Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tariq Aziz | -- | 2572 | 2023-07-03 12:10:21 | | | |
| 2 | Fanny Huang | Meta information modification | 2572 | 2023-07-04 04:43:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Aziz, T.; Khan, A.A.; Tzora, A.; Voidarou, C.(.; Skoufos, I. Common Inflammatory Diseases and Gut Microbiota with Diet. Encyclopedia. Available online: https://encyclopedia.pub/entry/46344 (accessed on 07 February 2026).
Aziz T, Khan AA, Tzora A, Voidarou C(, Skoufos I. Common Inflammatory Diseases and Gut Microbiota with Diet. Encyclopedia. Available at: https://encyclopedia.pub/entry/46344. Accessed February 07, 2026.
Aziz, Tariq, Ayaz Ali Khan, Athina Tzora, Chrysoula (Chrysa) Voidarou, Ioannis Skoufos. "Common Inflammatory Diseases and Gut Microbiota with Diet" Encyclopedia, https://encyclopedia.pub/entry/46344 (accessed February 07, 2026).
Aziz, T., Khan, A.A., Tzora, A., Voidarou, C.(., & Skoufos, I. (2023, July 03). Common Inflammatory Diseases and Gut Microbiota with Diet. In Encyclopedia. https://encyclopedia.pub/entry/46344
Aziz, Tariq, et al. "Common Inflammatory Diseases and Gut Microbiota with Diet." Encyclopedia. Web. 03 July, 2023.
Copy Citation
Dietary choices can have an immense impact on the microbial flora of the gut in people with inflammatory diseases.
polymyalgia rheumatica (PMR)
spinal muscular atrophy (SMA)
vasculitis
sarcopenia
cirrhosis
cancer
fibromyalgia
common inflammatory diseases
gut microbiota
1. Polymyalgia Rheumatica (PMR)
Polymyalgia rheumatica (PMR) is one of the types of inflammatory disorders that mostly affects individuals aged over 50. The exact cause of PMR is unclear, but there is evidence that shows that dietary factors have a role in the progression and disease development. Some studies have suggested that certain dietary supplements, such as vitamin D, may be beneficial for individuals with PMR. For example, in 2015, a study found that vitamin D supplementation may reduce the need for corticosteroid therapy in patients with PMR [1].
A 2019 study found that a diet containing processed foods, red meat, and refined sugars might be associated with a higher risk of developing PMR (Zhao et al., 2019) [2]. On the other hand, a diet that includes vegetables, fruits, protein sources, and flatbread may have a protective effect against PMR. Some of the bacterial species that may be associated with this condition include Prevotella, Bacteroides, and Ruminococcus, which are drastically abundant in the gut of the patients compared to the microbial flora of the gut in normal healthy individuals [3]. Consuming a healthy diet that may include fruits, whole grains, a variety of vegetables, nuts, seeds, and legumes can increase the intake of prebiotic fibers that feed beneficial bacteria in the gut. For probiotic bacteria, such fermented foods as yogurt are good as these can also support the gut microbiota. Apart from that, avoid the intake of processed foods, added sugars, and saturated and trans fats because they may largely contribute to inflammation [4]. Thus, all of the mentioned foods can alleviate the symptoms of inflammatory disease rather than cure the disease.
2. Spinal Muscular Atrophy (SMA)
Nutrition plays a significant part in the care of patients suffering from SMA. These patients face the progressive wasting of muscles, and their functional impairment has a profound and devastating influence on the outcomes of diet [5]. As many patients with this disease must face the problem of malnutrition, a special diet and dietary fiber intake become crucial to maintaining their good health conditions [6]. A study was conducted in Boston, Massachusetts, that included 60 subjects; after the uptake of a special diet and dietary for 3 years, the cases of malnutrition decreased from 65% to 27% [7].
