Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lis, K.; Bartuzi, Z. Allergy Diagnosis Based on the Measurement of sIgE. Encyclopedia. Available online: https://encyclopedia.pub/entry/46334 (accessed on 07 February 2026).
Lis K, Bartuzi Z. Allergy Diagnosis Based on the Measurement of sIgE. Encyclopedia. Available at: https://encyclopedia.pub/entry/46334. Accessed February 07, 2026.
Lis, Kinga, Zbigniew Bartuzi. "Allergy Diagnosis Based on the Measurement of sIgE" Encyclopedia, https://encyclopedia.pub/entry/46334 (accessed February 07, 2026).
Lis, K., & Bartuzi, Z. (2023, July 03). Allergy Diagnosis Based on the Measurement of sIgE. In Encyclopedia. https://encyclopedia.pub/entry/46334
Lis, Kinga and Zbigniew Bartuzi. "Allergy Diagnosis Based on the Measurement of sIgE." Encyclopedia. Web. 03 July, 2023.
Copy Citation
Diagnosis of allergic diseases is a complex, multi-stage process. It often requires the use of various diagnostic tools. The in vitro diagnostics (IVD), which includes various laboratory tests, is one of the stages of this process. Standard laboratory tests include the measurement of the serum concentration of specific immunoglobulin E (sIgE) for selected allergens, full allergen extracts and/or single allergen components (molecules). The measurement of IgE sIgE to the allergen components is called molecular allergy diagnosis.
specific IgE
allergy
molecular diagnostics
allergen extract
1. Introduction
Allergy and hypersensitivity are increasingly becoming serious global public health problems. These reactions are based on complex pathophysiological mechanisms leading to the dysfunction of various organs and even leading to a life-threatening condition. Both allergy and hypersensitivity can develop at any age and have a significant impact on the quality of life of patients and their families [1]. The terms “hypersensitivity” and “allergy” are not synonymous. Hypersensitivity is defined as conditions clinically resembling an allergy that cause objectively reproducible signs or symptoms, initiated by exposure to a specific stimulus at a dose tolerated by the body of a healthy person. Allergy is a hypersensitivity reaction based on certain or highly probable immunological mechanisms. An allergic reaction occurs when it is triggered by allergens to which the affected person is allergic (i.e., has specific antibodies or immunologically competent cells directed against these allergens) [1].
