Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Naveed Ahmed | -- | 1461 | 2023-07-01 09:32:06 | | | |
| 2 | Jessie Wu | + 5 word(s) | 1466 | 2023-07-03 05:31:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Anwar, F.; Haider, F.; Khan, S.; Ahmad, I.; Ahmed, N.; Imran, M.; Rashid, S.; Ren, Z.; Khattak, S.; Ji, X. Clinical Manifestation, Pathogenesis and Diagnosis of Monkeypox Virus. Encyclopedia. Available online: https://encyclopedia.pub/entry/46298 (accessed on 01 March 2026).
Anwar F, Haider F, Khan S, Ahmad I, Ahmed N, Imran M, et al. Clinical Manifestation, Pathogenesis and Diagnosis of Monkeypox Virus. Encyclopedia. Available at: https://encyclopedia.pub/entry/46298. Accessed March 01, 2026.
Anwar, Faheem, Fatima Haider, Sarmir Khan, Ibrar Ahmad, Naveed Ahmed, Muhammad Imran, Summya Rashid, Zhi-Guang Ren, Saadullah Khattak, Xin-Ying Ji. "Clinical Manifestation, Pathogenesis and Diagnosis of Monkeypox Virus" Encyclopedia, https://encyclopedia.pub/entry/46298 (accessed March 01, 2026).
Anwar, F., Haider, F., Khan, S., Ahmad, I., Ahmed, N., Imran, M., Rashid, S., Ren, Z., Khattak, S., & Ji, X. (2023, July 01). Clinical Manifestation, Pathogenesis and Diagnosis of Monkeypox Virus. In Encyclopedia. https://encyclopedia.pub/entry/46298
Anwar, Faheem, et al. "Clinical Manifestation, Pathogenesis and Diagnosis of Monkeypox Virus." Encyclopedia. Web. 01 July, 2023.
Copy Citation
Monkeypox virus is a double-stranded DNA virus species that causes disease in humans and mammals. It is a zoonotic virus belongs the genus Orthopoxviral, the family of Poxviridae, associated with the smallpox virus in many aspects.
monkeypox virus
vaccination
outbreak
1. Features of Monkeypox Epidemics
Monkeypox virus has evolved into two separate clades: West Africa and Congo Basin [1]. Different epidemiological and clinical characteristics distinguish the diseases caused by the two MPXV clades. The Congo Basin strain has a fatality rate of 10% [2]. West Africa has a fatality rate of approximately 1%, with a higher mortality rate among patients with HIV co-infection [3]. From 1986 to 1992, only 13 incidents were reported of monkeypox, and none were recorded from 1993 to 1995 [4]. In 1997, a total of 88 individuals were confirmed to have monkeypox (MPX) infection, whereas in 1996, there was a sudden increase in the number of MPXV-infected human cases reported in Democratic Republic of the Congo (DRC) [4][5]. MPX outbreak occurred in the United States in 2003. This is the first outbreak of MPX outside of Africa, and it has been linked to the importation of marmots from Africa into the United States. A total of 47 people have been detected in five states [6][7]. From September to December of 2005, Sudan reported ten confirmed cases and nine suspected cases of MPXV due to an outbreak of MPX [8]. Between 2006 and 2007, human MPX infection was again detected in DRC. Since the 1980s, MPX transmission has increased by 20, while smallpox vaccination reduces disease risk by 21 [9].
In addition, monkeypox from Central Africa inhibits T-cell receptor-mediated activation, suppressing proinflammatory cytokine production within human cells from earlier infected monkeypox patients [10]. The MPXV inhibitor supplementing the enzymes or gene inhibits enzymes missing in West African strains and has been recognized as an essential immune modulating factor contributing to the increased virulence of Central African strains [11]. Additionally, the central African strain selectively regulates host responses, particularly in host cell death [12]. Infection with central African monkeypox appears to preferentially inhibit the transcription of genes essential in host immunity, according to transcriptional research [9][13].
2. Nomenclature of Monkeypox Virus
The Orthopoxvirus genus of the Poxviridae family includes numerous zoonotic infections, particularly monkeypox [14]. To replace monkeypox, the WHO will adopt the new preferred name, “Mpox” [15]. Poxviruses’ primary hosts are lemmings, rabbits, and primates, which can be infrequently passed to humans, allowing the incidence of human-to-human transmission [16]. The Poxviridae family is divided into two taxonomic groups: Entomopoxvirinae and Chorodopoxvirinae. The subfamily classification depends upon whether the virus infects insects, as in Entemopovirinae, or vertebrates, as in Chorodopoxvirinae [17].
