 79–85.
- Burns, M.; Wiseman, G.; Knight, A.; Bramley, P.; Foster, L.; Rollinson, S.; Damant, A.; Primrose, S. Measurement issues associated with quantitative molecular biology analysis of complex food matrices for the detection of food fraud. Analyst 2016, 141, 45–61.
- Narsaiah, K.; Jha, S.N.; Bhardwaj, R.; Sharma, R.; Kumar, R. Optical biosensors for food quality and safety assurance—A review. J. Food Sci. Technol. 2012, 49, 383–406.
- Perumal, J.; Wang, Y.; Attia, A.B.E.; Dinish, U.S.; Olivo, M. Towards a point-of-care SERS sensor for biomedical and agri-food analysis applications: A review of recent advancements. Nanoscale 2021, 13, 553–580.
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687.
- Meher, A.K.; Chen, Y.-C. Combination of Raman Spectroscopy and Mass Spectrometry for Online Chemical Analysis. Anal. Chem. 2016, 88, 9151–9157.
- Petersen, M.; Yu, Z.; Lu, X. Application of Raman Spectroscopic Methods in Food Safety: A Review. Biosensors 2021, 11, 187.
- Neng, J.; Zhang, Q.; Sun, P. Application of surface-enhanced Raman spectroscopy in fast detection of toxic and harmful substances in food. Biosens. Bioelectron. 2020, 167, 112480.
- Jiang, L.; Gu, K.; Liu, R.; Jin, S.; Wang, H.; Pan, C. Rapid detection of pesticide residues in fruits by surface-enhanced Raman scattering based on modified QuEChERS pretreatment method with portable Raman instrument. SN Appl. Sci. 2019, 1, 627.
- Wang, P.; Wu, L.; Lu, Z.; Li, Q.; Yin, W.; Ding, F.; Han, H. Gecko-Inspired Nanotentacle Surface-Enhanced Raman Spectroscopy Substrate for Sampling and Reliable Detection of Pesticide Residues in Fruits and Vegetables. Anal. Chem. 2017, 89, 2424–2431.
- Wu, Y.; Yu, W.; Yang, B.; Li, P. Self-assembled two-dimensional gold nanoparticle film for sensitive nontargeted analysis of food additives with surface-enhanced Raman spectroscopy. Analyst 2018, 143, 2363–2368.
- He, S.; Xie, W.; Zhang, W.; Zhang, L.; Wang, Y.; Liu, X.; Liu, Y.; Du, C. Multivariate qualitative analysis of banned additives in food safety using surface enhanced Raman scattering spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1092–1099.
- Yan, S.; Wang, S.; Qiu, J.; Li, M.; Li, D.; Xu, D.; Li, D.; Liu, Q. Raman spectroscopy combined with machine learning for rapid detection of food-borne pathogens at the single-cell level. Talanta 2021, 226, 122195.
- Maruthamuthu, M.K.; Raffiee, A.H.; De Oliveira, D.M.; Ardekani, A.M.; Verma, M.S. Raman spectra-based deep learning: A tool to identify microbial contamination. MicrobiologyOpen 2020, 9, e1122.
- Martinez, L.; He, L. Detection of Mycotoxins in Food Using Surface-Enhanced Raman Spectroscopy: A Review. ACS Appl. Bio Mater. 2021, 4, 295–310.
- Guo, Y.; Girmatsion, M.; Li, H.-W.; Xie, Y.; Yao, W.; Qian, H.; Abraha, B.; Mahmud, A. Rapid and ultrasensitive detection of food contaminants using surface-enhanced Raman spectroscopy-based methods. Crit. Rev. Food Sci. Nutr. 2021, 61, 3555–3568.
- Xu, M.-L.; Gao, Y.; Han, X.-X.; Zhao, B. Innovative Application of SERS in Food Quality and Safety: A Brief Review of Recent Trends. Foods 2022, 11, 2097.
