Food safety analysis currently relies on the enzyme-linked immunosorbent assay (ELISA), polymerase chain reaction (PCR), HPLC, GC, UV-visible spectrophotometry, and MS, all of which require significant time to train qualified food safety testing laboratory operators. These factors have hindered the development of rapid food safety monitoring systems, especially in remote areas or areas with a relative lack of testing resources. Surface-enhanced Raman spectroscopy (SERS) has emerged as one of the tools of choice for food safety testing that can overcome these dilemmas. SERS offers advantages over chromatographic mass spectrometry analysis due to its portability, non-destructive nature, and lower cost implications. However, as it currently stands, Raman spectroscopy is a supplemental tool in chemical analysis, reinforcing and enhancing the completeness and coverage of the food safety analysis system. SERS combines portability with non-destructive and cheaper detection costs to gain an advantage over chromatographic mass spectrometry analysis.
1. Introduction
In recent years, there has been an increased awareness of food safety issues, highlighting the importance of food analysis [
1]. While chemical methods have been the standard for food safety penalties [
2], their high costs, lengthy wait times, and limited coverage have become more apparent. This has led to a renewed focus on developing versatile devices for food safety management, such as rapid screening methods [
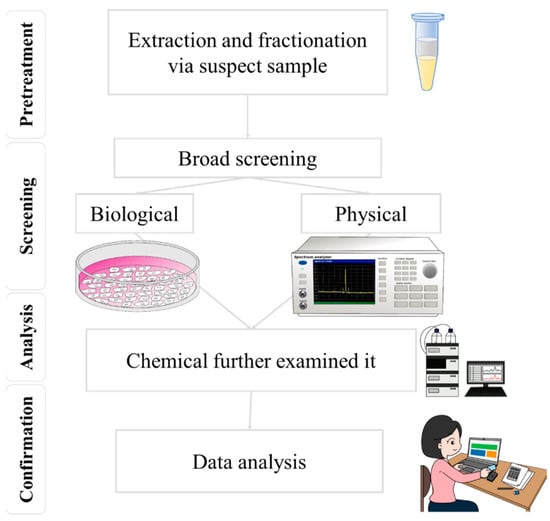
3]. Rapid screening techniques for food safety primarily utilize biological and physical processes, as shown in
Figure 1 [
4,
5]. In food safety analysis, the bioassay method generally involves analyzing the effects of biological or chemical contaminants on intracellular genes or utilizing cellular metabolic mechanisms, for detection, through an antibody-and-antigen match and a series of biochemical reactions [
6]. Instead of focusing on the concentrations of a few select chemicals, bioassays can be a supplementary approach to conventional chemical monitoring methods in the future. They can achieve this by evaluating cumulative effects, such as those caused by mixtures, and addressing the chemicals present at concentrations below the detection limits of chemical analysis [
6]. This method can detect harmful substances—rapidly and sensitively—in food, offering advantages such as fast detection, user-friendly operation, and low cost [
7]. Nonetheless, the bioassay method presents multiple disadvantages. For instance, if the sample preprocessing has little rigor, it could disrupt the identification of substances, resulting in false positives or negatives, owing to limited specificity [
8,
9]. Additionally, the precision of the detection outcomes might be influenced by environmental factors within the laboratory, including the integrity of laboratory partitions, temperature, and humidity. Moreover, the bioassay technique demonstrates a particular reliance on the sample’s composition, and its sensitivity might prove less effective than alternative chemical detection methods when examining components.
Figure 1. By combining screening and mass spectrometry techniques, the accuracy and efficiency of food safety testing can be effectively improved.