DNA viruses usually increase and express their genomes in the nucleus, making substantial use of cellular proteins; unfortunately, this is not the case for poxviruses [18][19]. The main portion of the genome will contain genes participating the important function, such as virus assembly and transcription, while some are located at termini which participate in virus–host interactions [20]. Poxviruses encode more than 150 genes, and 49 genes are common across all sequence members of the provirus family, while 90 are frequent inside the subfamily of chorodopoxviruses [21]. Further, poxvirus genera are divided into eight vertebrates: Yatapoxvirus, Orthopoxvirus, Caprioxviru, Molluscipoxvirus, Suipoxvirus, Capripoxvirus, Avpoxvirus, and Parapoxvirus (Figure 1). These viruses share the same DNA sequence with the same antigens for reactivity [22].
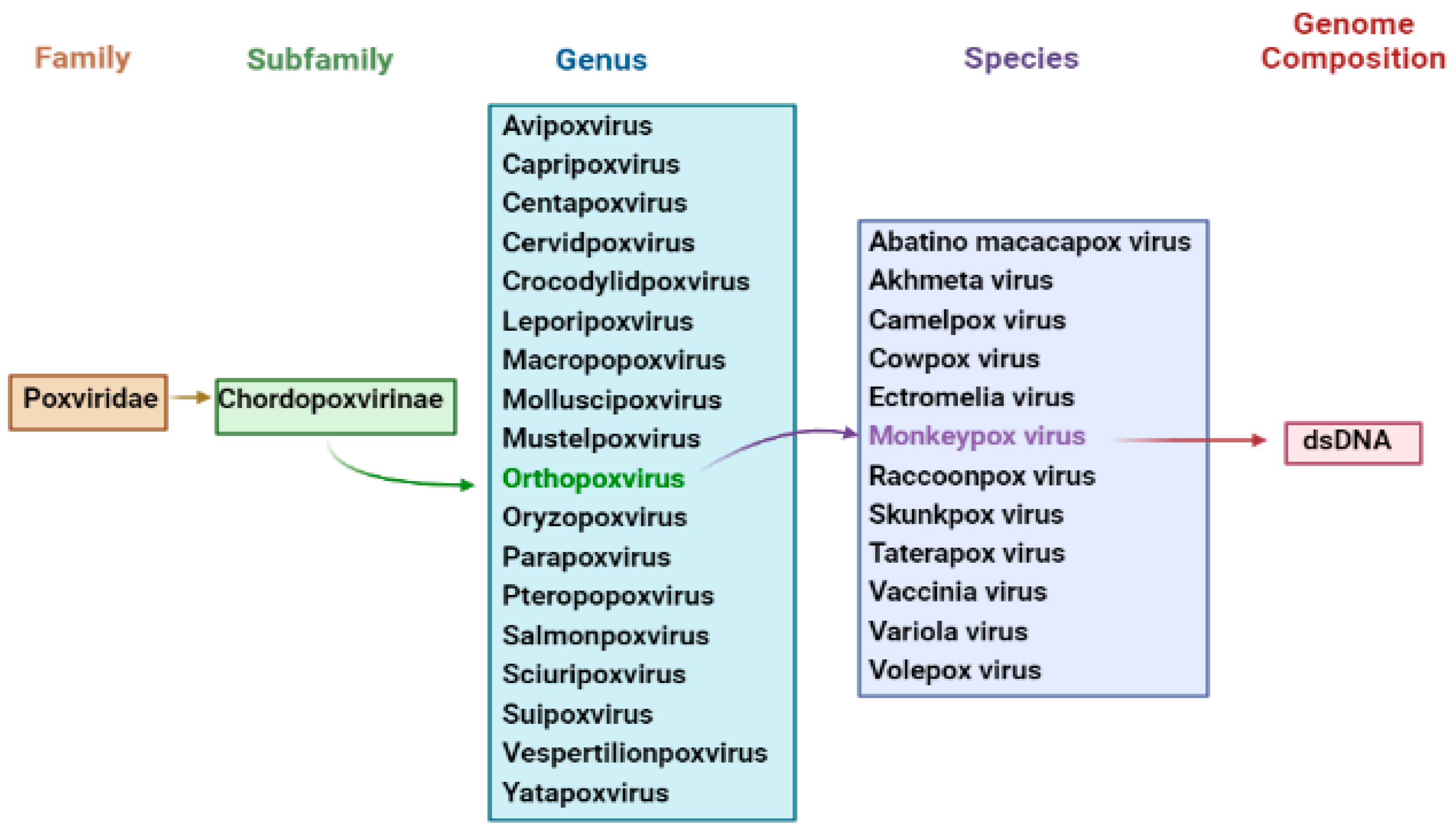
Figure 1. Taxonomy of Monkeypox Virus.