The currently used division of hypersensitivity reactions was proposed in 1963 by Gell and Coombs. This classification divides hypersensitivity reactions into four distinct groups based on the mechanism of tissue damage (Figure 1): Type I (immediate, IgE mediated), Type II (cytotoxic or IgG/IgM mediated), Type III (immediate mediated by the immune complex) and type IV (late, T-cell mediated, antibody-independent) [2].
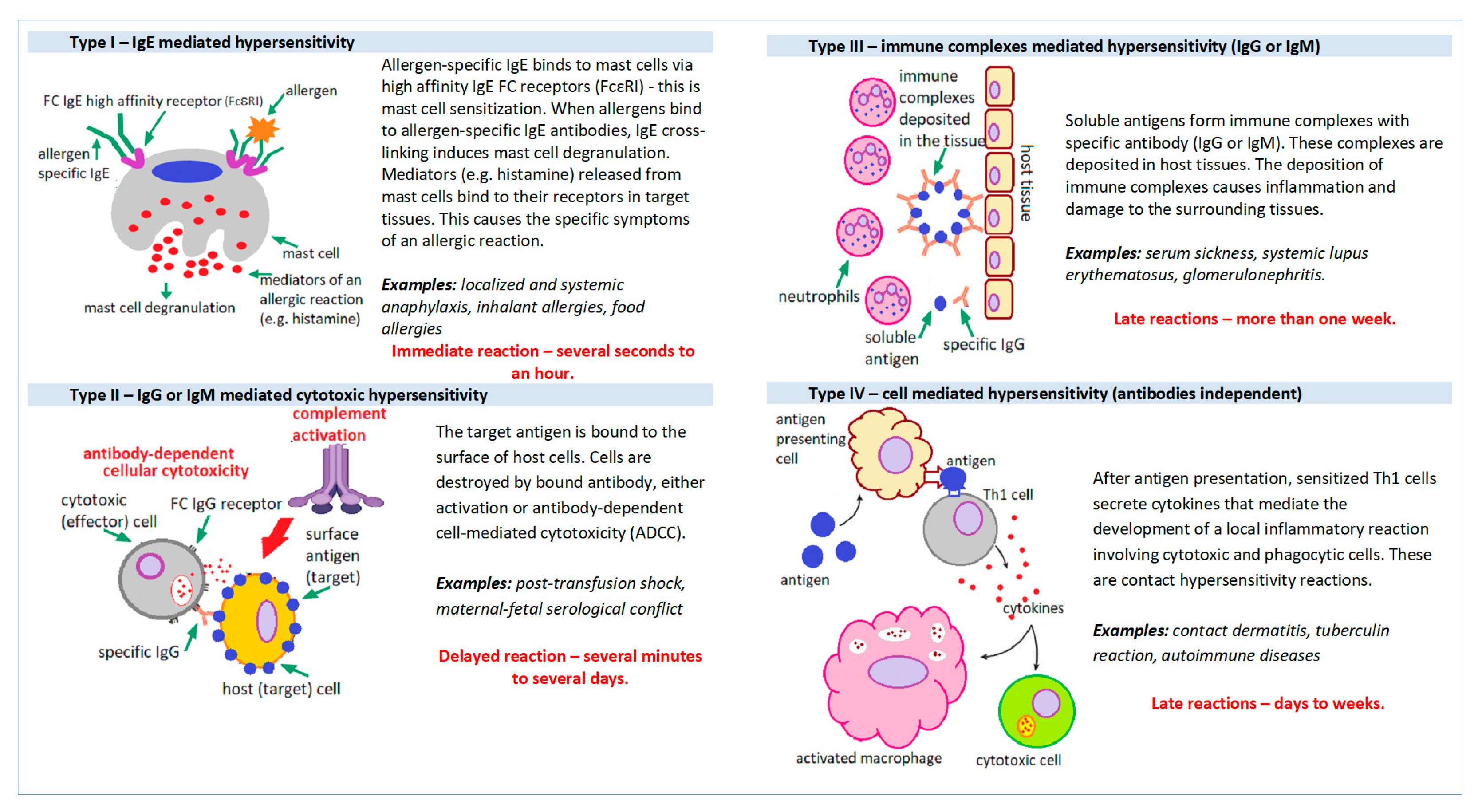
Figure 1. Mechanisms of hypersensitivity reactions.
Allergy diagnosis is a complex and multi-stage process. It begins with a clinical interview, physical examination of the patient and additional tests. Additional tests used in the diagnosis of allergic diseases can be divided into in vivo tests (e.g., skin prick tests, challenge tests) and in vitro tests (laboratory tests). Allergy tests validated for IVD can in principle only confirm or rule out Type I (IgE-mediated) hypersensitivity reactions. The in vitro tests include, among others, serological diagnostics based on the measurement of serum levels of allergen-specific immunoglobulins IgE (sIgE) [3][4].
The possibilities of modern serological diagnostics of allergy are very extensive. The IgE specific for both whole allergen extracts and individual allergen components can be measured in the patient’s blood serum. It is possible to determine sIgE both for single allergens and for mixes of various allergens (so-called allergen mixes). Currently available serological tests can also be used to measure the serum sIgE level for a single allergen (monspecific tests, so-called singleplex tests), as well as for many different allergens in one test (multispecific tests, so-called multiplex tests) [5][6].
2. Allergy Diagnosis Based on the Measurement of Allergen-Specific IgE (sIgE)
This type of laboratory test can be performed with test kits from various manufacturers that have been validated for in vitro diagnostics (IVD) and have certificates required by dedicated legal regulations in force in a specific country. The manufacturers of the test kits guarantee that the results of laboratory tests measured using the analytical reagents produced by them are reliable and repeatable. The test kits are provided with instructions for use, which also define the conditions for the storage of reagents and the collection of biological material for testing and storage. The manual also specifies possible factors interfering with the analytical procedure and the patient’s preparation for a specific laboratory test, including the possible need to discontinue various drugs or other clinically significant parameters that may interfere with the analytical procedure.