A systemic review was conducted on 36 studies in Australia that has shown a very important role of dietary fibers in the normal growth and muscle development of SMA patients [8]. Another study was conducted on the Chinese population in 2016; it showed that lower calcium uptake and malnutrition in 84% of the subjects could flare up SMA. They concluded that a special diet and calcium uptake could improve muscle and body growth [9]. Research has shown the link between SMA and changes in the gut microbial flora with reference to diet. One study that was conducted in the SMA animal model showed that the changes in the microbial flora of the gut were associated with disease severity and progression. Specifically, the abundance of certain bacterial taxa was altered in mice with SMA compared to control mice. The authors also found that modulating the microbiota of the gut by using probiotics has improved motor function as well as extended lifespan in mice with SMA [10]. In another study, the examination of the gut microbiota was performed in some patients who suffered from SMA. The researchers found that these patients had altered microbial diversity compared to healthy controls, which might be a key factor in the pathophysiology of SMA. The gut microbiota is targeted to represent a novel therapeutic approach to the disease [11].
3. Vasculitis
The involvement of gastrointestinal is very common in patients suffering from vasculitis. In chronic systemic inflammation, patients frequently experience weight loss and cachexia; therefore, the diet should be altered accordingly [12]. Another experiment was conducted involving two groups of mice based on the differences in diet. One group contained all the necessary nutrients, and the other group lacked beta-glucan. Group two, which was free from beta-glucan, was comparatively having less survival time. Moreover, the Bacteroides were present in large numbers, which caused inflammation in the microbial flora of the gut in the second group [13].
In some cross-sectional studies, researchers have found that levels of antioxidants are inversely related to the levels of inflammatory markers. A study group was designed with a restricted diet that included simple carbohydrates and fried foods and lacked fruits and vegetables. Their results showed an increased level of CRP, explaining the link between diet and disease [14]. One of the types of vasculitis is AAV (anti-neutrophil cytoplasmic antibody-associated vasculitis), in which the patient suffers from the inflammation of small blood vessels. Neutrophils are the main members in the development of disease, and their activity is strongly influenced by some metabolites produced by the fermentation of non-digestible carbohydrates by the microbial flora of the gut [15]. For the treatment of vasculitis, patients take steroids that can lead to osteoporosis. Increased calcium in the diet helps prevent it from developing. Moreover, it is recommended to consume broccoli, skimmed milk, and yogurt [16].
4. Sarcopenia
Diet may play a significant role in the onset and progression of sarcopenia, according to the available evidence. It has been demonstrated that getting enough protein, especially from high-quality sources, is necessary for older adults to keep their muscles intact, as well as their function. Omega-3 fatty acids and vitamin D may also have the potential to prevent sarcopenia. For instance, a precise survey and meta-examination of 20 investigations carried out in aged people having sarcopenia discovered that protein supplementation showed improvement in maintaining muscle mass, strength, and function [17]. According to another research, an omega-3-rich diet was linked to greater muscle mass and lower levels of inflammation in older women. At last, a randomized controlled preliminary trial led to the conclusion that vitamin D supplementation kept the activity of muscles intact and helped capability in aged people with sarcopenia [18].
Moreover, in one review, it was observed that dietary supplementation with whey protein, a high-quality protein source, can improve muscle strength, mass, functioning, and physical activity in adults with sarcopenia aged over 60 [19]. Additionally, a cross-sectional study found that a higher intake of fruits and vegetables was associated with greater muscle mass and strength in older adults [20]. Furthermore, a randomized controlled trial demonstrated that a Mediterranean-style diet improved muscle strength and physical function in older adults suffering from sarcopenia [21]. Overall, these studies suggest that a balanced diet with adequate protein, fruits, and vegetables and adherence to a Mediterranean-style diet may be beneficial for preventing and managing sarcopenia in older adults.
