Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manop Suphantharika | -- | 5297 | 2023-07-01 04:39:36 | | | |
| 2 | Camila Xu | Meta information modification | 5297 | 2023-07-03 03:33:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kumar, S.R.; Tangsrianugul, N.; Suphantharika, M. Isolation, Characterization, Modification, and Applications of PMS. Encyclopedia. Available online: https://encyclopedia.pub/entry/46294 (accessed on 07 February 2026).
Kumar SR, Tangsrianugul N, Suphantharika M. Isolation, Characterization, Modification, and Applications of PMS. Encyclopedia. Available at: https://encyclopedia.pub/entry/46294. Accessed February 07, 2026.
Kumar, Simmi Ranjan, Nuttinee Tangsrianugul, Manop Suphantharika. "Isolation, Characterization, Modification, and Applications of PMS" Encyclopedia, https://encyclopedia.pub/entry/46294 (accessed February 07, 2026).
Kumar, S.R., Tangsrianugul, N., & Suphantharika, M. (2023, July 01). Isolation, Characterization, Modification, and Applications of PMS. In Encyclopedia. https://encyclopedia.pub/entry/46294
Kumar, Simmi Ranjan, et al. "Isolation, Characterization, Modification, and Applications of PMS." Encyclopedia. Web. 01 July, 2023.
Copy Citation
Proso millet starch (PMS) as an unconventional and underutilized millet starch is becoming increasingly popular worldwide due to its health-promoting properties. PMS can be isolated from proso millet grains by acidic, alkaline, or enzymatic extraction. PMS exhibits typical A-type polymorphic diffraction patterns and shows polygonal and spherical granular structures with a granule size of 0.3–17 µm. PMS is modified by chemical, physical, and biological methods. The native and modified PMS are analyzed for swelling power, solubility, pasting properties, thermal properties, retrogradation, freeze–thaw stability, and in vitro digestibility.
proso millet starch
extraction
characterization
modification
in vitro digestibility
application
1. Introduction
Global production of cereal grains has reached record levels. Cereal grains play an important role in the human diet as a primary source of energy. The Food and Agriculture Organization (FAO) [1] reported in 2020 that the global production of cereal grains in 2019 reached a record high of 2715 million tons. At the same time, the global community is facing climatic changes, pollution, water scarcity, rising food costs, population growth, and other socioeconomic issues. These negative aspects can affect regional agricultural progress and limit grain production, leading to high food prices and serious food security concerns worldwide [2]. Moreover, smallholder farmers facing these conditions become economically vulnerable due to their limited resources and have difficulty maintaining their yields and profitability [3]. As a consequence of unfavorable global phenomena and their adverse impacts that constrain agricultural production, there is an urgent need among experts in nutrition and technology to identify a suitable cereal crop that could serve as a viable food source to address these challenges [4]. Under these circumstances, millets may be a nutritious option to supplement the nutritional needs of a growing world population in an uncertain global environment [5].
Millet belongs to the Poaceae family and is cultivated in subtropical and tropical regions of marginal drylands. Over 10,000 years ago, prior to the widespread consumption of wheat, maize, barley, and rice, this food item served as a staple for the people of that era. Currently, the most commonly cultivated species include proso millet (Panicum miliaceum), pearl millet (Pennisetum glaucum), and finger millet (Setaria italica) [6]. Millet is abundant in proteins, fats, carbohydrates, fiber, minerals, vitamins, and phenolic compounds [7]. Nutritionally, millet contains proteins (6–19%), carbohydrates (60–70%), fats (1.5–5%), minerals (2–4%), dietary fiber (12–20%), and various phytochemicals [8]. In addition, millet is gluten-free. This is desirable for people with celiac disease, and because of millet’s blood sugar-lowering properties, it is also effective in treating type II diabetes [8].
Proso millet (Panicum miliaceum L.) is also known as common millet, hog millet, Russian millet, and broomcorn millet in certain areas [9]. Proso millet is characterized by its adaptability to unfavorable environmental conditions (such as salt, drought, temperature, and pH). It also has a short lifecycle (about 12 weeks) and is grown in slightly acidic, saline, sandy, and low-fertility soils with limited nitrogen and carbon dioxide [10][11][12]. Proso millet contains carbohydrates (70–74%), proteins (9.4–9.9%), ash (1.2–3.8%), and fats (1.2–3.8%), along with a variety of phytochemicals and vital minerals [13].
Starch is a major constituent of millet and is divided into two types, namely, amylose and amylopectin. Based on the amylose content, millets are classified into nonwaxy (high amylose content) and waxy (low amylose content) [14]. Yang et al. [15] measured the range of starch content in nonwaxy (high amylose content) proso millet as 59–77% and for waxy millet as 55–69%. Starch serves as a crucial energy source for humans and is extensively utilized in the food and food-related industries. It is a renewable, biodegradable, economical, and natural material used to modify the textural properties of various foods. It can be modified into thickeners, stabilizers, and sweeteners, and can also serve as a water-retention agent [9].
A number of researchers conducted an analysis comparing various types of millet starches, but, unfortunately, they did not provide a thorough study of proso millet starch (PMS) [6][16][17]. According to Banger et al. [18], a comprehensive account of PMS, including its physiochemical and functional properties, modification, and applications, was presented. However, it was noted that more detailed information on isolation, digestibility, and recent advances in its applications is lacking.