Routinely, under standard conditions, the measurement of IgE is measured in the serum of venous blood. Blood for the test should be collected in the morning, although in the case of IgE it is not an obligatory recommendation, as this parameter is not subject to significant daily fluctuations. In these types of laboratory tests, there is also no need to stop treatment with medicines normally used to treat allergies, because they do not interfere with the analytical procedures used. The patient does not have to be fasting, although it is definitely better to fast before each laboratory test, even if it is not absolutely required. It may be important to refrain from ingestion dietary supplements, mainly vitamins (especially vitamin C and biotin), which may significantly interfere with some laboratory procedures and may cause false-negative or false-positive results. Vitamins should be stopped at least 7 days before taking blood for laboratory testing. It is important that each laboratory prepares appropriate guidelines for the patient on how to prepare for the collection of biological material for a specific laboratory test [7][8].
The technique of all currently available serum sIgE assays is based on the Enzyme-Linked Immunosorbent Assay (ELISA) method, which involves the binding of IgE antibodies from the patient’s serum to the allergens for which these antibodies are specific (Figure 2).
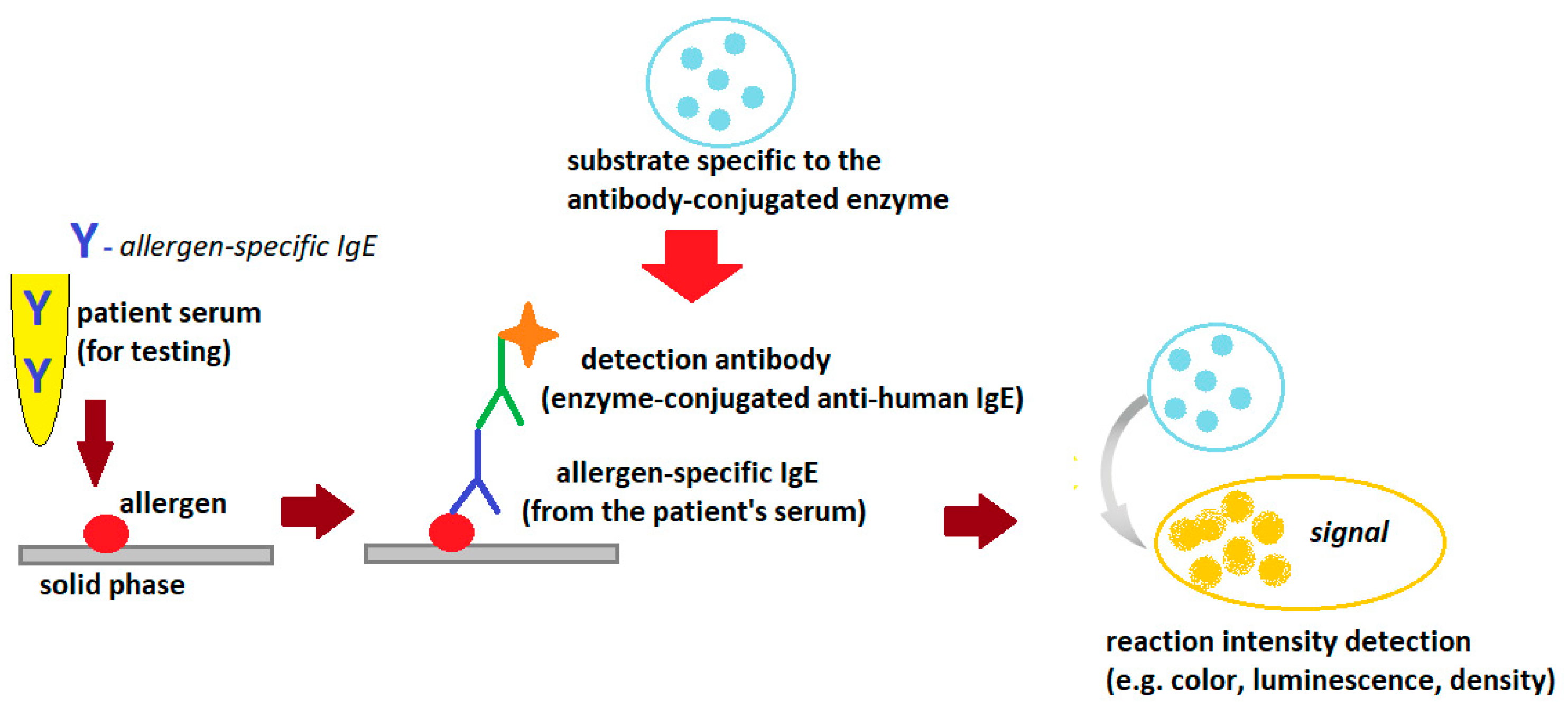
Figure 2. General scheme of the ELISA reaction detecting allergen-specific.