5. Cirrhosis
Cirrhosis is a chronic liver illness that is characterized by the growth of scar tissue in lieu of healthy liver tissue. Research has revealed that nutrition has a significant impact on the onset and progression of cirrhosis. For instance, a high protein diet has been linked to a higher chance of developing hepatic encephalopathy, a cirrhosis consequence that impairs brain function [22]. Conversely, a larger diet of polyunsaturated fats has been connected to a decreased risk of liver fibrosis, which is a major cause of cirrhosis, whereas a higher intake of saturated and monounsaturated fats led to an increased risk of liver fibrosis [23].
Moreover, non-alcoholic fatty liver disease (NAFLD), a frequent cause of cirrhosis, has been related to a diet rich in sugar and refined carbohydrates [24]. Additionally, cirrhosis and its side effects, such as hepatic encephalopathy and variceal hemorrhage, have been linked to reducing intakes of dietary fiber [25]. According to a study performed in 2019, a diet rich in red and processed meat led to an increased chance of liver fibrosis, which can result in cirrhosis [26]. According to a different 2020 study, people with cirrhosis may benefit from a Mediterranean-style diet that is high in fruits, vegetables, whole grains, fish, and olive oil because it enhances liver function and lowers the risk of complications [27]. These data collectively imply that dietary changes may be crucial in the prevention and treatment of cirrhosis.
6. Cancer
There are some research studies that show the link between diet and cancer. According to a 2015 study, a Western-style diet was linked to a higher risk of colorectal cancer [28]. A plant-based diet lowers the risk of breast cancer [29]. Various nutrients, such as vitamin D and omega-3 fatty acids, may also act as a preventative measure against some cancers, according to other research [30].
7. Fibromyalgia
Fibromyalgia is a complex chronic pain condition that affects many aspects of a person’s life, including diet and nutrition. Numerous studies imply that dietary elements may influence the onset and treatment of fibromyalgia. For instance, a low-FODMAP diet, which limits specific carbohydrate types that may cause digestive problems, may be useful in easing fibromyalgia symptoms [31]. Other studies have shown that people with fibromyalgia may also benefit from a diet high in anti-inflammatory foods, such as fruits, vegetables, whole grains, and lean protein may also be beneficial for individuals with fibromyalgia [32][33].
8. Alzheimer’s Disease
Alzheimer’s disease is a degenerative neurological condition marked by memory loss and cognitive deterioration. While the precise origin of Alzheimer’s disease is unknown, evidence indicates that food and gut flora may have an impact on the onset and course of the condition. According to one study, people with Alzheimer’s disease exhibited a different gut microbiota composition from those in general, with lower concentrations of helpful bacteria and greater concentrations of possibly dangerous bacteria [34]. According to other research, alterations in the gut microbiota might cause an increase in inflammation and oxidative stress, both of which have been associated with the onset of Alzheimer’s disease [35]. In terms of food, a Mediterranean-style diet lowers the incidence of Alzheimer’s disease. This diet places an emphasis on whole grains, fruits, vegetables, and healthy fats. This is assumed to be because many of the items of this kind of diet have anti-inflammatory and antioxidant qualities. A sugary diet, refined carbohydrates, and trans fats have a higher probability of Alzheimer’s disease [35]. Overall, there is evidence to suggest that a good diet and a balanced microbial flora of the gut may be significant factors in lowering the risk of acquiring Alzheimer’s disease, even though the relationship between Alzheimer’s disease, food, and gut microbiota is currently being investigated.
9. Parkinson’s Disease
A neurodegenerative ailment called Parkinson’s disease is characterized by the brain’s dopamine-producing neurons dying out. According to recent studies, the development and course of the illness may be influenced by food and gut bacteria. Studies have revealed that the gut microbiota in people with Parkinson’s disease differs from those in health, with a reduced number of helpful bacteria and a larger abundance of possibly dangerous bacteria [36]. The course of Parkinson’s disease is assumed to be aided by increased inflammation due to the alterations in gut microbiota in another research [37]. A high-fiber diet was linked to a lower incidence of Parkinson’s disease, according to research on food [38]. Fiber is considered to encourage the development of good gut flora, which may assist in lessening oxidative stress and inflammation. On the other hand, a diet with a high potency of saturated fats and processed foods is associated with a greater chance of Parkinson’s disease [39]. There is evidence to suggest that a healthy diet and a balanced gut microbiota may be significant factors in lowering the chance of acquiring Parkinson’s disease, even though the relationship between Parkinson’s disease, food, and gut microbiota is still being investigated.