- Yaseen, T.; Pu, H.; Sun, D.-W. Functionalization techniques for improving SERS substrates and their applications in food safety evaluation: A review of recent research trends. Trends Food Sci. Technol. 2018, 72, 162–174.
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166.
- Kerker, M.; Wang, D.S.; Chew, H. Surface Enhanced Raman-Scattering (SERS) by Molecules Adsorbed at Spherical-Particles. Appl. Opt. 1980, 19, 4159–4174.
- Creighton, J.A. Surface Raman Electromagnetic Enhancement Factors for Molecules at the Surface of Small Isolated Metal Spheres-The Determination of Adsorbate Orientation From SERS Relative Intensities. Surf. Sci. 1983, 124, 209–219.
- Lombardi, J.R.; Birke, R.L.; Lu, T.; Xu, J. Charge-transfer theory of surface enhanced Raman spectroscopy: Herzberg–Teller contributions. J. Chem. Phys. 1986, 84, 4174.
- Laurent, G.; Félidj, N. Evidence of multipolar excitations in surface enhanced Raman scattering. Phys. Rev. B 2005, 71, 1045430.
- Le Ru, E.C.; Etchegoin, P.G. Rigorous justification of the |E|4 enhancement factor in Surface Enhanced Raman Spectroscopy. Chem. Phys. Lett. 2006, 423, 63–66.
- Lombardi, J.R.; Birke, R.L. A Unified Approach to Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617.
- Lombardi, J.R.; Birke, R.L. Theory of Surface-Enhanced Raman Scattering in Semiconductors. J. Phys. Chem. C 2014, 118, 11120–11130.
- Yang, B.; Jin, S.; Guo, S.; Park, Y.; Chen, L.; Zhao, B.; Jung, Y.M. Recent Development of SERS Technology: Semiconductor-Based Study. ACS Omega 2019, 4, 20101–20108.
- Singh, P.D.D.; Murthy, Z.V.P.; Kailasa, S.K. Metal nitrides nanostructures: Properties, synthesis and conceptualization in analytical methods developments for chemical analysis and separation, and in energy storage applications. Coord. Chem. Rev. 2023, 481, 215046.
- Samriti; Rajput, V.; Gupta, R.K.; Prakash, J. Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: Fundamentals and emerging SERS applications. J. Mater. Chem. C 2021, 10, 73–95.
- Wang, S.Y.; Hao, Q.X.; Zhao, Y.N.; Chen, Y.S. Two-Dimensional Printed Swab for SERS Screening of Pesticide Residues on Apples and Pears. J. Agric. Food Chem. 2023, 71, 4982–4989.
- Guo, Z.M.; Gao, L.B.; Yin, L.M.; Arslan, M.; El-Seedi, H.R.; Zou, X.B. Novel mesoporous silica surface loaded gold nanocomposites SERS aptasensor for sensitive detection of zearalenone. Food Chem. 2023, 403, 134384.
- Zhang, Q.; Mi, S.N.; Xie, Y.F.; Yu, H.; Guo, Y.H.; Yao, W.R. Core-shell (Fe) as an enhanced substrate for flunixin meglumine ultra-sensitive detection. Spectrochim. Acta Part A 2023, 287, 122018.
- Song, Y.; Xiao, K.Y.; Chen, Q.; Zhang, X.D.; Yu, Z.; Chen, W.W.; Zhang, X.B.; Zhang, D.; Ni, D.J.; Liang, P. Fabrication of GO/Fe3O4@Au MNPs for Magnetically Enriched and Adsorptive SERS Detection of Bifenthrin. Chemosensors 2023, 11, 73.
- Chen, Y.M.; Zhu, L.X.; Yang, Y.L.; Wu, D.; Zhang, Y.; Cheng, W.W.; Tang, X.Z. Fabrication of a metal organic framework (MOF)-modified Au nanoparticle array for sensitive and stable SERS sensing of paraquat in cereals. J. Food Sci. 2023, 88, 1769–1780.