Poxviruses contain the human smallpox virus, declared eradicated in 1980 by the World Health Organization. Other poxviruses that can infect humans include the cowpox virus, smallpox virus, and Akhmeta virus, as well as viruses from different animal species that can cause a pandemic when combined with the monkeypox virus. The monkeypox virus is the most prevalent of these in humans. Bovine pox is another zoonotic disease with a large population in the animal kingdom, a clear relationship to livestock exposure in humans with variable prevalence and severity and a progression of several weeks. Paraboxvirus is a viral genus in the poxvirus family that includes species widespread among sheep, goats, cattle, camels, and the cervix. They are emollients in humans (breast nodules) infected by oral or pharyngeal (papular stomatitis) or skin contact (cellular dermatitis), typically with a moderate granulomatous reaction, causing a variety of clinical manifestations in animals.
Moreover, Paraboxvirus results in the formation of large nodular or vascular lesions. The species generates less immunity than Orthopoxviruses, increasing the likelihood of reinfection. Molluscum contagiosum is a common disease in humans, children, and adults caused by species of the genus Molluscipoxvirus, with specific human swellings and erythematous papules mimicking soft tumors, usually with few and variable numbers of immunizations. In the planting area, it is mainly distributed in roots, trunk, and limbs. Immunity is self-limited. The spreading of Molluscum contagiosum disease via direct contact with the genital lesions is facilitated by sexual contact and is also spread by Candida.
In contrast to other viruses, smallpox viruses have a much lower mutation ability than RNA viruses, such as influenza viruses. However, these viruses have extensive genetic ability, which explains their ability to attack and evade the virus and the immune system [23][24].
3. Morphology and Pathogenesis of Monkeypox
Poxvirus mature structure (Figure 2) has a distinctive dumbbell-shaped nucleoprotein core composed of a double-stranded DNA genome [6]. Monkeypox virions consist of 30 structural and membrane viral proteins, virus-encoded DNA-dependent RNA polymerase, and associated transcription enzymes [25][26]. The virus genome comprises 197,000 bp and contains hairpin termini along with >190 non-overlapping open reading frames (ORFs) [27][28]. The genome’s