2. Isolation, Yield, and Composition of Proso Millet Starch
The starch granules within proso millet grains exhibit strong binding affinity to the surrounding protein matrix. Various methods and chemical reagents are used to extract starch and solubilize the proteins in the grain [19]. Generally, starch extraction methods consist of three phases, i.e., fragmentation, cell disruption, and purification or separation [20]. Millet starch is usually isolated by the wet milling method. The grain or flour is soaked in an aqueous solution (water, alkali, or acid) for a certain time, depending on its chemical properties and composition [16]. The particular method of starch extraction (e.g., acidic, alkaline, or enzymatic) has a significant effect on starch yield. Starch isolation methods vary widely and depend on the inherent starch content of the grain and the initial soaking conditions (neutral, alkaline, or acidic) [21]. The procedure for isolating proso millet starch (PMS) is depicted in Figure 1.
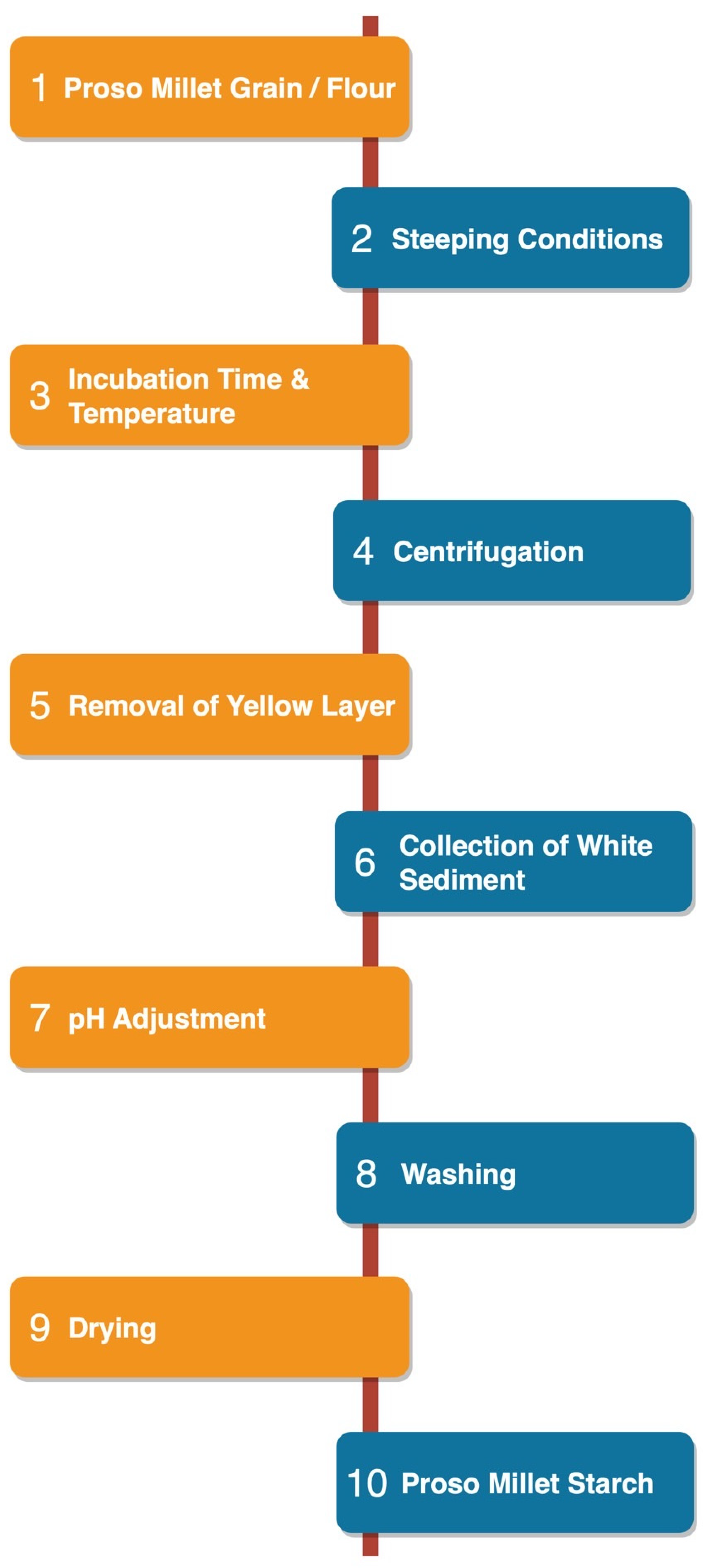
Figure 1. Isolation of proso millet starch.
In the alkaline steeping method, the grains are soaked with 0.3% sodium hydroxide (NaOH) solution for 24 h at 4 °C. The soaked grains are then ground to a wet slurry using a mill and then sieved through a 100-mesh sieve. After this, the samples are centrifugated at 3000 rpm for 15 min, the supernatant is removed, and the remaining contents are resuspended in water. This washing step is repeated for a total of 3 cycles; the slurry is then neutralized with hydrochloric acid (HCl). After washing/neutralization, the starch cake is dehydrated at 40 °C for 48 h [22]. In the acid steeping method of PMS extraction, the grains are soaked with 0.15% sulfur dioxide (SO2) solution for 48 h at 52 °C. The soaked grains are then crushed with a blender, sieved through a 40-mesh sieve, and washed with water. The residual material is then crushed using a mortar and pestle and filtered through a 200-mesh sieve and then through a 270-mesh sieve. The residue is again washed with water, filtered through a Buchner funnel with No. 2 Whatman filter paper, centrifuged, and dried overnight at 45 °C [6].