- Chen, R.P.; Wang, H.; Sun, C.Q.; Zhao, Y.G.; He, Y.; Nisar, M.S.; Wei, W.S.; Kang, H.Q.; Xie, X.L.; Du, C.M.; et al. 2 SERS nanotags based lateral flow immunoassay for simultaneous detection of aflatoxin B1 and ochratoxin A. Talanta 2023, 258, 124401.
- Qiu, J.Y.; Chu, Y.J.; He, Q.H.; Han, Y.K.; Zhang, Y.; Han, L. A self-assembly hydrophobic oCDs/Ag nanoparticles SERS sensor for ultrasensitive melamine detection in milk. Food Chem. 2023, 402, 134241.
- Zhang, D.; Fan, Y.S.; Sun, X.X.; Wei, X.O.; Lin, Z.T.; Zhang, X.A.; Shi, J.Y.; Battino, M.; Gong, Y.Y.; Shi, B.L.; et al. SERS determination of hydroxy-?-sanshool in spicy hotpot seasoning: The strategy to restrain the interference of capsaicin and its mechanism. Food Chem. 2023, 413, 135644.
- Zhao, Y.; Xu, Y.J.; Jing, X.H.; Ma, W. SERS-active plasmonic metal NP-CsPbX3 films for multiple veterinary drug residues detection. Food Chem. 2023, 412, 135420.
- Asgari, S.; Dhital, R.; Mustapha, A.; Lin, M.S. Duplex detection of foodborne pathogens using a SERS optofluidic sensor coupled with immunoassay. Int. J. Food Microbiol. 2022, 383, 109947.
- Li, J.X.; Wu, T.; Wang, C.G.; Tu, J.; Song, X.J.; Shao, Y.; Wang, C.W.; Qi, K.Z.; Xiao, R. Nanogapped Fe3O4@Au Surface-Enhanced Raman Scattering Tags for the Multiplex Detection of Bacteria on an Immunochromatographic Strip. ACS Appl. Nano Mater. 2022, 5, 12679–12689.
- Tu, Z.J.; Cheng, S.Y.; Dong, H.; Wang, W.Q.; Yang, X.S.; Gu, B.; Wang, S.Q.; Wang, C.W. Universal and ultrasensitive detection of foodborne bacteria on a lateral flow assay strip by using wheat germ agglutinin-modified magnetic SERS nanotags. RSC Adv. 2022, 12, 27344–27354.
- Shen, W.Z.; Wang, C.G.; Zheng, S.; Jiang, B.; Li, J.X.; Pang, Y.F.; Wang, C.W.; Hao, R.Z.; Xiao, R. Ultrasensitive multichannel immunochromatographic assay for rapid detection of foodborne bacteria based on two-dimensional film-like SERS labels. J. Hazard. Mater. 2022, 437, 129347.
- He, Q.; Yang, J.Y.; Zabotina, O.A.; Yu, C.X. Surface-enhanced Raman spectroscopic chemical imaging reveals distribution of pectin and its co-localization with xyloglucan inside onion epidermal cell wall. PLoS ONE 2021, 16, e0250650.
- Hu, S.; Gu, F.; Chen, M.; Wang, C.W.; Li, J.; Yang, J.; Wang, G.Y.; Zhou, Z.; Yang, Y. A novel method for identifying and distinguishing Cryptococcus neoformans and Cryptococcus gattii by surface-enhanced Raman scattering using positively charged silver nanoparticles. Sci. Rep. 2020, 10, 12480.
- Prakash, O.; Sil, S.; Verma, T.; Umapathy, S. Direct Detection of Bacteria Using Positively Charged Ag/Au Bimetallic Nanoparticles: A Label-free Surface-Enhanced Raman Scattering Study Coupled with Multivariate Analysis. J. Phys. Chem. C 2020, 124, 861–869.