In contrast, physical methods more directly detect physical properties in food samples, such as optical and acoustic properties, and they have a more comprehensive application range that is not limited by sample type. Physical methods also have a lower dependence on sample components. Laser technology is a physical method that offers benefits such as fast detection speed, high sensitivity, and good selectivity [
10]. By controlling the wavelength and power of the laser, it is possible to detect and analyze different components in the sample. Laser technology also allows for non-destructive testing, minimizing damage to the model. Raman spectroscopy, particularly surface-enhanced Raman spectroscopy (SERS) technology, is an emerging physical method that offers ultra-trace analysis capabilities. SERS technology is likely to become the preferred option for preliminary screening under the “screening first and testing later” food safety management approach [
11]. The microstructure fabrication and morphology of SERS significantly impact its sensitivity, specificity, selectivity, and anti-interference ability for food safety screening. Raman spectroscopy is a non-destructive spectroscopic technique that enables the rapid and accurate identification and semi-quantification of chemical and biological substances [
12]. Mass spectrometry is a technique that allows for the high-sensitivity and high-resolution analysis of complex samples. The combination of Raman spectroscopy and mass spectrometry in food safety detection can enhance the accuracy and efficiency of testing [
13]. Leveraging a tandem approach of Raman spectroscopy and mass spectrometry in food safety inspection provides several benefits, including accelerated detection [
14], increased precision [
13], preservation of sample integrity [
12], and real-time surveillance [
15]. The initial screening via Raman spectroscopy, coupled with mass spectrometry confirmation and quantification, enhances testing efficiency. These complementary techniques provide diverse chemical insights, corroborating and bolstering the accuracy of results. Importantly, the non-invasive characteristic of Raman spectroscopy and its swift operation allow for timely monitoring to control safety risks. Practical applications span from detecting pesticide residues [
16,
17] and food additives [
18,
19], to identifying microbial contamination [
20,
21] and food poisoning agents [
15,
22], and to verifying food authenticity. Prompt identification and the quantification of potential hazards assure compliance with safety regulations.
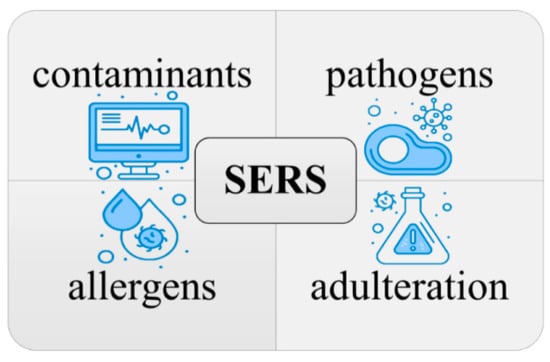
Surface-enhanced Raman spectroscopy (SERS) is a promising technique for food safety testing. It is highly sensitive, rapid, non-destructive, and versatile, and it can be used to detect a wide range of contaminants, including food-borne pathogens, contaminants (pesticides, heavy metals, and antibiotics), adulteration, and allergens [
66], as shown in
Figure 3. SERS has been applied to food safety in several ways, and there is a growing body of research on its use in this field [
11,
67]. However, some challenges are associated with using SERS for food safety testing, such as the need to develop SERS substrates that are specific for detecting the target contaminants [
68] and the need to standardize SERS protocols to ensure that results are comparable between different laboratories. The key lies in the close relationship between the microstructure of SERS and these challenges. Despite these challenges, SERS is a highly promising and complementary technique to conventional chemical analysis in the field of food safety testing.
Figure 3. Utilizing SERS for the detection and identification of contaminants, pathogens, allergens, and adulteration in food safety.
2. Design, Fabrication, and Applications of SERS
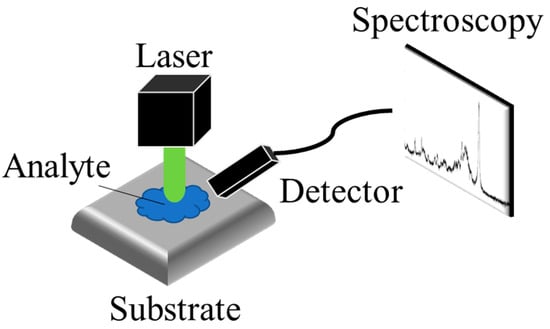
Raman spectroscopy is an analytical technique that detects the inelastic scattering of photons to observe the vibrational and rotational modes of molecules. This interaction between photons and molecules provides a unique spectroscopic “fingerprint” that can be used to identify different molecular species. As
Figure 5 shows, SERS was first discovered in 1974 by Fleischmann et al. [
69] when they observed a significant enhancement of the Raman signal from pyridine molecules adsorbed on a rough silver electrode [
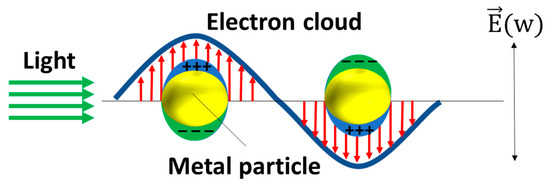
69]. The enhancement in SERS can be attributed to two mechanisms: electromagnetic mechanisms (EM) [
70,
71] and chemical mechanisms (CM) [
72,
73]. The EM arises from the increased local electric field at the metal surface, which occurs due to the generation of local surface plasmon resonance (LSPR) when the metal is illuminated with light. Surface plasmons refer to the collective oscillations of unbound electrons within a region confined to the interface between a metal and a dielectric material. As
Figure 6 shows, the free electrons within the metal nanostructures oscillate in response to the incident light, and these oscillations are confined to the isolated nanostructures, leading to the resonance of the electrons and a subsequent increase in the local electric field. The enhancement factor of the surface-enhanced Raman spectroscopy (SERS) substrate can be estimated using the approximate formula
EF ≈ (|
Eloc|/|
E0|)
4, taking into account a squared factor for the excitation and another squared factor for the emission [
74]. The significant amplification of the local enhanced electric field contributes to the enhancement of the SERS signal, leading to the major development of EM techniques in SERS applications.