To extract starch from proso, pearl, kodo, foxtail, little, and barnyard millets, a practically neutral solution (pH 6.5) containing a minute amount of either sodium azide (0.01%) or mercury (II) chloride (0.01%) is used to prevent bacterial growth and inhibit amylase activity [6]. However, it should be noted that the use of sodium azide and/or mercury (II) chloride can cause serious health problems if ingested. A small amount of sulfur dioxide (0.5 g/L) and lactic acid (0.15 g/L) are added to isolate starch from proso millet in the acid steeping procedure. Similarly, the addition of a small amount of NaOH (0.1%) and sodium borate buffer comprising SDS (0.5%) and Na2S2O5 (0.5%) are used to isolate starch from these treatments. The use of these isolating solutions can significantly impact the chemical composition and characteristics of the extracted starch. In a comparative study between acid and alkaline steeping, acid steeping has a higher residual protein content (4.3%) than alkaline steeping (0.7%) [23].
The amount of millet starch obtained and the resulting chemical compositions differ significantly in the studies presented in Table 1. Millet contains about the same amount of starch as other cereal grains. The millet starch usually contains 20–30% amylose and 70–80% amylopectin. The presence of impurities in millet starch grains has significant implications for achieving some desired functional objectives [16]. For example, millet starch contains mainly nonpolar and polar lipids. The majority (89%) of the overall lipid content is attributed to polar phospholipids, whereas the rest primarily comprises nonpolar triglycerides [24]. These lipids can combine with the amylose component of starch to form complexes, which can lead to a decrease in the starch’s swelling capacity and flowability. This is caused by the lipids’ hydrophobic bonds and cohesive nature [16].
Table 1. Starch yield and chemical composition of proso millet starch.
| Source | Starch Yield (%) |
Protein (%) |
Amylose (%) |
Lipid (%) |
Reference |
|---|---|---|---|---|---|
| Proso millet | 93.7 | - | 33.9 | - | [25] |
| 92.19–94.60 | 0.27–0.67 | 14.92–17.37 | 0.13–0.25 | [26] | |
| - | 0.69–4.31 | 27.2–29.1 | 0.59–0.6 | [23] | |
| 61.8–68.2 | 1.1–2.1 | 29.2–32.6 | - | [27] | |
| 87.27–94.60 | 1.07–1.30 | 2.80–32.80 | 0.01 | [14] | |
| - | 0.48 | 1.61 | 0.01 | [22] | |
| 54.1 | 1.21 | 28.51 | 0.27 | [28] | |
| - | 0.45 | 0.38 | - | [29] |
3. Morphology and Crystallinity of Proso Millet Starch
The size of starch granules in millet varies depending on the plant species. Despite being generally spherical and polygonal in shape (as indicated in Table 2), the dimensions of these granules range from 0.3–17 µm. The polygonal shapes are also larger and have more indentations than the spherical shapes [25], and the morphology of the starch is strongly influenced by its treatment and/or biomodification [30]. In addition, differences in particle size of PMS obtained from proso millet grown in different regions can be due to local environmental aspects. An increase in altitude and reduced mean temperature can lead to bigger granules [26]. Additionally, the morphology of starch is influenced by the arrangement of starch granules inside the endosperm of the grain [31]. Cavities are dispersed randomly throughout the entire outer layer of the starch granules due to surface pores and protein bodies. These pores are connected to the central cavity of the granules, enabling specific molecules from the external environment to penetrate the granules [16]. From a starch modification perspective, this phenomenon is helpful. These pores allow OH ions or water to enter the granules, destroying the amylose-containing amorphous region. Consequently, the restrictive qualities of amylose are reduced, leading to enhanced starch swelling and hydration properties [32].
Table 2. Proso millet starch’s native and modified morphological properties.
| Starch Source | Type | Size (µm) | Shape | Reference |
|---|---|---|---|---|
| Proso millet | NS | 3–10 | Oval, polygonal, irregular, and spherical | [33] |
| UHP | - | Structural disruption, gel-like structure formed | [33] | |
| NS | 2.5–17 | Few spherical and mostly polygonal | [25] | |
| NS | 0.3–12 | Few small spherical granules and mainly uniform large or small polygonal | [26] | |
| NS | 3–10 | Few small spheres and large polygonal shape | [29] | |
| NS | 4.3–8.9 | Mostly polygonal with some elliptical granules having rounded edges and surface pores | [34] | |
| NS | 5–12 | Round and smooth | [22] | |
| DHT | - | Smooth and plump surface with large lumps | [22] | |
| NS | 1.8–13.5 | Bimodal distribution, small spherical and large polygonal | [27] | |
| NS | 1.3–8 | Bimodal distribution, large polygonal, small and large spherical | [35] | |
| NS | 4.49–4.70 | Regular, polygonal, and round shape, along with the characteristic Maltese cross structure | [14] | |
| NS | 1.54–11.7 | Mainly polygonal and round shape, larger and smaller granules make honey-comb structure | [9] |
Key: NS, native starch; DHT, dry-heat-treated; UHP, ultra-high-pressure-treated.
Millet starches are semi-crystalline and are similar to other starches that contain both crystalline and amorphous regions. Millet exhibits typical A-type polymorphic diffraction patterns [16]. The relative crystallinity observed for native starch is 35.7% with a diffraction peak at 2θ values of 15.3–23.1° for a single peak and about 17°–8° for a double peak [36]. Sun et al. [22] observed that the native starch of the proso millet exhibited A-type X-ray diffraction patterns with 2θ of 15°, 17°, 18°, and 23.5°, confirming a previous report by Kim et al. [34], which also confirmed an A-type diffraction pattern for PMS. In a 2019 study, the relative crystallinity of PMS was measured to range from 37.6% to 38.4% [14]. The differences in the degree of relative crystallinity can be attributed to a variety of factors, including the biological origin, plant variety, composition of amylose and amylopectin, conditions during cultivation, and maturity stage of the parent plant at the time of harvest [37]. Impurities present in the starch, such as other millet constituents, result in a shift of the peaks and a decline in intensity. This is because the occurrence of impurities alone increases the size of the amorphous region compared to the crystalline portion [38]. These general differences in starch granules all affect the degree of crystallinity, and due to the absence of amylose, this occurs without affecting the granular size [39]. A stable crystalline structure for starch is formed by long amylopectin chains, in contrast to the less stable shorter amylopectin chains, which are easily broken down by high temperatures [24]. Food processing techniques, such as milling, frequently cause damage to the physical structure of starch. The crystalline amylopectin is transformed into amorphous amylopectin during these processes, and the resulting material develops low-molecular-weight fractions. These changes in crystallinity affect food functionality [16].