highly conserved central coding region is guarded by variable ends comprising inverted terminal repeats. At least ninety open reading frames (ORFs) are required for the virion’s replication and morphogenesis. Many non-essential ORFs play a role in the differences in poxvirus host tropism, immunomodulation, and pathogenesis, with many ORFs yet to be functionally categorized [29].
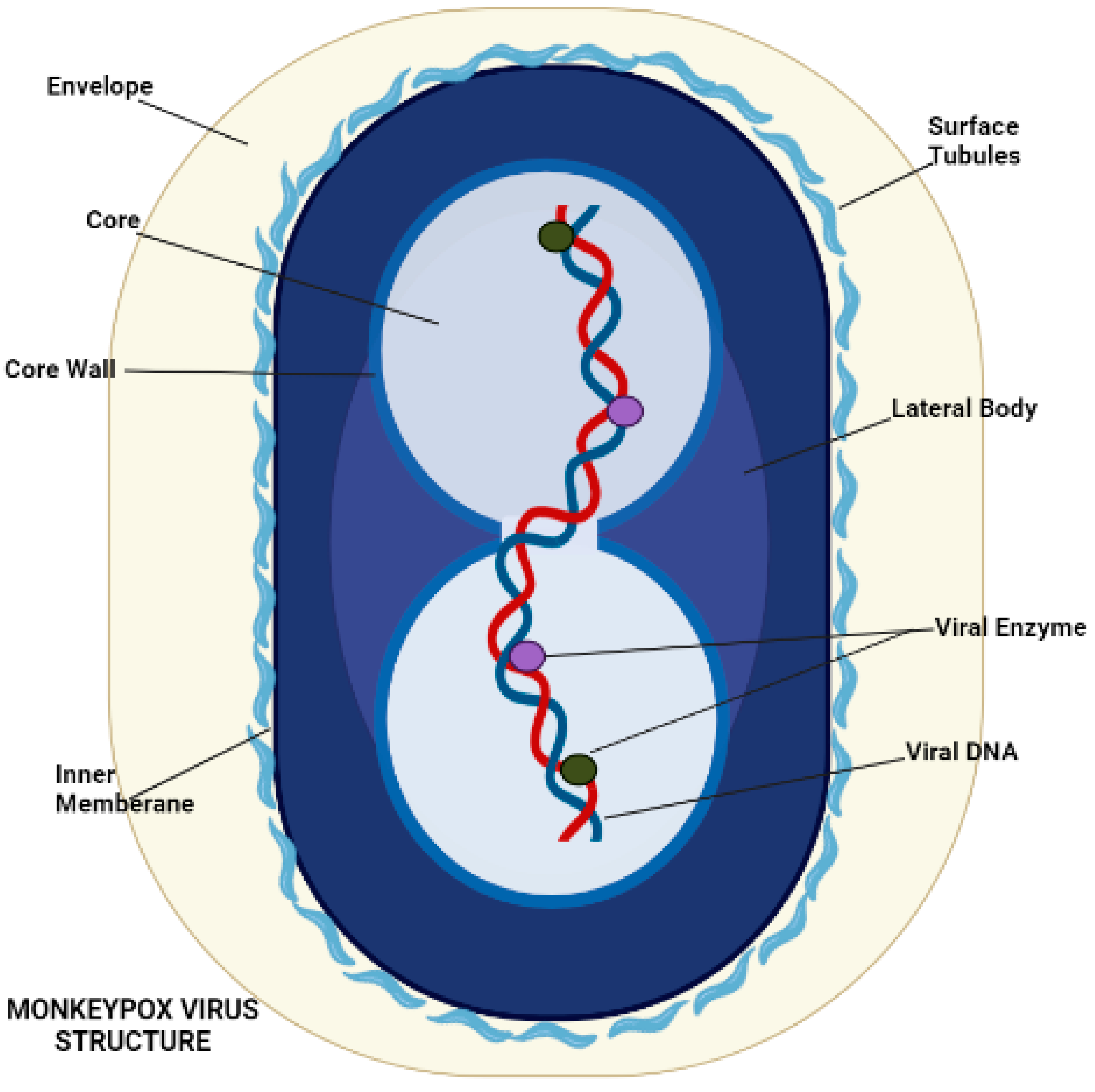
Figure 2. Morphology of Monkeypox Virus.
The pathogenicity and pathophysiology of the monkeypox virus begin with virus transmission, which starts with close contact of animal to human or human-to-human. Smallpox and monkeypox infect through the host’s respiratory or oropharyngeal mucosa. The virus enters through the inoculation site and begins replication in the respiratory and oropharyngeal mucosa. Viruses spread to the local lymph nodes in primary viremia. However, these viruses enter lymph nodes and organs via blood circulation in secondary viremia. This process represents the incubation period of 7 to 14 or 21 days [30].
External virion proteins, cellular glycosaminoglycans on the target cell’s surface, and extracellular matrix components are most likely involved in monkeypox virion attachment. Poxviruses enter host cells via a low-pH endosomal pathway or direct fusion with the plasma membrane at neutral pH, releasing the viral core in the cytoplasm. A complex of 12 non-glycosylated viral membrane proteins is required for intracellular mature virions and enveloped extracellular virions to fuse with the cell [31]. Following entry, the virus-encoded multi-subunit DNA-dependent RNA polymerase initiates viral transcription, followed by the translation of early, intermediate, and late proteins on host ribosomes [32]. Poxvirus DNA synthesis occurs in cytoplasmic structures known as “factories”, which gradually transition from compact DNA-containing structures wrapped in Endoplasmic reticulum membranes to crescent-shaped structures where virion assembly takes place. While most mature virions remain within the cell (intracellular mature virions), some are transported via microtubules and enveloped by two Endoplasmic reticulum or Golgi-derived membranes. These enveloped virions can either initiate actin polymerization, which propels the particle on an actin tail toward an adjacent cell, or exit the cell via cytoplasmic membrane fusion and become enveloped extracellular virions (Figure 3) [33].