Figure 5. The schematic of SERS detection. The Raman scattering of the analyte, enhanced by the substrate, is detected by the detector.
Figure 6. The schematic of the plasmonic resonance of the MNPs. The red arrows stand for the direction of the electron cloud and the green arrows stand for the direction of the light.
The CM, on the other hand, arises from the charge transfer (CT) between the analyte molecule and the substrate. This charge transfer can increase the Raman cross-section and, consequently, enhance the intensity of the Raman signal. Lombardi and Brike elucidated the Herzberg–Teller surface selection rules as a means to elucidate the role of CT, which could enhance the intensities of non-totally symmetric modes, thereby exhibiting a pronounced dependence on wavelength or voltage [
75,
76]. Recently, several research groups have undertaken comprehensive investigations into the surface enhancement of semiconductor materials [
77,
78,
79].
3.1. The Design of the SERS Substrates
The selection of substrates in SERS technology is critical for achieving optimal sensing performance. The design of SERS substrates should be tailored to the specific target substances. In food safety applications, the target substances can be categorized into two groups: small amount molecules [
80,
81,
82,
83,
84,
85,
86,
87,
88] and tiny biomaterials, such as cells [
89,
90,
91,
92], cell walls [
93,
94,
95,
96], and spores [
97,
98,
99,
100]. The key distinction between these two categories lies in their size. Molecules are typically smaller than 100 nm and can be accommodated within the hotspot of the SERS substrate [
80,
81,
82,
83,
84,
85,
86,
87,
88]. On the other hand, tiny biomaterials are generally larger than 100 nm and cannot fit into the small hotspot of the SERS substrate [
89,
90,
91,
92,
93,
94,
95,
96,
97,
98,
99,
100].
The choice of SERS substrate significantly affects the Raman enhancement, prompting researchers to invest in the development of diverse substrates for SERS measurements over the past few decades. These SERS substrates can generally be classified into two categories: metal nanoparticles (MNPs) in suspension and metal nanostructures on solid substrates. The utilization of MNPs in suspension offers several advantages for SERS applications, including easier chemical synthesis (typically without the need for complex equipment) and the production of excellent enhanced Raman signals.
Currently, various shapes of metal MNPs have been proposed, including the nanorods [
111,
116], nanostars [
109], nanocubes [
110], hyperbranched Au nanocorals [
102], flower-like Au NPs [
103], Pt-Au triangular nanoprisms [
104], Au popcorns [
105], decahedral Ag NPs [
106], Au nanofoams [
107], and dual-gap Au nanodumbbells [
108]. These different shapes are aimed at creating more branches, pores, or tips on the MNPs, which are believed to generate high-intensity electromagnetic regions, also known as anisotropic hot spots.
Nanostructures such as nanorods, nanopillars, and nanowires, decorated with MNPs, have the capability to generate controllable hotspots by tuning the distance between each nanostructure. This configuration offers advantages in terms of sensitivity, uniformity, and stability. However, the fabrication processes for these nanostructures are often complex and time-consuming. Therefore, it is crucial to develop simple and facile methods for fabricating nanostructure supporters. Porous materials present another option as SERS supporters. These materials possess a large specific surface area, controllable porosity, and can be easily fabricated on a large scale. Various porous materials have been proposed, including porous SiO
2 [
81], porous Si [
128], porous TiO
2 [
129], porous Al
2O
3 [
130,
131,
135], Cu foam [
136], and Ni foam [
137]. These materials serve as a platform for supporting MNPs or metal nanofilms to generate high-density electric fields.