The allergy laboratory tests use standardized allergens, extracts or single allergen components (native or recombinant), which can be suspended in the liquid phase or bound in the solid phase (e.g., cellulose paper, cellulose sponge, polymers, glass, paramagnetic particles and others). If the patient’s serum contains IgE antibodies specific to the allergen used in the test, the allergen/allergen-specific IgE complexes are formed. The complexes are bound to the solid phase and unbound serum components are washed out of the reaction tube with a washing solution (e.g., phosphate buffer). These complexes are then visualized using antibodies specific to human IgE (mouse, goat or rabbit) labeled with the enzyme and a substrate compatible with the labeling enzyme used (chromogen or fluorochrome). The intensity of the reaction is expressed as color intensity or fluorescence intensity (depending on the marker used) and is proportional to the concentration of specific IgE in the tested serum. If appropriate calibration standards are used, the method can be quantitative. Quantitative or semi-quantitative methods are usually used to detect sIgE [5][9][10].
The solid-phase immunoassays based on the ELISA model for routine immunochemical diagnostics, including the measurement of specific IgE levels, were available as early as the 1970s. Initially, radioimmunoassays (radioallergosorbent test, RAST) were used. RAST tests consisted of immobilizing allergen extracts on activated paper discs to bind specific IgE from the patient’s serum. Paper discs were the solid phase of the RAST test. Radiolabels were used to detect allergen/sIgE immune complexes in RAST tests. Currently, radioactive tracers are not used, mainly due to their harmfulness. They have been effectively replaced by colorimetric markers, and above all by fluorescent markers (chemiluminescent and electrochemiluminescent techniques) [5][10].
The materials of the solid phase of testing have also changed. In addition to paper discs, which are rarely used anymore, various types of polymers and multidimensional cellulose matrices (cellulose sponges) are more often used. There are also reaction models based on allergens suspended in the liquid phase. In this assay model, the first step of the reaction (formation of allergen/sIgE complexes) takes place in the liquid phase. In the next step, the formed allergen/sIgE complexes are immobilized on a solid phase matrix. Matrices made of glass fibers or nanoparticles with paramagnetic properties are most often used [11][12].
Qualitative, semi-quantitative and quantitative tests are used to measure the concentration of specific IgE in allergy diagnostics. Quantitative immunoassays for sIgE antibodies require the inclusion of a standard curve. Calibrators for both specific and tIgE measurements should be traceable to the World Health Organization (WHO) International Reference Preparation for Human IgE, 75/502 [10].
Extract-Based and Component (Molecular) Immunological Allergy Diagnostic
Traditional immunological (or serological) diagnosis of allergies is based on the determination of IgE antibodies specific for extracts of native allergens. This type of allergy diagnosis is called allergy diagnosis based on extracts. Allergen extract is a mixture of allergenic and non-allergenic proteins derived from a specific allergenic source (Figure 3).