10. Arthritis
Joint discomfort and inflammation are often referred to as arthritis. According to research, food and gut bacteria are involved in the onset and progression of different types of arthritis. Research has revealed that the intestinal microflora of rheumatoid arthritis (RA) patients differs from that of controls, with a reduced quantity of helpful bacteria and a larger abundance of possibly dangerous bacteria [40]. The development of RA is mostly influenced by inflammation, which has been linked to alterations in gut microbiota in another research [41]. A study on food discovered that a decreased risk of having RA was linked to a Mediterranean-style diet [42]. This is assumed to be because many of the items in this kind of diet have anti-inflammatory and antioxidant qualities. As with all other inflammatory diseases, saturated fat and sugar also play an important role in the development of RA [43]. Additionally, it has been demonstrated that several foods and supplements contain anti-inflammatory characteristics, which may be helpful for treating the symptoms of arthritis. For instance, omega-3 fatty acids, which are included in fish oil, have been demonstrated to lower inflammation and may assist people with arthritis to have less pain and stiffness in their joints [44]. Overall, there is evidence to suggest that a good diet and a balanced gut microbiota may be significant factors in lowering the risk of developing arthritis and controlling its symptoms, even though the relationship between food, arthritis, and gut microbiota is currently being investigated.
11. Inflammatory Bowel Diseases
Inflammatory bowel diseases (Crohn’s disease, peptic ulcer, gastritis, ulcerative colitis diverticular disease, irritable bowel syndrome) are chronic conditions manifested by gastrointestinal tract inflammation. These diseases have a significant impact on the patients’ lifestyle quality and require long-term management. In recent years, research efforts have focused on understanding the underlying mechanisms and developing targeted therapies (Figure 1). Advances in genetic studies have identified key genetic variants associated with susceptibility to these diseases, shedding light on their complex etiology. Additionally, emerging technologies, such as microbiome analysis and precision medicine approaches, hold promise for personalized treatment strategies. Furthermore, ongoing clinical trials are exploring novel therapeutic agents targeting specific immune pathways and cytokines involved in the inflammatory process. The integration of these advancements in the diagnosis, monitoring, and treatment of IBD shows potential for improved outcomes and a brighter future for patients affected by these conditions [45][46].
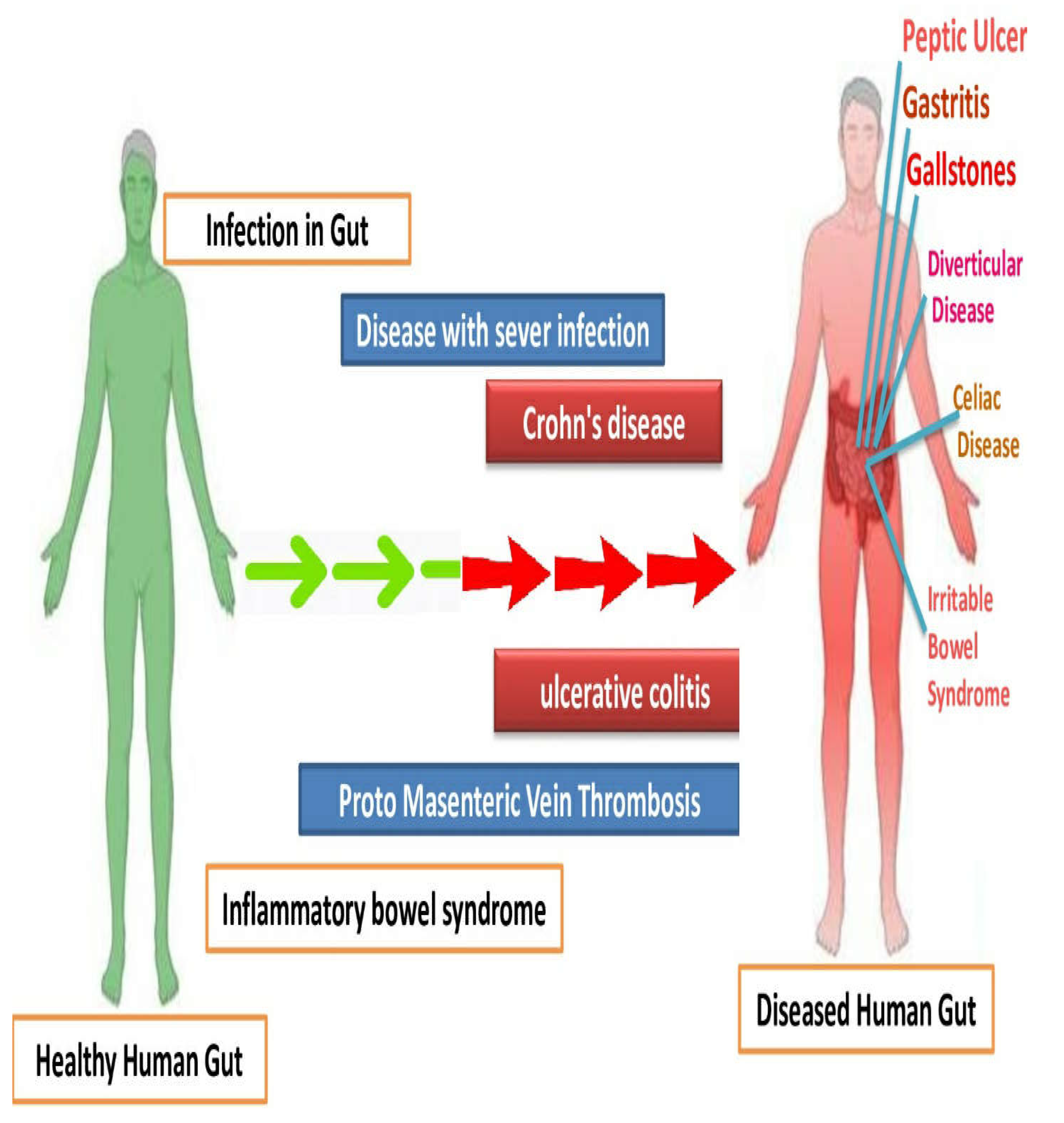
Figure 1. Comparison of human gut with bowel inflammatory diseases, with the control having healthy gut microbiome.
Table 1 below summarizes the healthy, beneficial diet to prevent or alleviate the symptoms of the disease, and the harmful diet that triggers the disease by altering the gut microbial flora is given below.
Table 1. Summary of the common inflammatory diseases and gut microbiota with reference to diet.