- Uusitalo, S.; Popov, A.; Ryabchikov, Y.V.; Bibikova, O.; Alakomi, H.L.; Juvonen, R.; Kontturi, V.; Siitonen, S.; Kabashin, A.; Meglinski, I.; et al. Surface-enhanced Raman spectroscopy for identification and discrimination of beverage spoilage yeasts using patterned substrates and gold nanoparticles. J. Food Eng. 2017, 212, 47–54.
- Liu, S.J.; Zhu, Y.D.; Li, M.Y.; Liu, W.J.; Zhao, L.J.; Ma, Y.Y.; Xu, L.N.; Wang, N.; Zhao, G.M.; Liang, D.; et al. Rapid Identification of Different Pathogenic Spore-Forming Bacteria in Spice Powders Using Surface-Enhanced Raman Spectroscopy and Chemometrics. Food Anal. Methods 2022, 15, 2810–2820.
- Jiang, G.Z.; Xu, W.H.; Huang, X.X.; Dai, Z.Q.; Zhou, C.X.; Hong, P.Z.; Li, C.Y. Detection of Bacillus cereus Spore Biomarkers Using SERS-Based Cuttlebone-Derived Organic Matrix/Silver Nanoparticles. ACS Sustain. Chem. Eng. 2023, 11, 4145–4154.
- Ikeno, S.; Maekawa, T.; Hara, N. Multi-Functional Silver Nanoparticles for High-Throughput Endospore Sensing. Biosensors 2022, 12, 68.
- Chen, Q.; Yang, Y.; Ilnur, M.; Liang, W.W.; Shen, A.G.; Hu, J.M. ‘Mixing-and-measuring’ surface-enhanced Raman scattering (SERS) detection of Bacillus cereus for potentially aiding gold mine field exploration. Talanta 2019, 204, 44–49.
- Litti, L.; Reguera, J.; Berganza, L.B.; Meneghetti, M.; Lanceros-Mendez, S. Enhancement of Magnetic Surface-Enhanced Raman Scattering Detection by Tailoring Fe3O4@Au Nanorod Shell Thickness and Its Application in the On-site Detection of Antibiotics in Water. ACS Omega 2022, 7, 45493–45503.
- Singh, M.K.; Singh, A.K.; Dunmore, T.J.; Singh, J. epsilon-Poly-L-lysine conjugated gold nanorod probe to monitor antimicrobial activity and mechanism of action by surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2023, 54, 13–23.
- Guo, H.L.; Li, Y.; Pi, F.W. Sensitive and reproducible gold framework-based SERS membranes for the online monitoring of the freshness of shrimps. Analyst 2023, 148, 2081–2091.
- Dikmen, G. Ultrasensitive detection of amoxicillin using the plasmonic silver nanocube as SERS active substrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 278, 121308.
- Ramos, R.M.C.R.; Jiang, W.B.; Heng, J.Z.X.; Ko, H.Y.Y.; Ye, E.Y.; Regulacio, M.D. Hyperbranched Au Nanocorals for SERS Detection of Dye Pollutants. ACS Appl. Nano Mater. 2023, 6, 3963–3973.
- Nguyen, T.A.; Do, A.N.K.; Lo, T.N.H.; Park, I.; Vo, K.Q. Single-step controlled synthesis of flower-like gold nanoparticles stabilized by chitosan for sensitive detection of heparin using a surface-enhanced Raman scattering method. RSC Adv. 2022, 12, 34831–34842.
- Li, J.Y.; Zhu, J.; Li, X.; Weng, G.J.; Li, J.J.; Zhao, J.W. Tuning the structure and plasmonic properties of Pt-Au triangular nanoprisms: From deposition to etching. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130081.
- Ba, J.W.; Huang, Z.Z.; Yang, W.S. 3-Aminopropyltriethoxysilane-directed formation of Au popcorns for colorimetric and SERS dual detection of cysteine. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129033.