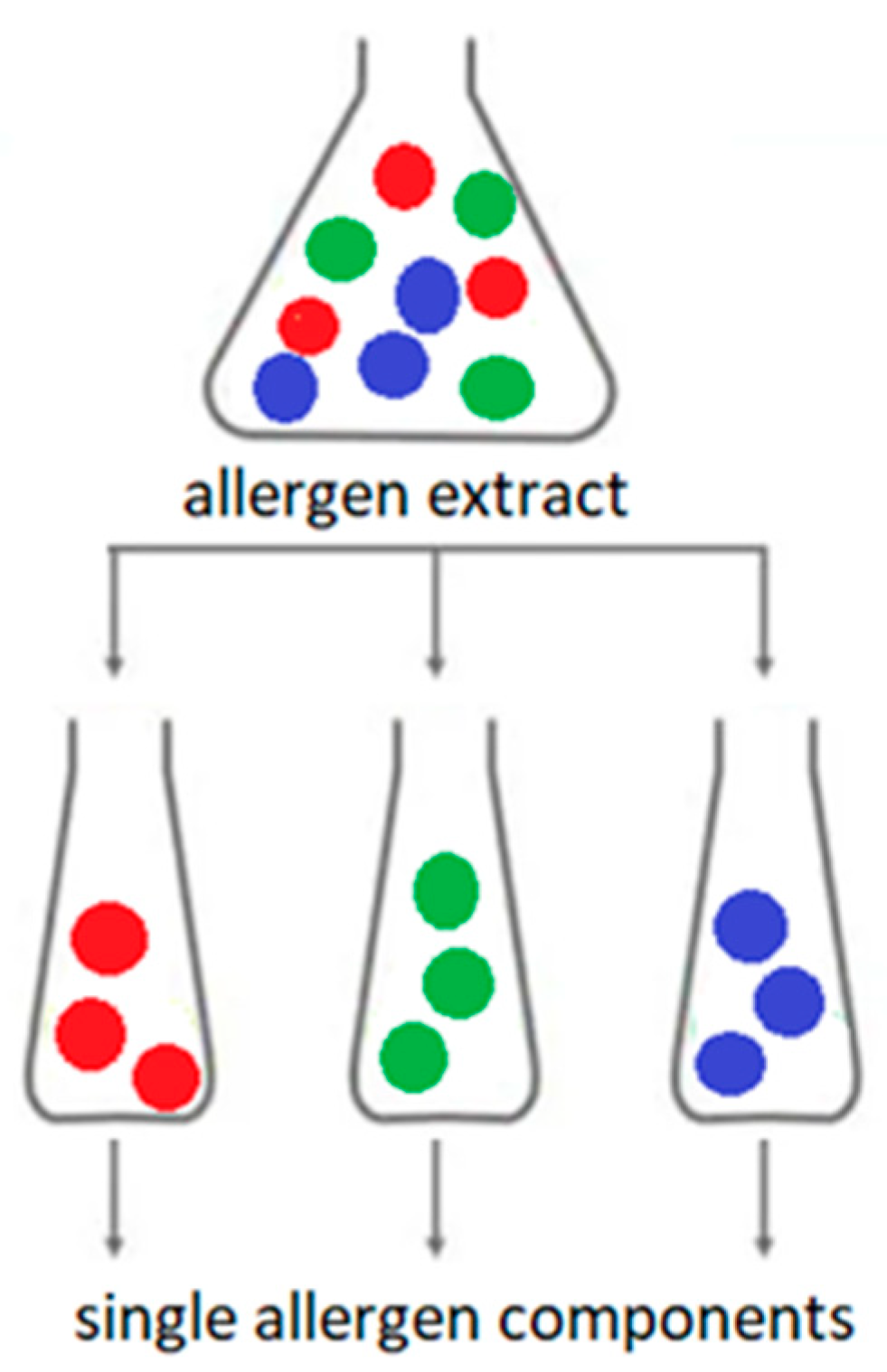
Figure 3. Allergen extract versus allergen components; The colored dots are individual allergen molecules.