4. Physiological and Functional Properties
4.1. Swelling Power and Solubility
With an appropriate quantity of water, the starch is subjected to heating, causing the granules to absorb moisture and undergo swelling. In this process, the components of the starch granule are leached out and largely dissolved in the form of amylose. Eventually, the swollen starch granules break down and disintegrate when they continue to be exposed to high temperatures. This activity is influenced by several factors, including the physical associations of the chemical components in the granules, the molecular structure of amylose and amylopectin, the intrinsic phosphorus groups, and the restricting entanglement of the lipid–amylose complex [40]. Starch granules undergo swelling when exposed to temperatures between 50–90 °C in the presence of water. Studies have shown that the swelling power (SP) of millet is lower compared to that of rye, potato, and wheat. This indicates that millet starch has greater resistance towards swelling due to its relatively strong binding force between the granules [16]. Much research has been conducted on SP of PMS, and some representative results are presented below. Singh and Adedeji [28] studied the SP of PMS at different temperatures (70–90 °C) and recorded the percentage range of their size changes, i.e., native starch (4.69–24.99%), acid-modified starch (4.94–21.26%), and hydrothermally modified starch (5.29–10.37%). At 95 °C, Xiao et al. [41] studied the SP of native PMS (13.77 g/g) and PMS with proanthocyanidins (14.15–19.83 g/g). Wu et al. [29] reported that the SP of PMS in their research was greater than other millet varieties, such as foxtail, barnyard, and finger millet, as well as a hybrid of barnyard and pearl millet. Li et al. [33] studied the SP of PMS (2–35%) at 50–90 °C and found that after ultra-high pressure, the treated starch showed lower SP than native starch.
The following solubility of PMS at different temperatures (70–90 °C) was observed for native starch (2.62–34.88%), acid-modified starch (18.97–86.17%), and hydrothermally modified starch (1.71–12.45%) [28]. The solubility of acid-modified starch was higher than that of native starch, which is due to the fact that increasing temperature causes structural weakening and depolarization of starch granules in the former [28]. Li et al. [33] observed the solubility of PMS in a temperature range of 50–90 °C and found that the ultra-high-pressure-treated starch exhibited lower solubility than the native starch at a higher temperature. At 95 °C, Xiao et al. [41] investigated the solubility of native PMS (5.32%) and found a higher solubility of PMS with proanthocyanidins (8.64–16.35%). Wu et al. [29] found that the solubility of PMS was higher than other millets such as foxtail, barnyard, hybrid barnyard, and pearl millets, but lower than finger millet. However, all millet starches exhibited lower SP and solubility patterns in the temperature range of 60–90 °C than other commonly used starch sources (e.g., wheat and potato), suggesting stronger swelling resistance and binding strength within the starch granules [21]. It is thought that the interaction between starch and water molecules upon heating is the cause of the increased solubility and swelling power, and that the starch exposes additional groups that become associated with water molecules [41].
4.2. Pasting Properties
In the majority of cases, rheological evaluation of starch was carried out using both the Rapid Visco Analyzer (RVA) and the Brabender Visco-Amylograph (BVA), and the findings are presented in Table 3. This technique involves heating starch with a substantial quantity of water under continuous shear. The viscosity changes at a given temperature cycle are recorded. Pasting is affected by several parameters, including starch structure, water content, temperature program, and shear rate, which are closely monitored. The amount of starch used in the studies that was examined ranged from 6 to 10% [6]. Three sections can be identified in a typical pasting curve, each representing a specific phase of starch granule transformation during the pasting process [9]. The first phase involves the gradual absorption of water by the starch granules, causing them to expand; the second phase involves the leaching of the amylose component; and the final phase involves the loss of structural integrity of the expanded starch granules, causing them to disintegrate into fragments [42]. The pasting properties and attributes of starch paste are subject to the influence of several factors, including the concentration of starch, its composition in terms of amylose content and amylose-to-amylopectin ratio, and cooking and cooling temperatures, as well as the presence of solutes such as pH, lipids, and sugars. For instance, waxy starch has a greater tendency to absorb water and expand quickly, enabling it to attain its maximum pasting temperature in a shorter duration as compared to starches with a higher amylose content [43]. Yang et al. [14] reported that the peak viscosity (PV), trough viscosity (TV), and breakdown viscosity (BD) of waxy proso millet starch were greater, while the setback viscosity (SB) and pasting temperature (PT) were lower compared to nonwaxy millet starch. The study conducted on proso millet starch revealed that amylose content had a strong negative correlation with PV, TV, and BD, but a substantial positive correlation with SB and PT. A lower SB indicates better stability, and a lower BD indicates high shear resistance. Waxy proso millet starch demonstrates superior stability, making it a desirable choice for frozen food and thickening applications. On the other hand, nonwaxy proso millet starch exhibits higher temperature stability and improved shear resistance, indicating its potential suitability for medicinal resources [14].