| Common Inflammatory Diseases | Beneficial Diet | Harmful Diet | Changes in Gut Microbial Flora due to Harmful Diet | References |
|---|---|---|---|---|
| PMR | Vitamin D, plant-based diet, lean proteins | Processed foods, refined sugars, fats | Increase in Prevotella, Bacteroides, Ruminococcus sp. | Muratore et al., 2015 [47] Zhao et al., 2019 [2] |
| SMA | Calcium, dietary fibers, probiotics | Processed foods | Increase in harmful bacteria. | Zhou et al., 2022 [48] Brzozowski et al., 2016 [49] |
| Vasculitis | Calcium, broccoli, yogurt, skimmed milk, plant-based diet, beta-glucan | Food additives, fried foods, non-digestible carbohydrates | Increase in bacteroides | Lunardi et al., 1992 [50] Sato et al., 2017 [3] Snelson et al., 2023 [5] |
| Sarcopenia | Proteins, vitamin D, omega 3 fatty acids, fruits, vegetables | Western-style foods, including fats and processed sugars and foods | Increase in harmful bacteria. | Smith et al., 2015 [7] Beaudart et al., 2016 [8] Morley et al., 2020 [11] |
| Cirrhosis | Polyunsaturated fats, dietary fibres, whole grains, omega-3 foods (fish, olive oil) | Saturated and monounsaturated fats, high intake of processed and red meat, sugars, and refined carbohydrates | Increase in harmful gut bacteria | Lee et al., 2016 [12] Han et al., 2017 [14] De la Fuente et al., 2020 [17] |
| Cancer | Vitamin D, omega-3 fatty acids, plant-based diet | High intake of processed and red meat | Increase in inflammation-causing bacteria | Fung et al., 2015 [18] Harvie and Howell, 2018 [19] Larsson and Wolk, 2018 [20]; Norris and Dennis, 2019 [21] |
| Fibromyalgia | Low FODMAP diet, fruits, vegetables, lean proteins, whole grains | Processed foods and carbohydrates | Increase in inflammation-causing flora | Pedersen et al., 2017 [24] Bagis et al., 2015 [22]; Castro et al., 2019 [23] |
References
- Dellaripa, P.F.; Howard, D. Nutritional Issues in Vasculitis. In Nutrition and Rheumatic Disease. Nutrition and Health; Coleman, L.A., Ed.; Humana Press: Totowa, NJ, USA, 2008.
- Zhao, Y.; Lv, L.; Cui, L.; Yao, Q.; Zhang, H.; Yu, X. Dietary patterns and polymyalgia rheumatica: A case-control study. Clin. Rheumatol. 2019, 38, 197–205.
- Sato, W.; Ishibashi, K.I.; Yamanaka, D.; Adachi, Y.; Ohno, N. Effects of Natural and Chemically Defined Nutrients on Candida albicans Water-soluble Fraction (CAWS) Vasculitis in Mice. Med. Mycol. J. 2017, 58, E47–E62.
- Perkins, A.; Sontheimer, C.; Otjen, J.P.; Shenoi, S. Scurvy Masquerading as Juvenile Idiopathic Arthritis or Vasculitis with Elevated Inflammatory Markers: A Case Series. J. Pediatr. 2020, 218, 202.
- Snelson, M.; Nguyen, J.; Huang, S.; Le, A.; Cheong, D.; Coughlan, M.; O’Sullivan, K. Resistant starch supplementation limits kidney injury in an experimental model of anti-neutrophil cytoplasmic antibody associated vasculitis. Proc. Nutr. Soc. 2023, 82, E67.
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Volpi, E. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2015, 16, 531–546.
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122.
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Bruyère, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2016, 101, 533–541.
- Liao, C.D.; Tsauo, J.Y.; Huang, S.W.; Hsiao, D.J.; Liou, T.H.; Chen, H.C. Effects of protein supplementation combined with exercise intervention on frailty indices, body composition, and physical function in frail older adults. Nutrients 2018, 10, 1916.
- Kim, S.J.; Ju, Y.S.; Lee, J.Y.; Hong, K.J. Fruit and vegetable intake is associated with muscle mass and strength among Korean adults: The Korea National Health and Nutrition Examination Survey 2014–2016. J. Nutr. Gerontol. Geriatr. 2020, 39, 95–108.
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Fried, L.P. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2020, 21, 674–678.
- Lee, J.; Lee, H.R.; Kang, B.; Kim, J.; Kim, S.; Kim, Y.S.; Yoon, H. Association between dietary fat intake and liver fibrosis in nonalcoholic fatty liver disease. Liver Int. 2016, 36, 806–815.
- Tandon, P.; Garcia-Tsao, G. Dietary protein and the risk of hepatic encephalopathy in cirrhosis. Hepatology 2015, 61, 1666–1672.
- Han, M.A.; Nguyen, M.H.; Nguyen, K.P.; Tran, T.T.; Shire, A.M. High dietary fructose intake on cardiovascular and metabolic risk factors in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Ann. Hepatol. 2017, 16, 212–220.