To further simplify the fabrication of the SERS substrate, some biological or commercial substrates were applied to be a simple SERS substrate, such as a cuttlebone-derived organic matrix [
98], diatomite [
138], beetle wings [
139], cotton swabs [
140,
141], and filter paper [
142]. The biological materials usually have complex structures such as the “wall-septa” on the cuttlebone [
98], 3D periodic microstructures on the beetle wings, and the micro porous structure on the diatomite are naturally formed micro-to-nano structures that can be directly decorated with MNPs [
98,
139] or that used to be the mold to translate the nanostructure to PDMS [
139]. Other simple SERS substrate supporters are commercial substrates such as cotton swabs [
140,
141] and filter paper [
142]. Those commercial substrates are constructed with fibers, which allows the NPs adhesion and generates the hotspots on the fibers. The fiber-based SERS substrates have the advantages of the good hydrophilicity, flexibility, and the low-cost. However, the sensitivity and uniformity of fiber-based SERS substrates are a challenge. In brief, those commercial simple SERS supporters may suffer from the lower sensitivity because they lack the well-defined nanostructures.
3.2. The Fabrication of the SERS Substrates
In the bottom-up approaches, the nanostructures can be done by chemical deposition [
120,
129,
143,
144], 3D printing [
124,
145], self-assembly particles [
123], and chemical synthesis [
126,
130,
131,
132,
133,
134,
135]. During the chemical deposition, controlling the size and the shape of the deposited nanostructures is a challenge. Durastanti et al. used SiH
4 and H
2 as precursors to grow the Si nanowire with the length of 2–3 μm and the diameter of 40–70 nm by plasma-enhanced chemical vapor deposition (PECVD) [
120]. Malik et al. deposited TiO
2 shells on the carbon soot layer to generate the TiO
2 fractal nanostructure [
129], due to the carbon soot layer usually consisting of small particles with sizes of 1–100 nm [
146]. The 3D printing has the advantage of a well-defined microstructure [
147]; however, the 3D printer cannot construct the nanostructure directly, and a decent investment is necessary for high resolution 3D printers [
147].
In the top-down approaches, etching is a common method to generate nanostructures, such as Si micropyramid [
119], porous Au [
149], Si nanowires [
121], and Si nanopillar [
150]. The Si micropyramid can be simply fabricated by etching the Si wafer in the KOH due to the anisotropic properties of the Si wafer [
119]. Kochylas et al. fabricated Si nanowires by metal-assisted chemical etching, and they decorated the Si nanowires with Ag nanoparticles. By tuning the etching time, the morphology of the Ag nanoparticles formed dendritic structures instead of aggregating together, which shows higher sensitivity [
121]. The reactive ion etching (RIE) is a suitable option when aiming for uniform surface structure coverage over large areas. The RIE system uses a plasma source consisting of highly reactive ion species, and when they bombard the sample, a chemical reaction takes place that selectively erodes away the sample surface [
150].
3.3. The SERS Application on Food Safety
As Figure 10 shows, The SERS applications on food safety can be divided into four kinds, depending on the sensing targets: the pesticide, antibiotic, microorganic, and metal ions. To detect various analytes, SERS substrates fabricated for the different functions are required.
Figure 10. Schematic of food safety detection.
Antibiotics are widely applied in various fields of food safety, such as poultry production, livestock production, and aquaculture, to protect the production from microorganism infections. However, the antibiotic residues are an important issue for multiple food products, such as meat, milk, fish, and honey [
160,
161,
162,
163,
164]. The antibiotic residues can enter human bodies through food chains and the bioaccumulation. High concentration antibiotic residues could damage human organs and lead the human body develop antibiotic resistances.
Table 5 lists the currently reported SERS substrates for sensing antibiotics and their substrates, metal particles, target substances, and performances.
Heavy metal ions, which cannot be degraded by organisms and can accumulate through the food chain, posing a great threat to human health, are widely distributed in the environment and food [
170]. Therefore, the determination of heavy metal ions is a significant issue for food safety, and tremendous efforts have been made to detect various heavy metal ions. SERS is a powerful sensing technique; however, the metal ions have no Raman signal, which means that the SERS can only detect the metal ions with chelation pre-treatment. In addition, the difficult preparation of aptamer for heavy metal ions also limits the SERS application on metal detection. It is worth noting that some metal ions detections were realized by SERS recently.
This entry is adapted from the peer-reviewed paper 10.3390/mi14071343