Table 3. Pasting properties of proso millet starch.
| Starch (g/mL) |
Method | Unit | PV | BD | SB | PT (°C) | Reference |
|---|---|---|---|---|---|---|---|
| - | BVA | BU | 72.5–74.5 | [27] | |||
| - | BVA | BU | 520 | 50 | 330 | 75.8 | [35] |
| 3.5/25 | DHR | Pa.s | 4.60 | 2.60 | 1.69 | 79.23 | [28] |
| 3/25 | RVA | cP | 2807 | 1746 | 1634 | 57.40 | [33] |
| 3/25 | RVA | cP | 2372 | 1792 | 582 | - | [29] |
| 3/25 | RVA | cP | 2822 | 1854 | 501 | 76 | [22] |
| 2.5/25 | RVA | cP | 2284.5 | 913 | 372.5 | 79.18 | [41] |
| 2/25 | RVA | cP | 2134–3515 | 488–967 | 197–1102 | 63.60–63.80 | [37] |
| 3/25 | RVA | cP | 2110–3286 | 1114–2189 | 279–1478 | 77.8–80.9 | [14] |
| 2/26 | RVA | cP | 2215–3585 | 511–1437 | 752–1435 | 78.8–82.8 | [44] |
| - | BVA | BU | 219–457 | 79.5–240 | 115.05–201.5 | - | [26] |
Key: RAV = Rapid Visco-Analyzer, DHR = Discovery Hybrid Rheometer, BVA = Brabender Visco-Amylograph. The viscosity units for RVA, BVA, and DHR are cP, BU, and Pa.s, respectively; PV = peak viscosity; BD = breakdown viscosity; SB = setback viscosity; PT = pasting temperature (°C).
4.3. Thermal Properties
The process of gelatinization occurs when starch granules are subjected to a specific temperature range and a sufficient quantity of water, leading to an order–disorder phase transition. Gelatinization is characterized by radial swelling of the granules, water absorption by the amorphous region, leaching of starch molecules, and collapse of the crystalline region with breakup of the double helices [40]. Differential scanning calorimetry (DSC) is most commonly used for the analysis of millet starch, where the initial (To), peak (Tp), and conclusion (Tc) gelatinization temperatures, as well as the enthalpy change (ΔH), are regularly recorded [6]. Table 4 presents the thermal properties of PMS. The characteristics of gelatinization in starch vary not only between different species of millet, but also among various genotypes within the same species [16]. Various factors, including the granule size and the ratio of amylose to amylopectin, have an impact on the gelatinization properties of diverse types of millet starch. Moreover, these differences are also observed between different varieties of the same plant species. The gelatinization temperature of waxy and low-amylose starches takes a longer time to reach compared to nonwaxy, high-amylose starches [45]. Gelatinization temperatures are also important in the selection of specific starch properties for various food applications [21]. Thermal properties of PMS observed by Yang et al. (2019) [14] in both nonwaxy and waxy starch are as follows: To (64.6–71.1 °C); Tp (70.5–77.9 °C); Tc (77.4–82.3 °C); and ΔH (9.6–10.8 J/g). A higher gelatinization temperature indicates a perfect crystal structure of starch, while a higher enthalpy indicates that the gelatinization of starch requires more energy [46].
Table 4. Thermal properties of proso millet starch determined by differential scanning calorimetry (DSC).
| Starch Water Ratio (w/w) | Heating Rate (°C/min) |
To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | References |
|---|---|---|---|---|---|---|
| 1:3 | 5 | 68.4 | 72.2 | 13.1 | [25] | |
| 1:3 | 10 | 72.7–73.6 | 75.8–77.6 | 84.4–89.5 | 13.2–14.8 | [23] |
| 1:2 | 5 | 65.8–80.2 | 6.4–11.4 | [47] | ||
| 1:2.7 | 10 | 67.8–69.0 | 69–73.9 | 75.5–81.8 | 13.2–14.8 | [27] |
| 1:2 | 5 | 62–69 | 67–74 | 77–78 | 9.6–12.6 | [48] |
| 1:2 | 10 | 68.65 | 71.37 | 80.04 | 15.03 | [22] |
| 1:2 | 10 | 71.95 | 77.36 | 87.42 | 14.98 | [41] |
| 1:2 | 10 | 68.56 | 74.53 | 82.43 | 5.16 | [29] |
| 1:3 | 10 | 73.1–76.4 | 78.0–81.5 | 79.3–86.0 | 0.81–4.48 | [34] |
| 1:3 | 10 | 67.4–75.5 | 71.5–79.0 | 76.5–84.0 | 11.9–17.6 | [44] |
| 1:3 | 10 | 66.81–70.01 | 72.79–76.55 | 78.30–82.44 | 10.40–14.46 | [26] |
| 1:2 | 10 | 64.6–71.1 | 70.5–77.9 | 77.4–82.3 | 9.6–10.8 | [14] |
| 1:3 | 10 | 67.9–72.7 | 74.6–76.1 | 80.4–81.2 | 10.37–12.65 | [37] |
| 1:2 | 10 | 72.93 | 78.61 | 94.55 | 3.83 | [28] |
| 1:4 | 10 | 64.16 | 68.45 | 79.09 | 10.58 | [33] |
4.4. Retrogradation
After gelatinization, when starch is cooled, the amylose and amylopectin molecules bind to each other, leading to recrystallization and forming a more structured organization than previously. Retrogradation is a process, which demonstrates the ability of starch to thicken and create rigid gels. Amylose content, water content, amylopectin’s molecular structure, storage parameters (time, temperature), and the presence of other elements (proteins, fiber, lipids) are all factors that influence retrogradation [40]. Annor et al. [25] reported that retrograded PMS had higher onset gelatinization temperature (To) and gelatinization enthalpy (ΔH) values than foxtail and pearl millets, but lower than finger millet. Retrograded millet starch, including retrograded pearl millet starch, typically exhibits reduced enthalpies and melting temperatures. This could be due to the fact that the stored gel exhibits unstable recrystallization of the branched chains of amylopectin compared to native gels [16]. Yang et al. [14] stated that retrogradation of waxy PMS increases in the first 2 h and then stabilizes, whereas in nonwaxy PMS it increases in the first 40 h before stabilizing. Retrogradation reflects the stability of a starch, with higher retrogradation indicating deterioration of stability [49]. Li et al. [44] reported both the highest (28.3%) and lowest (0.1%) retrogradation of PMS. These were different cultivars of proso millet with different origins, calling into question a consistent, measurable correlation between locations. Chao et al. [37] conducted a comparative study of waxy PMS and nonwaxy PMS. Their study showed higher retrogradation rates in the first 4 h; after 8 h, the retrogradations slowed down and stabilized after 32 h for both varieties. However, the percentage of retrogradation of waxy PMS was lower than that of nonwaxy PMS. As a crucial factor limiting the use of starch, higher retrogradation may lead to undesirable changes in the biomechanical properties of starch-based foods and affect their nutritional and sensory aspects. For this reason, and because of its better transparency, waxy millet starch with lower retrogradation could be suitable as a raw material source for beverage production [37].