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2017, 53, 372–384.
- Karanjia, R.N.; Crossey, M.M.; Cox, I.J.; Fye, H.K.; Njie, R.; Goldin, R.D.; Taylor-Robinson, S.D. Hepatic steatosis and fibrosis: Non-invasive assessment. World J Gastroenterol. 2016, 22, 9880–9897.
- de la Fuente, Á.D.; Hernández-Contreras, M.E.; Rodríguez-Gutiérrez, R. Mediterranean diet as a complementary therapy in adults with chronic liver disease: A review. Nutrients 2020, 12, 1436.
- Fung, T.T.; Chiuve, S.E.; Willett, W.C.; Hankinson, S.E.; Hu, F.B. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am. J. Clin. Nutr. 2015, 101, 172–184.
- Harvie, M.; Howell, A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects—A narrative review of human and animal evidence. Behav. Sci. 2017, 7, 4.
- Larsson, S.C.; Wolk, A. Vitamin D and risk of multiple cancers: A systematic review and meta-analysis. Eur. J. Cancer 2018, 50, 2736–2748.
- Norris, P.C.; Dennis, E.A. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Adv. Biol. Regul. 2019, 71, 187–196.
- Bagis, S.; Karabiber, M.; As, I.; Tamer, L.; Erdogan, C.; Atalay, A. Is magnesium citrate treatment effective on pain, clinical parameters and functional status in patients with fibromyalgia? Rheumatol. Int. 2015, 35, 393–400.
- Castro, K.L.; Karpinski, K.P.; McFarlin, B.K.; Isiguzo, M.A. Changes in dietary intake after an immune-based elimination diet in fibromyalgia patients: A pilot study. J. Evid. Based Integr. Med. 2019, 24, 2515690X19864356.
- Pedersen, A.M.; Holst, R.; Jakobsen, J.P.; Overgaard, A.; Krogh-Madsen, R. Treatment of fibromyalgia syndrome with a low fermentable oligo-, di-, and monosaccharides and polyols diet (FODMAP): A randomized controlled trial. J. Clin. Gastroenterol. 2017, 51, 139–147.
- Cheng, S.; Li, Y.; Liang, Y.; Li, Y.; Wang, Y. The association between dietary patterns and sarcoidosis risk: A case-control study in China. BMC Pulm. Med. 2020, 20, 10.
- Gerke, A.K.; Hunninghake, G.M.; Theel, E.S. The role of environmental exposures in the pathogenesis of sarcoidosis. Semin. Respir. Crit. Care Med. 2015, 36, 349–363.
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S. Dietary intake of fruits and vegetables and risk of sarcoidosis: A case-control study. Nutrients 2019, 11, 1779.
- Gupta, V.K.; Mehrotra, S.; Misra, R.; Agarwal, V. Altered gut microbiota composition in patients with idiopathic pulmonary sarcoidosis. Microb. Pathog. 2019, 127, 263–270.
- Tomas, I.; Diz, P.; Tobias, A.; Scully, C.; Donos, N. Periodontal health status and bacteraemia from daily oral activities: Systematic review/meta-analysis. J. Clin. Periodontol. 2018, 45, 1340–1359.
- Li, W.; Han, J.; Hu, Q.; Liang, Y. Role of the Gut Microbiota in the Pathogenesis of Psoriasis: Implications for Treatment. Am. J. Clin. Dermatol. 2020, 21, 749–764.
- Kakuta, Y.; Naito, T.; Kinouchi, Y.; Masamune, A. Current Status and Future Prospects of Inflammatory Bowel Disease Genetics. Digestion 2023, 104, 7–15.
- Flamant, M.; Rigaill, J.; Paul, S.; Roblin, X. Advances in the development of Janus kinase inhibitors in inflammatory bowel disease: Future prospects. Drugs 2017, 77, 1057–1068.