- Chen, J.C.; Chu, Y.T.; Chang, S.H.; Chuang, Y.T.; Huang, C.L. Physical Properties and the Reconstruction of Unstable Decahedral Silver Nanoparticles Synthesized Using Plasmon-Mediated Photochemical Process. Nanomaterials 2022, 12, 1062.
- Koster, H.J.; Rojalin, T.; Carney, R.P.; O’Toole, H.J.; Chiu, K.L. Homogenous high enhancement surface-enhanced Raman scattering (SERS) substrates by simple hierarchical tuning of gold nanofoams. Colloids Interface Sci. Commun. 2022, 47, 100596.
- Kim, J.M.; Kim, J.; Choi, K.; Nam, J.M. Plasmonic Dual-Gap Nanodumbbells for Label-Free On-Particle Raman DNA Assays. Adv. Mater. 2023, 35, e2208250.
- Muthukumar, D.; Shtenberg, G. SERS-based immunosensor for E. coli contaminants detection in milk using silver-coated nanoporous silicon substrates. Talanta 2023, 254, 124132.
- Malik, U.; Korcoban, D.; Mehla, S.; Kandjani, A.E.; Sabri, Y.M.; Balendhran, S.; Bhargava, S.K. Fabrication of fractal structured soot templated titania-silver nano-surfaces for photocatalysis and SERS sensing. Appl. Surf. Sci. 2022, 594, 153383.
- Yu, C.Y.; Chung, C.K. Novel irregular pore peripheral plasmonic mechanism of nanocomposite metal-nanoporous AAO using new facile one-step anodization and pore widening for high SERS enhancement. Appl. Surf. Sci. 2022, 580, 152252.
- Chung, C.K.; Yu, C.Y. Unique high-performance metal-nanoparticle-free SERS substrate with rapid-fabricated hybrid 3D-Nano-Micro-Cavities anodic alumina for label-free detection. Appl. Surf. Sci. 2023, 635, 157731.
- Lee, S.M.; Nam, D.H.; Lee, D.; Lim, S.H.; Son, S.J.; Lee, S. Gold Nanoparticles Deposited on a Conical Anodic Aluminum Oxide Substrate for Improved Surface-Enhanced Raman Scattering. ACS Appl. Nano Mater. 2022, 4, 12905–12912.
- Bao, Z.Y.; Zhou, Y.; Du, Y.L.; Zhang, M.F.; Liu, Y.K.; Wang, J.H.; Lv, J.; Cai, J.; Zhang, Y.; Wu, Y.C. Porous Copper Foam-Based Plasmonic Nanocrystals Modified 3D Semiconductor Nanoflowers for Multifold, Recyclable, and Portable Detection of Environmental Contaminant. Part. Part. Syst. Charact. 2022, 39, 2200072.
- Xu, F.G.; Lai, H.S.; Xu, H. Gold nanocone arrays directly grown on nickel foam for improved SERS detection of aromatic dyes. Anal. Methods 2018, 10, 3170–3177.
- Cvjetinovic, J.; Merdalimova, A.A.; Kirsanova, M.A.; Somov, P.A.; Nozdriukhin, D.V.; Salimon, A.I.; Korsunsky, A.M.; Gorin, D.A. A SERS platform based on diatomite modified by gold nanoparticles using a combination of layer-by-layer assembly and a freezing-induced loading method. Phys. Chem. Chem. Phys. 2022, 24, 8901–8912.
- Lu, C.H.; Cheng, M.R.; Chen, S.; Syu, W.L.; Chien, M.Y.; Wang, K.S.; Chen, J.S.; Lee, P.H.; Liu, T.Y. Flexible PDMS-Based SERS Substrates Replicated from Beetle Wings for Water Pollutant Detection. Polymers 2023, 15, 191.
- Rafiq, F.; Wang, N.; Li, K.Y.; Hong, Z.J.; Cao, D.D.; Du, J.J.; Sun, Z.L. Au-NP-Decorated Cotton Swabs as a Facile SERS Substrate for Food-Safety-Related Molecule Detection. ACS Omega 2023, 8, 8541–8547.