4.5. Freeze–Thaw Stability
Starchy foods are exposed to multiple cycles of freezing and thawing during different stages of their preparation and storage. The freeze–thaw stability of starch is evaluated by measuring the quantity of water that separates from starch gels as a result of freeze–thaw cycles (syneresis) [6]. Freeze–thaw stability of starch depends on the amount of amylose and amylopectin, water content, thermal history, and molecular structure. The better freeze–thaw stability of native starch depends on a higher proportion of shorter unit chains of amylopectin and a lower quantity of amylose chains [50]. The stability of the starch gel from proso millet was lower than that of the maize starch gel, but a partially dried starch gel from proso millet could rapidly reabsorb water and restore its original structure [27]. However, Wu et al. [29] reported that the freeze–thaw stability of proso millet starch is much higher than that of other millet species such as foxtail, barnyard, hybrid barnyard, pearl, and finger millets. Therefore, the starch of proso millet is more appropriate for use in the frozen food industry than that of other types of millet.
4.6. Digestibility
Starch digestibility is an important nutritional indicator that influences consumer perception of the acceptability or unacceptability of a product [16]. The rate and extent of digestibility, as indicated by time-dependent blood glucose levels in the intestinal tract, determine the starch digestibility factor of a food and are an important metabolic measurement in health care [51]. Rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS) are the three types of digestible starch [52]. RDS is rapidly broken-down during digestion and absorbed by the small intestine. Due to this rapid response, blood glucose levels spike, which in turn increases the likelihood of obesity, type 2 diabetes, and cardiovascular disease [53]. Conversely, SDS takes a long time to be digested. While SDS is also digested in the small intestine, it provides a slower, sustained release of glucose into the bloodstream, resulting in healthier glycemic responses. The low glycemic index of SDS makes it useful in mitigating the risk of obesity and type 2 diabetes, as well as regulating undesirable cycles of excitability and fatigue. SDS is also helpful in many other diseases, such as elevated cholesterol, poor mineral absorption, gallstones, cancer, and reducing fat formation. Conversion of RDS to SDS can have a significant impact on improving the health of millions of people, as it enables a sustained release of glucose into the bloodstream, as opposed to a spiked release. Therefore, it is imperative to include PMS in research trials exploring this area. RS resists breakdown in the small intestine and instead undergoes fermentation in the large intestine, serving as a prebiotic that nourishes beneficial bacteria in the gut [54]. It does not increase blood sugar. Chang et al. [55] reported that nonwaxy proso millet starch has lower RDS content and higher SDS and RS contents than waxy proso millet starch, normal corn starch, and potato starch, indicating a potential functional food formula.
5. Starch Modifications
The structure and functions of native starch depend on the plant from which the starch is obtained. There are some limitations of native starch that restrict its direct application in the food and nonfood industry, especially the low thermal stability and shear strength and the high degree of retrogradation [56]. Notwithstanding these poor gelling properties of their pastes, native starches may also be prone to syneresis, which further reduces water content by increasing coagulation. To address this deficiency of native starch, physical, chemical, and enzymatic processes are commonly used in the food, paper, and textile industries to stimulate and enhance certain functional properties of native starch, including PMS [21]. Derivatization (e.g., etherification, esterification, and crosslinking), disintegration (by acid hydrolysis and oxidation of starch), enzymatic treatments (e.g., fermentation), and physical modifications (e.g., heat–moisture treatment (HMT) and ultra-high-pressure treatment (UHP)), or combinations of these, are the most commonly used methods for starch modification [16].
5.1. Acid Treatment
Acid modification is a chemical process used to modify starch that involves hydrolysis of the α-glucan chains through the use of mineral acids. This process results in the breaking of glycosidic bonds, leading to the alteration of the structure and properties of native starch [57]. Acid hydrolysis is a widely utilized process for the modification of starch. It involves the exposure of starch to mineral acids, such as HCl, H2SO4, H3PO4, and HNO3, resulting in an increase in the number of short starch chains, as found in amylose. Despite being commonly employed, the primary limitation of this technology is its heavy reliance on chemical agents, which can result in adverse environmental impacts [16]. In addition to altering the structure of native starch, acid modification also changes its physicochemical properties, making it suitable for use in various industries such as food and textiles. This modified starch is utilized in the production of starch gum candy, paper, and cationic and amphoteric starches, among other applications [21]. Compared with native PMS, acid-modified starch reduces water binding capacity. It is suggested that this reduction is due to the fact that acid modification reduces the size of the amorphous region, thereby reducing accessibility to the binding sites [28].