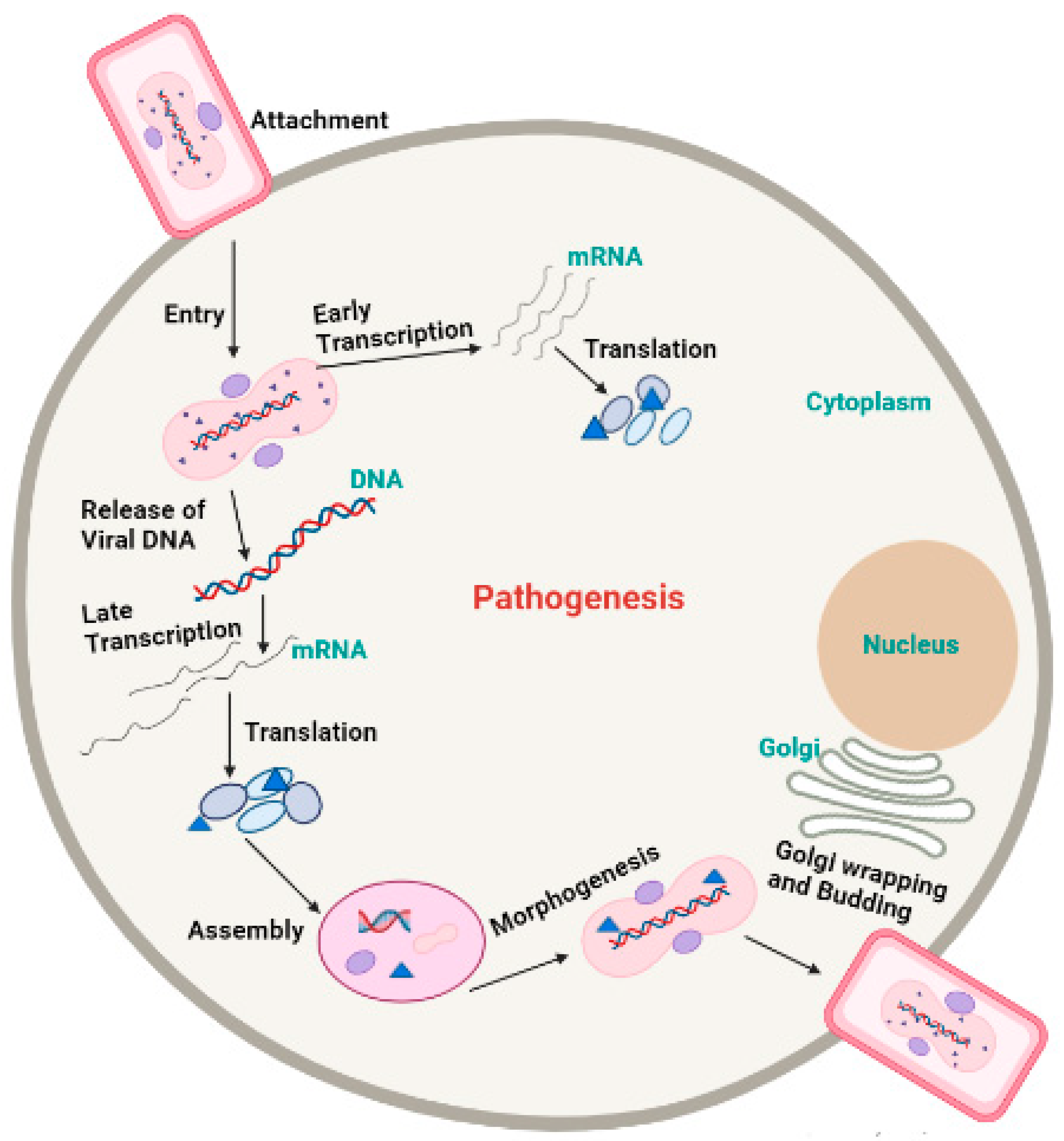
Figure 3. Pathogenesis of Monkeypox virus.
4. Symptoms
In non-human primates, MPVX typically causes a short-lived rash. Plague and 1–4 mm epidermal papules that develop into pustules and crust over are the first clinical signs. A typical lesion has a red, necrotic, and depressed center surrounded by epidermal hyperplasia. These “smallpox pustules” can appear anywhere on the body, but the face and extremities are the most common. Most infected animals recover quickly; however, fatal cases occur, particularly in neonatal monkeys. Following vaccination, MPVX-caused illness was discovered in various rodents, including prairie dogs, dormice, and squirrels. Clinical symptoms in each of these cases (Figure 4) vary, but fever, weight loss, nasal discharge, coughing, respiratory involvement, and mouth ulcers may establish [26][34][35]. MSM have been reported to represent the majority of cases in the current outbreak, often resulting in genital lesions or a vesicular–pustular rash [36][37][38][39][40][41]. The fact that the perineum and vaginal area are commonly affected by the rash suggests that sexual contact may have been the cause of the condition. Monkeypox is often confused with other sexually transmitted infections (STIs), such as granuloma inguinale, molluscum contagiosum, chancre, or herpes simplex infection [42][43].