Extracts from natural allergen sources are often heterogeneous and may contain many non-allergenic molecules. Extracts of the same allergen from different lots may differ in the composition and amount of allergenic proteins. The detailed composition of full extracts is not clearly possible to determine. Extracts from the same allergen source from different manufacturers may have different component compositions. The composition of extracts from food allergens also depends on the method and conditions of production of a particular food, cultivation and storage of plants or animal husbandry technology. In addition, the presence of bioactive molecules (e.g., proteolytic enzymes) in natural extracts may affect the stability of the allergen extract. Contamination of the natural allergen extract with proteins from other sources is also possible [13][14][15][16][17][18][19].
Molecular diagnosis of allergy is based on the detection in the blood serum of IgE specific against single allergen components (molecules) from specific allergen source. An allergenic component is a single protein or, less commonly, a carbohydrate moiety that can induce an allergic immune response [17][20][21][22]. In the molecular diagnosis of allergy, native or recombinant allergen components are used. Native components are obtained directly from the natural allergen extract using various physicochemical or biochemical techniques. Recombinant components are produced by genetic engineering. Recombinant molecules produced in this way are synthesized by a different organism than the one from which they originate. Escherichia coli (E. coli), or yeast cells, are most commonly used for the production of recombinant allergens. This technology makes it possible to produce unlimited amounts of perfectly purified allergens with precisely defined molecular structures. The downside of recombination is that proteins produced in this way do not undergo post-translational modifications (e.g., glycosylation). This may change their spatial structure and immunological specificity. Such molecules may not bind antibodies produced by immunization with natural proteins. In turn, the advantage of recombinant allergenic components is that as they are devoid of carbohydrate groups, they do not bind clinically insignificant antibodies specific for cross-reactive carbohydrate determinants (anti-CCD). This prevents false-positive results with sIgE tests [13][14][15][16].
Traditional in vitro allergy diagnosis based on allergen extracts makes it possible to determine the source of sensitizing allergens, but it does not allow for the identification of cross-reactions or estimation of the severity of a possible reaction after contact with this allergen. This is especially important for patients allergic to food allergens. Molecular diagnosis of allergy enables accurate identification of the allergen molecule to which the patient is allergic. This gives the opportunity to precisely identify a harmful allergen, determine possible cross-reactions, predict the severity of a possible reaction after contact with this allergen, including severe anaphylactic reactions, or estimate the expected effectiveness of allergen immunotherapy. It also helps to determine the dietary management of a patient with food allergy [17][20][21].
References
- Tanno, L.K.; Calderon, M.A.; Smith, H.E.; Sanchez-Borges, M.; Sheikh, A.; Demoly, P. Dissemination of definitions and concepts of allergic and hypersensitivity conditions. World Allergy Organ J. 2016, 9, 24.
- Dispenza, M.C. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2019, 40, 470–473.
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; Dutoit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025.
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J. 2020, 13, 100080.
- Kleine-Tebbe, J.; Jakob, T. Molecular allergy diagnostics using IgE singleplex determinations: Methodological and practical considerations for use in clinical routine: Part 18 of the Series Molecular Allergology. Allergo J. Int. 2015, 24, 185–197.
- Jakob, T.; Forstenlechner, P.; Matricardi, P.; Kleine-Tebbe, J. Molecular allergy diagnostics using multiplex assays: Methodological and practical considerations for use in research and clinical routine: Part 21 of the Series Molecular Allergology. Allergo J. Int. 2015, 24, 320–332.
- Wauthier, L.; Cabo, J.; Eucher, C.; Rosseels, C.; Elsen, M.; Favresse, J. Biotin interference in immunoassays: Water under the bridge? Clin. Chem. Lab. Med. 2023. advance online publication.
- Liu, D.; Gebreab, Y.B.; Hu, J.; Zhou, L.; Zhang, N.; Tong, H.; Chen, B.; Wang, X. Development and Evaluation of an Anti-Biotin Interference Method in Biotin-Streptavidin Immunoassays. Diagnostics 2022, 12, 1729.