5.2. Hydrothermal Treatment
Heat–moisture treatment (HMT) is a hydrothermal processing method carried out under high temperatures ranging from 90–120 °C, and low moisture levels of 35% or below. The process of HMT involves subjecting starch to a controlled temperature above its glass transition temperature for a specified duration, typically between 1 to 24 h, to achieve a low moisture content. The period of the procedure is dependent on the desired outcome and specific treatment process [58]. Annealing (ANN) refers to the hydrothermal process of heating starch with a water content of 40–65% at temperatures lower than the onset of gelatinization. This treatment facilitates the interaction and reassociation of amylose and amylopectin chains inside the starch granules, leading to the repair of structural defects in the crystalline portion [6]. HMT decreases the swelling power, the solubility, the PV, the BD and SB values, and the gelatinization enthalpy of PMS, while the pasting and gelatinization temperatures increase [59][60]. A reduction in the swelling power is desirable for some applications such as noodle production, while a reduction in the breakdown and setback values improves the hot and cold paste stability, respectively, of PMS. Kumar et al.’s [59] findings indicate that the in vitro digestibility of PMS demonstrated an increase in the rapidly digestible starch (RDS) and slowly digestible starch (SDS) fractions, with a decrease in the resistant starch (RS) fraction after undergoing HMT. The effect of HMT on enzymatic digestibility of starch is influenced by various factors such as (i) type of starch source, (ii) moisture content, (iii) treatment temperature and duration, and (iv) interactions between different starch fractions, including amylopectin–amylopectin, amylose–amylose, and amylose–amylopectin interactions [61][62].
5.3. Dry Heat Treatment (DHT)
DHT is a form of physical modification that induces alterations in the physicochemical properties of starch, while preserving its granular structure. Dry heat represents a simple, nontoxic, and healthful substitute for chemical-based techniques. The new properties that occur with thermally treated starch are similar to the results of the chemical crosslinking process. Dry heat improves the pasting and functional properties of starch as effectively as the chemical alternative [22]. In the case of proso millet starch, DHT (8% moisture content, 130 °C, 2 and 4 h) resulted in a decrease in peak (PV) and breakdown (BD) viscosities and pasting temperature (PT) and an increase in final (FV) and setback (SB) viscosities compared to the native starch, the extent of which increased with increasing treatment time. The decrease in BD demonstrated that DHT-modified starch became more resilient to thermal and mechanical shear, thus exhibiting heightened hot paste stability comparable to that of chemically crosslinked starch. The DHT starch could be used in the products which require higher final viscosity and hot paste stability. DHT of PMS also led to an increase in the onset (To) and peak (Tp) gelatinization temperatures and a decrease in gelatinization enthalpy (ΔH). A decrease in ΔH could be attributed to a decrease in crystallinity in the starch granule after DHT, which was determined by X-ray diffraction [22].
5.4. Ultra-High-Pressure (UHP) Treatment
UHP is a nonthermal modification approach that can be employed to induce gelatinization or physical modification of diverse starch types. The degree of gelatinization attained through UHP, as an alternative to conventional thermal processing, is influenced by several factors, including starch type, pressure levels, water content, temperature ranges, and duration of treatment. UHP completely gelatinizes starch when it is under sufficient pressure at specified ambient temperatures [33]. The use of UHP to change the composition of millet starch or its molecular structure has hardly been explored so far. With the exception of proso millet starch treated at a maximum pressure of 600 MPa for 15 min, which caused a decrease in all viscosities of the paste during pasting, the application of UHP at different pressures between 150 and 450 MPa increased the trough and final viscosities and the pasting temperature, but decreased the peak and breakdown viscosities compared to native starch [33]. The exerted pressure potentially enhanced the infiltration of water molecules into the starch granules, disrupted hydrogen bonds, and instigated alterations in the starch configuration. The pasting properties of starch treated at 600 MPa were dissimilar to those of starch treated at 150–450 MPa, implying complete gelatinization of the starch at higher pressure [63][64].
5.5. Fermentation
Through the process of fermentation, it is feasible to alter the chemical and physical characteristics of starch. This methodology serves to augment the structural attributes of starch. Bian et al. [65] conducted a study on the impact of lactic acid bacteria fermentation on the physical properties and structure of glutinous (waxy) PMS. During fermentation, microbial enzymes and acids hydrolyze the starch molecules predominantly located in the amorphous region into smaller molecules. The fermented PMS had higher amylose content and crystallinity, but lower molecular weight, swelling power, and solubility than the nonfermented PMS. All RVA pasting parameters of PMS decreased after fermentation, except for the setback viscosity (SB), which increased. The gelatinization enthalpy (ΔH) of the fermented PMS increased compared to that of the nonfermented PMS, reflecting the increase in crystallinity after fermentation.
5.6. Dual Modifications
Dual modification of starches is gaining popularity in current scientific research. This is because single modification methods may not always fulfill the necessary functional requirements for food and industrial applications. Dual modification of starch offers the ability to customize starch properties for specific applications and to enhance the functionality of single modified starch, thereby increasing the range of applications for starch. Sun et al. [66] studied the effects of dual physical modification with UHP and cold plasma (CP) on the properties and digestibility of PMS. CP is an environmentally friendly and nonthermal method that utilizes gas ionization to produce various free radicals which react with starch, leading to either crosslinking or depolymerization of the starch without the use of chemicals [67]. Application of dual modification with UHP at 600 MPa and CP resulted in gelatinization and depolymerization of PMS and an elevated content of resistant starch (RS). In addition, the dual modified starch exhibited lower breakdown viscosity, indicating higher stability of the paste to heat and mechanical shear [66].