- Huang, W.C.; Chen, H.R. Application of Cotton Swab-Ag Composite as Flexible Surface-Enhanced Raman Scattering Substrate for DMMP Detection. Molecules 2023, 28, 520.
- Mai, Q.D.; Nguyen, H.; Dinh, N.X.; Thuy, N.T.T.; Tran, Q.H.; Thanh, P.C.; Pham, A.T.; Le, A.T. Versatile and high performance in-paper flexible SERS chips for simple and in-situ detection of methylene blue in river water and thiram on apple skin. Talanta 2023, 253, 124114.
- Durastanti, C.; Cirillo, E.N.M.; De Benedictis, I.; Ledda, M.; Sciortino, A.; Lisi, A.; Convertino, A.; Mussi, V. Statistical Classification for Raman Spectra of Tumoral Genomic DNA. Micromachines 2022, 13, 1388.
- Mussi, V.; Ledda, M.; Polese, D.; Maiolo, L.; Paria, D.; Barman, I.; Lolli, M.G.; Lisi, A.; Convertino, A. Silver-coated silicon nanowire platform discriminates genomic DNA from normal and malignant human epithelial cells using label-free Raman spectroscopy. Mater. Sci. Eng. C 2021, 122, 111951.
- Prabhu, B.R.; Varier, M.M.; John, N.S. Fabrication of sandwich structures of Ag/analyte/MoO3 sea urchins for SERS detection of methylene blue dye molecules. Nanotechnology 2023, 21, 215701.
- Yi, X.; Yuan, Z.S.; Yu, X.; Zheng, L.J.; Wang, C.Y. Novel Microneedle Patch-Based Surface-Enhanced Raman Spectroscopy Sensor for the Detection of Pesticide Residues. ACS Appl. Mater. Interfaces 2023, 5, 4873–4882.
- Sakalys, R.; Kho, K.W.; Keyes, T.E. A reproducible, low cost microfluidic microcavity array SERS platform prepared by soft lithography from a 2 photon 3D printed template. Sens. Actuators B Chem. 2021, 340, 129970.
- Gu, X.F.; Wang, K.Y.; Tian, S.; Shao, X.Y.; Li, J.G.; Deng, A.P. A SERS/electrochemical dual-signal readout immunosensor using highly-ordered Au/Ag bimetallic cavity array as the substrate for simultaneous detection of three beta-adrenergic agonists. Talanta 2023, 254, 124159.
- Sai, C.D.; Nguyen, Q.H.; Tran, T.N.A.; Pham, V.; Nguyen, T.B.; Do, H.H.; Vu, T.D. CuO nanorods decorated gold nanostructures as an ultra-sensitive and recyclable SERS substrate. Mater. Chem. Phys. 2022, 293, 126962.
- Chung, C.K.; Chang, W.T.; Liao, M.W.; Chang, H.C.; Lee, C.T. Fabrication of Enhanced Anodic Aluminum Oxide Performance at Room Temperatures Using Hybrid Pulse Anodization With Effective Cooling. Electrochim. Acta 2011, 56, 6489–6497.
- Chung, C.K.; Liu, T.Y.; Chang, W.T. Effect of Oxalic Acid Concentration on the Formation of Anodic Aluminum Oxide Using Pulse Anodization at Room Temperature. Microsyst. Technol. 2010, 16, 1451–1456.
- Chung, C.K.; Tsai, C.H.; Hsu, C.R.; Kuo, E.H.; Chen, Y.; Chung, I.C. Impurity and Temperature Enhanced Growth Behaviour of Anodic Aluminium Oxide from AA5052 Al-Mg alloy Using Hybrid Pulse Anodization at Room Temperature. Corros. Sci. 2017, 125, 40–47.