- ELISA Handbook Principle, Troubleshooting, Sample Preparation and Assay Protocols. Available online: https://www.bosterbio.com/media/pdf/ELISA_Handbook.pdf (accessed on 3 June 2023).
- Yunginger, J.W.; Ahlstedt, S.; Eggleston, P.A.; Homburger, H.A.; Nelson, H.S.; Ownby, D.R.; Platts-Mills, T.A.; Sampson, H.A.; Sicherer, S.H.; Weinstein, A.M.; et al. Quantitative IgE antibody assays in allergic diseases. J. Allergy Clin. Immunol. 2000, 105 Pt 1, 1077–1084.
- Technical Guide for ELISA. Available online: https://www.seracare.com/globalassets/seracare-resources/tg-protocols-and-troubleshooting.pdf (accessed on 3 June 2023).
- Hamilton, R.G.; Matsson, P.N.J.; Chan, S.; Cleve, M.; van Hovanec-Burns, D.; Magnusson, C.; Quicho, R.; Adkinson, N.F. Analytical performance characteristics, quality assurance and clinical utility of immunological assays for human immunoglobulin E (IgE) antibodies of defined allergen specificities. J. Allergy Clin. Immunol. 2015, 135, AB8. Available online: https://clsi.org/media/1414/ila20ed3_sample.pdf (accessed on 3 June 2023).
- Schmidta, M.; Hoffmanb, D.R. Expression systems for production of recombinant allergens. Int. Arch. Allergy Immunol. 2002, 128, 264–270.
- Tscheppe, A.; Breiteneder, H. Recombinant Allergens in Structural Biology, Diagnosis, and Immunotherapy. Int. Arch. Allergy Immunol. 2017, 172, 187–202.
- Curin, M.; Garib, V.; Valenta, R. Single recombinant and purified major allergens and peptides: How they are made and how they change allergy diagnosis and treatment. Ann. Allergy Asthma. Immunol. 2017, 119, 201–209.
- Jutel, M.; Solarewicz-Madejek, K.; Smolinska, S. Recombinant allergens: The present and the future. Hum. Vaccin. Immunother. 2012, 8, 1534–1543.
- Kleine-Tebb, J.; Jakob, T. (Eds.) Molecular Allergy Diagnostics Innovation for a Better Patient Management; Springer International Publishing: New York, NY, USA, 2017; ISBN 978-3-319-42498-9.
- Siekierzynska, A.; Piasecka-Kwiatkowska, D.; Litwinczuk, W.; Burzynska, M.; Myszka, A.; Karpinski, P.; Zygala, E.; Piorecki, N.; Springer, E.; Sozanski, T. Molecular and Immunological Identification of Low Allergenic Fruits among Old and New Apple Varieties. Int. J. Mol. Sci. 2021, 22, 3527.
- Ferrari, E.; Breda, D.; Spisni, A.; Burastero, S.E. Component-Resolved Diagnosis Based on a Recombinant Variant of Mus m 1 Lipocalin Allergen. Int. J. Mol. Sci. 2023, 24, 1193.
- Luengo, O.; Labrador-Horrillo, M. Molecular Allergy Diagnosis in Clinical Practice: Frequently Asked Questions. J. Investig. Allergol. Clin. Immunol. 2022, 32, 1–12.
- Barber, D.; Diaz-Perales, A.; Escribese, M.M.; Kleine-Tebbe, J.; Matricardi, P.M.; Ollert, M.; Santos, A.F.; Sastre, J. Molecular allergology and its impact in specific allergy diagnosis and therapy. Allergy 2021, 76, 3642–3658.
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Gomez, R.M.; Jensen-Jarolim, E.; Luengo, O.; Caraballo, L.; Passalacqua, G.; Poulsen, L.K.; Savi, K.; et al. A WAO–ARIA–GA2LEN consensus document on molecular-based allergy diagnosis (): Update 2020. World Allergy Organ J. 2020, 13, 100091.
More
Information
Subjects:
Allergy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
991
Revisions:
2 times
(View History)
Update Date:
04 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