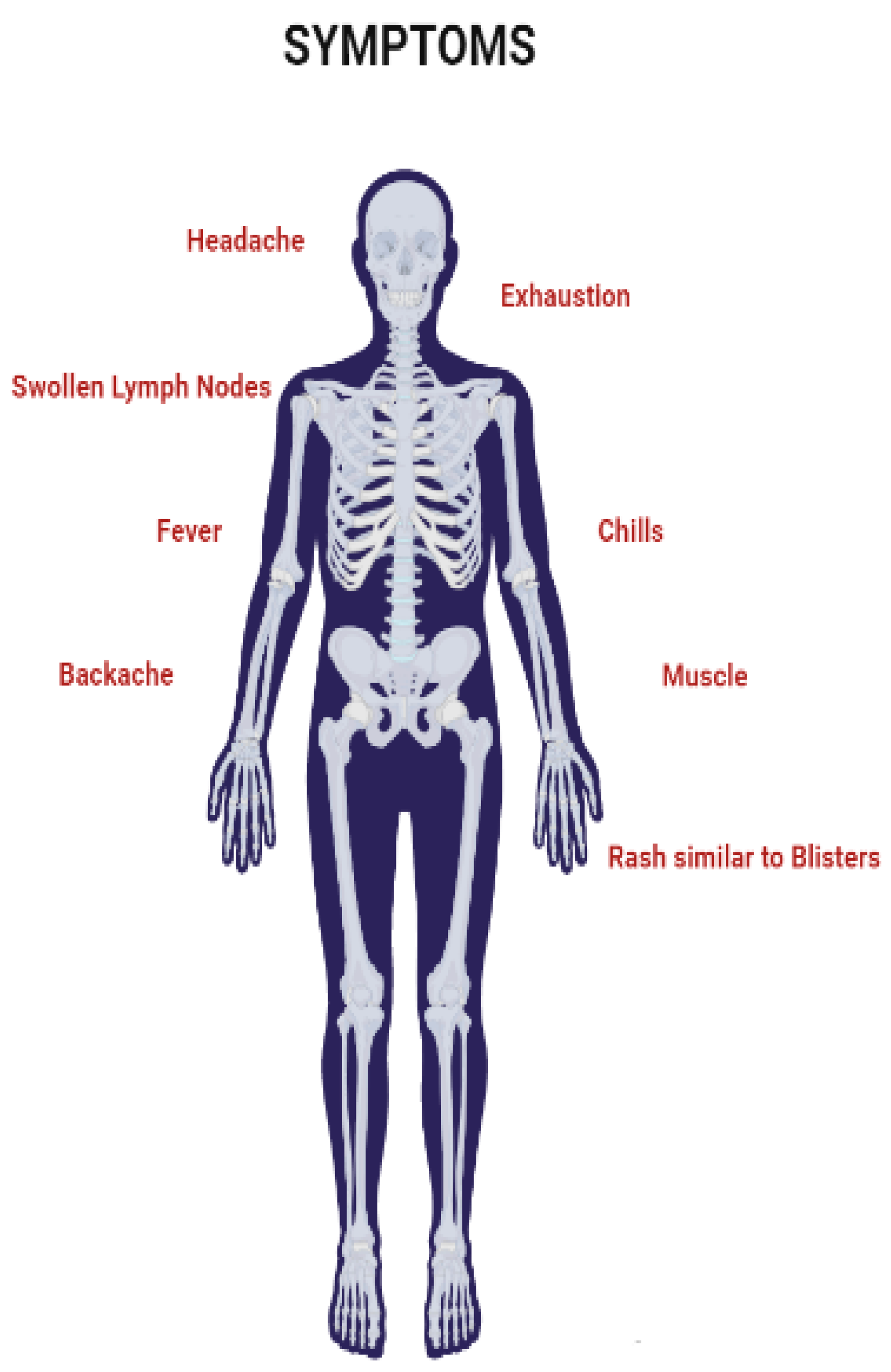
Figure 4. Symptoms of Monkeypox virus.
The clinical features of traditional smallpox with a high mortality rate are classified as typical, altered, variola sine eruption, hemorrhage, and flat type [44]. The hemorrhagic type, which causes widespread bleeding in the skin and mucosal membranes, and the flat type, in which the pustules remain flat, were both frequently fatal. The pathophysiology of these virulent strains of smallpox is poorly studied. Non-human primate models of smallpox and Orthopoxviral infections have been developed utilizing cynomolgus and rhesus macaques [19].
References
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 2005, 86, 2661–2672.
- Doshi, R.H.; Guagliardo, S.A.J.; Doty, J.B.; Babeaux, A.D.; Matheny, A.; Burgado, J.; Townsend, M.B.; Morgan, C.N.; Satheshkumar, P.S.; Ndakala, N.; et al. Epidemiologic and Ecologic Investigations of Monkeypox, Likouala Department, Republic of the Congo, 2017. Emerg. Infect. Dis. 2019, 25, 281–289.
- Ray, S.; Maunsell, J. Different Origins of Gamma Rhythm and High-Gamma Activity in Macaque Visual Cortex. PLoS Biol. 2011, 9, e1000610.