- Kandjani, A.E.; Sabri, Y.M.; Field, M.R.; Coyle, V.E.; Smith, R.; Bhargava, S.K. Candle-Soot Derived Photoactive and Superamphiphobic Fractal Titania Electrode. Chem. Mater. 2016, 28, 7919–7927.
- Guo, W.J.; Chen, Z.J.Q.; Feng, Z.T.; Li, H.N.; Zhang, M.Y.; Zhang, H.R.; Cui, X. Fabrication of Concave Microwells and Their Applications in Micro-Tissue Engineering: A Review. Micromachines 2022, 13, 1555.
- Gao, Y.M.; Zhu, H.Y.; Wang, X.X.; Shen, R.; Zhou, X.M.; Zhao, X.F.; Li, Z.; Zhang, C.; Lei, F.C.; Yu, J. Promising Mass-Productive 4-Inch Commercial SERS Sensor with Particle in Micro-Nano Porous Ag/Si/Ag Structure Using in Auxiliary Diagnosis of Early Lung Cancer. Small 2023, 19, e2207324.
- Shahine, I.; Mevellec, J.Y.; Richard-Plouet, M.; Humbert, B.; Tessier, P.Y. Nanoporous Gold Stacked Layers as Substrates for SERS Detection in Liquids or Gases with Ultralow Detection Limits and Long-Term. J. Phys. Chem. C 2022, 126, 17223–17233.
- Kochylas, I.; Dimitriou, A.; Apostolaki, M.A.; Skoulikidou, M.C.; Likodimos, V.; Gardelis, S.; Papanikolaou, N. Enhanced Photoluminescence of R6G Dyes from Metal Decorated Silicon Nanowires Fabricated through Metal Assisted Chemical Etching. Materials 2023, 16, 1386.
- Golubewa, L.; Rehman, H.; Padrez, Y.; Basharin, A.; Sumit, S.; Timoshchenko, I.; Karpicz, R.; Svirko, Y.; Kuzhir, P. Black Silicon: Breaking through the Everlasting Cost vs. Effectivity Trade-Off for SERS Substrates. Materials 2023, 16, 1948.
- Li, X.D.; Zhou, H.L.; Wang, L.H.; Wang, H.W.; Adili, A.; Li, J.T.; Zhang, J.G. SERS paper sensor based on three-dimensional nanoflowers assembling on polyester fiber membrane for rapid detection of florfenicol residues in chicken. J. Food Compos. Anal. 2023, 115, 104911.
- Zhao, Y.J.; Wang, X.; Chen, Y.Q.; Wang, Q.Z.; Wang, L.; Yao, Z.Y. Electrochemical synthesis of Co/Ni bimetal-organic frameworks: A high-performance SERS platform for detection of tetracycline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121843.
- Wang, P.X.; Wang, L.; Li, C.; Li, X.; Li, G.L. Reliable and Rapid Detection and Quantification of Enrofloxacin Using a Ratiometric SERS Aptasensor. Molecules 2022, 27, 8764.
- Zhao, B.B.; Liu, H.; Wang, H.; Zhang, Y.T.; Wang, X.L.; Zhou, N.D. Bilayer magnetic-plasmonic satellite nanoassemblies for SERS detection of tobramycin with exonuclease amplification. Biosens. Bioelectron. 2022, 218, 114789.
- Yu, Z.N.; Huang, L.; Zhang, Z.M.; Li, G.K. Simultaneous and Accurate Quantification of Multiple Antibiotics in Aquatic Samples by Surface-Enhanced Raman Scattering Using a Ti3C2Tx/DNA/Ag Membrane Substrate. Anal. Chem. 2021, 93, 13072–13079.
- Yao, L.; Chen, Y.L.; Wang, R.R.; Yan, C.; Xu, J.G.; Yao, B.B.; Cheng, J.G.; Chen, W. Rapid and sensitive detection of Hg2+ with a SERS-enhanced lateral flow strip. Analyst 2022, 147, 4337–4347.