- Kim, H.J.; Kim, J.H.; Noh, S.; Kwon, H.J. The association between diet and gut microbiota and its impact on disease progression in patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1855–1863.
- Navarro-López, V.; Martínez-Andrés, A.; Ramírez-Boscá, A. The role of the gut microbiome in the pathophysiology and potential treatment of psoriasis. Expert Rev. Clin. Immunol. 2018, 14, 979–988.
- Shen, L.; Liu, L.; Ji, H.F. Alzheimer’s Disease Histological and Behavioral Manifestations in Transgenic Mice Correlate with Specific Gut Microbiome State. J. Alzheimers Dis. 2017, 56, 385–390.
- Hussain, Z.; Hussain, N. Detection of nDNA antibodies in Rheumatoid Arthritis patients by an immunofluorescent technique. Afr. J. Biotechnol. 2014, 13, 3943–3949.
- Mehvish; Hussain, N. Association of ACE I/D polymorphism with diabetes. J. Adv. Biotechnol. 2014, 3, 248–255.
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.-F.; Huang, L.; et al. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, 1241–1257.
- Hussain, N. Clinical and Laboratory Manifestations of SLE in Pakistani Lupus Patients. Pakistan J. Zool. 2013, 45, 169–175.
- Hussain, N.; Kayani, H.Z. Mutational Analysis of DNASE I Gene in Diabetic Patients. J. Adv. Biotechnol. 2014, 3, 243–247.
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean Diet and Risk for Alzheimer’s Disease. Ann. Neurol. 2006, 59, 912–921.
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Schneider, J.; Wilson, R.S. Dietary Fats and the Risk of Incident Alzheimer Disease. Arch. Neurol. 2003, 60, 194–200.
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut Microbiota are Related to Parkinson’s Disease and Clinical Phenotype. Mov. Disord. 2015, 30, 350–358.
- Houser, M.C.; Tansey, M.G. The Gut-Brain Axis: Is Intestinal Inflammation a Silent Driver of Parkinson’s Disease Pathogenesis? NPJ Parkinsons Dis. 2017, 3, 3.
- Gao, X.; Cassidy, A.; Schwarzschild, M.A.; Rimm, E.B.; Ascherio, A. Habitual Intake of Dietary Flavonoids and Risk of Parkinson Disease. Neurology 2012, 78, 1138–1145.
- Chen, H.; O’Reilly, E.J.; Schwarzschild, M.A.; Ascherio, A. Peripheral Inflammatory Biomarkers and Risk of Parkinson’s Disease. Am. J. Epidemiol. 2014, 179, 279–288.
- Muratore, F.; Pipitone, N.; Salvarani, C.; Schmidt, W.A.; Warrington, K.J. Vitamin D and polymyalgia rheumatica: A systematic review and meta-analysis. Clin. Rheumatol. 2015, 34, 419–424.
- Zhou, Y.; Chen, J.; Gong, X.; Lu, Z.; Hua, H.; Zhu, X.; Shi, P.; Li, X.; Zhou, S.; Wang, Y. Nutrition status survey of type 2 and 3 spinal muscular atrophy in Chinese population. J. Nutr. Nutr. 2022, 25, 1488–1494.
- Brzozowski, B.; Mazur-Bialy, A.; Pajdo, R.; Kwiecien, S.; Bilski, J.; Zwolinska-Wcislo, M.; Mach, T.; Brzozowski, T. Mechanisms by which Stress Affects the Experimental and Clinical Inflammatory Bowel Disease (IBD): Role of Brain-Gut Axis. Curr. Neuropharmacol. 2016, 14, 892–900.
- Lunardi, C.; Bambara, L.M.; Biasi, D.; Zagni, P.; Caramaschi, P.; Pacor, M.L. Elimination diet in the treatment of selected patients with hypersensitivity vasculitis. Clin. Exp. Rheumatol. 1992, 10, 131–135.
More
Information
Subjects:
Nutrition & Dietetics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
670
Revisions:
2 times
(View History)
Update Date:
04 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