- Heymann, D.L.; Szczeniowski, M.; Esteves, K. Re-emergence of monkeypox in Africa: A review of the past six years. Br. Med Bull. 1998, 54, 693–702.
- Hutin, Y.J.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434.
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350.
- Sale, T.A.; Melski, J.W.; Stratman, E.J. Monkeypox: An epidemiologic and clinical comparison of African and US disease. J. Am. Acad. Dermatol. 2006, 55, 478–481.
- Formenty, P.; Muntasir, M.O.; Damon, I.; Chowdhary, V.; Opoka, M.L.; Monimart, C.; Mutasim, E.M.; Manuguerra, J.-C.; Davidson, W.B.; Karem, K.L.; et al. Human Monkeypox Outbreak Caused by Novel Virus Belonging to Congo Basin Clade, Sudan, 2005. Emerg. Infect. Dis. 2010, 16, 1539–1545.
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Lloyd Smith, J.O.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267.
- Chapman, J.L.; Nichols, D.K.; Martinez, M.J.; Raymond, J.W. Animal Models of Orthopoxvirus Infection. Vet. Pathol. 2010, 47, 852–870.
- Zaucha, G.M.; Jahrling, P.B.; Geisbert, T.W.; Swearengen, J.R.; Hensley, L. The pathology of experimental aerosolized mon-keypox virus infection in cynomolgus monkeys (Macaca fascicularis). Lab. Investig. 2001, 81, 1581–1600.
- Hahon, N.; Wilson, B.J. Pathogenesis of variola in Macaca irus monkeys. Am. J. Epidemiol. 1960, 71, 69–80.
- Rubins, K.H.; Hensley, L.E.; Relman, D.A.; Brown, P.O. Stunned Silence: Gene Expression Programs in Human Cells Infected with Monkeypox or Vaccinia Virus. PLoS ONE 2011, 6, e15615.
- Joklik, W.K. The poxviruses. Bacteriol. Rev. 1966, 30, 33–66.
- World Health Organization. WHO Recommends New Name for Monkeypox Disease; WHO News Release; World Health Organization: Geneva, Switzerland, 2022.
- Haller, S.L.; Peng, C.; McFadden, G.; Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 2014, 21, 15–40.
- Hughes, A.L.; Irausquin, S.; Friedman, R. The evolutionary biology of poxviruses. Infect. Genet. Evol. 2010, 10, 50–59.
- Norby, R.J.; Ledford, J.; Reilly, C.D.; Miller, N.E.; O’Neill, E.G. Fine-root production dominates response of a deciduous forest to atmospheric CO 2 enrichment. Proc. Natl. Acad. Sci. USA 2004, 101, 9689–9693.
- Johnson, R.F.; Dyall, J.; Ragland, D.R.; Huzella, L.; Byrum, R.; Jett, C.; St Claire, M.; Smith, A.L.; Paragas, J.; Blaney, J.E.; et al. Comparative Analysis of Monkeypox Virus Infection of Cynomolgus Macaques by the Intravenous or Intrabronchial Inoculation Route. J. Virol. 2011, 85, 2112–2125.
- Saijo, M.; Ami, Y.; Suzaki, Y.; Nagata, N.; Iwata, N.; Hasegawa, H.; Iizuka, I.; Shiota, T.; Sakai, K.; Ogata, M.; et al. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J. Gen. Virol. 2009, 90, 2266–2271.
- McConnell, S.; Hickman, R.L.; Wooding Jr, W.L.; Huxsoll, D.L. Monkeypox: Experimental infection in chimpanzee (Pan satyrus) and immunization with vaccinia virus. Am. J. Vet. Res. 1968, 29, 1675–1680.
- Esposito, J.J.; Sammons, S.A.; Frace, A.M.; Osborne, J.D.; Olsen-Rasmussen, M.; Zhang, M.; Govil, D.; Damon, I.K.; Kline, R.; Laker, M.; et al. Genome Sequence Diversity and Clues to the Evolution of Variola (Smallpox) Virus. Science 2006, 313, 807–812.
- Yong, S.E.; Ng, O.T.; Ho, Z.J.; Mak, T.M.; Marimuthu, K.; Vasoo, S.; Yeo, T.W.; Ng, Y.K.; Cui, L.; Ferdous, Z.; et al. Im-ported Monkeypox, Singapore. Emerg. Infect. Dis. 2020, 26, 1826.
- Vaughan, A.; Aarons, E.; Astbury, J.; Balasegaram, S.; Beadsworth, M.; Beck, C.R.; Chand, M.; O’Connor, C.; Dunning, J.; Ghebrehewet, S.; et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Eurosurveillance 2018, 23, 1800509.
- Resch, W.; Hixson, K.K.; Moore, R.J.; Lipton, M.S.; Moss, B. Protein composition of the vaccinia virus mature virion. Virology 2006, 358, 233–247.
- Manes, N.P.; Estep, R.D.; Mottaz, H.M.; Moore, R.J.; Clauss, T.R.W.; Monroe, M.E.; Du, X.; Adkins, J.N.; Wong, S.W.; Smith, R.D. Comparative Proteomics of Human Monkeypox and Vaccinia Intracellular Mature and Extracellular Enveloped Virions. J. Proteome Res. 2008, 7, 960–968.
- Shchelkunov, S.N.; Totmenin, A.V.; Babkin, I.V.; Safronov, P.F.; Ryazankina, O.I.; Petrov, N.A.; Gutorov, V.V.; Uvarova, E.; Mikheev, M.V.; Sisler, J.R.; et al. Human monkeypox and smallpox viruses: Genomic comparison. FEBS Lett. 2001, 509, 66–70.
- Kugelman, J.R.; Johnston, S.C.; Mulembakani, P.M.; Kisalu, N.; Lee, M.S.; Koroleva, G.; McCarthy, S.E.; Gestole, M.C.; Wolfe, N.D.; Fair, J.N.; et al. Genomic Variability of Monkeypox Virus among Humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014, 20, 232–239.
- Lalani, A.S.; Graham, K.; Mossman, K.; Rajarathnam, K.; Clark-Lewis, I.; Kelvin, D.; McFadden, G. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. J. Virol. 1997, 71, 4356–4363.
- Moore, M.J.; Rathish, B.; Zahra, F. Mpox (Monkeypox); StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Moss, B. Membrane fusion during poxvirus entry. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2016.
- Challberg, M.; Englund, P. Purification and properties of the deoxyribonucleic acid polymerase induced by vaccinia virus. J. Biol. Chem. 1979, 254, 7812–7819.
- Smith, G.L.; Vanderplasschen, A.; Law, M. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 2002, 83, 2915–2931.
- Khattak, S.; Qaisar, M.; Zaman, S.; Khan, T.A.; Ali, Y.; Wu, D.D.; Ji, X.Y. Monkeypox virus preparation in Paki-stan-Next viral zoonotic disease outbreak after COVID-19? Biomed. Lett. 2022, 8, 196–201.
- Pickup, D.J. Extracellular Virions: The Advance Guard of Poxvirus Infections. PLoS Pathog. 2015, 11, e1004904.
- Vivancos, R.; Anderson, C.; Blomquist, P.; Balasegaram, S.; Bell, A.; Bishop, L.; Brown, C.S.; Chow, Y.; Edeghere, O.; Florence, I.; et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Eurosurveillance 2022, 27, 2200422.
- Farahat, R.A.; Sah, R.; El-Sakka, A.A.; Benmelouka, A.Y.; Kundu, M.; Labieb, F.; Shaheen, R.S.; Abdelaal, A.; Abdelazeem, B.; Rodriguez-Morales, A.J.; et al. Human monkeypox disease (MPX). Infez. Med. 2022, 30, 372–391.
- Hammerschlag, Y.; MacLeod, G.; Papadakis, G.; Sanchez, A.A.; Druce, J.; Taiaroa, G.; Savic, I.; Mumford, J.; Roberts, J.; Caly, L.; et al. Monkeypox infection presenting as genital rash, Australia, May 2022. Eurosurveillance 2022, 27, 2200411.
- Bjekic, M.; Markovic, M.; Dejanovic, L. Genital rash as an initial presentation of monkeypox. An. Bras. Dermatol. 2023, 98, 114–115.
- Ortiz-Martínez, Y.; Rodríguez-Morales, A.J.; Franco-Paredes, C.; Chastain, D.B.; Gharamti, A.A.; Barahona, L.V.; Henao-Martínez, A.F. Monkeypox—A description of the clinical progression of skin lesions: A case report from Colorado, USA. Ther. Adv. Infect. Dis. 2022, 9.
- Griffiths-Acha, J.; Vela-Ganuza, M.; Sarró-Fuente, C.; López-Estebaranz, J.L. Monkeypox: A new differential diagnosis when addressing genital ulcer disease. Br. J. Dermatol. 2022, 187, 1050–1052.
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chate-lain, R.; et al. Monkeypox outbreak—Nine states, May 2022: Weekly/June 10, 2022/71 (23); 764–769. Am. J. Transpl. 2022, 22, 2104–2110.
- Soriano, V.; Corral, O. International outbreak of monkeypox in men having sex with men. Aids Rev. 2022, 24.
- Malacrida, L.M.; Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D. Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
03 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