References
- FAO. Cereal Supply and Demand Brief World Food Situation Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 26 March 2020).
- Yousaf, L.; Hou, D.; Liaqat, H.; Shen, Q. Millet: A review of its nutritional and functional changes during processing. Food Res. Int. 2021, 142, 110197.
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 2016, 6, 166–171.
- Adekunle, A.; Lyew, D.; Orsat, V.; Raghavan, V. Helping agribusinesses—Small millets value chain—To grow in India. Agriculture 2018, 8, 44.
- Kumar, A.; Tomer, V.; Kaur, A.; Kumar, V.; Gupta, K. Millets: A solution to agrarian and nutritional challenges. Agric. Food Secur. 2018, 7, 31.
- Zhu, F. Structure, physicochemical properties, and uses of millet starch. Food Res. Int. 2014, 64, 200–211.
- Kumar, S.R.; Sadiq, M.B.; Anal, A.K. Comparative study of physicochemical and functional properties of pan and microwave cooked underutilized millets (proso and little). LWT 2020, 128, 109465.
- Annor, G.A.; Tyl, C.; Marcone, M.; Ragaee, S.; Marti, A. Why do millets have slower starch and protein digestibility than other cereals? Trends Food Sci. Technol. 2017, 66, 73–83.
- Zhang, T.; Li, K.; Ding, X.; Sui, Z.; Yang, Q.Q.; Shah, N.P.; Liu, G.; Corke, H. Starch properties of high and low amylose proso millet (Panicum miliaceum L.) genotypes are differentially affected by varying salt and pH. Food Chem. 2021, 337, 127784.
- Boukail, S.; Macharia, M.; Miculan, M.; Masoni, A.; Calamai, A.; Palchetti, E.; Dell’Acqua, M. Genome wide association study of agronomic and seed traits in a world collection of proso millet (Panicum miliaceum L.). BMC Plant Biol. 2021, 21, 330.
- Habiyaremye, C.; Matanguihan, J.B.; D’Alpoim Guedes, J.; Ganjyal, G.M.; Whiteman, M.R.; Kidwell, K.K.; Murphy, K.M. Proso millet (Panicum miliaceum L.) and its potential for cultivation in the Pacific Northwest, US: A review. Front. Plant Sci. 2017, 7, 1961.
- Yuan, Y.; Liu, J.; Ma, Q.; Gao, Y.; Yang, Q.; Gao, X.; Feng, B. Cleaner production of proso millet (Panicum miliaceum L.) in salt-stressed environment using re-watering: From leaf structural alleviations to multi-omics responses. J. Clean. Prod. 2022, 334, 130205.
- Devisetti, R.; Yadahally, S.N.; Bhattacharya, S. Nutrients and antinutrients in foxtail and proso millet milled fractions: Evaluation of their flour functionality. LWT-Food Sci. Technol. 2014, 59, 889–895.
- Yang, Q.; Zhang, W.; Li, J.; Gong, X.; Feng, B. Physicochemical properties of starches in proso (non-waxy and waxy) and foxtail millets (non-waxy and waxy). Molecules 2019, 24, 1743.
- Yang, Q.; Zhang, P.; Qu, Y.; Gao, X.; Liang, J.; Yang, P.; Feng, B. Comparison of physicochemical properties and cooking edibility of waxy and non-waxy proso millet (Panicum miliaceum L.). Food Chem. 2018, 257, 271–278.
- Mahajan, P.; Bera, M.B.; Panesar, P.S.; Chauhan, A. Millet starch: A review. Int. J. Biol. Macromol. 2021, 180, 61–79.
- Thakur, K.; Sharma, S.; Sharma, R. Morphological and functional properties of millet starches as influenced by different modification techniques: A review. Starke 2023, 75, 2200184.
- Bangar, S.P.; Ashogbon, A.O.; Dhull, S.B.; Thirumdas, R.; Kumar, M.; Hasan, M.; Chaudhary, V.; Pathem, S. Proso-millet starch: Properties, functionality, and applications. Int. J. Biol. Macromol. 2021, 190, 960–968.
- El Halal, S.L.M.; Kringel, D.H.; da Rosa Zavareze, E.; Dias, A.R.G. Methods for extracting cereal starches from different sources: A review. Starke 2019, 71, 1900128.
- Liu, Q. Understanding starches and their role in foods. In Food Carbohydrates: Chemistry, Physical Properties and Applications; Cui, S.W., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 309–355.
- Punia, S.; Kumar, M.; Siroha, A.K.; Kennedy, J.F.; Dhull, S.B.; Whiteside, W.S. Pearl millet grain as an emerging source of starch: A review on its structure, physicochemical properties, functionalization, and industrial applications. Carbohydr. Polym. 2021, 260, 117776.
- Sun, Q.; Gong, M.; Li, Y.; Xiong, L. Effect of dry heat treatment on the physicochemical properties and structure of proso millet flour and starch. Carbohydr. Polym. 2014, 110, 128–134.
- Yanez, G.A.; Walker, C.E. Effect of tempering parameters on extraction and ash of proso millet flours, and partial characterization of proso starch. Cereal Chem. 1986, 63, 164–167.
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CyTA J. Food 2018, 16, 1003–1017.
- Annor, G.A.; Marcone, M.; Bertoft, E.; Seetharaman, K. Physical and molecular characterization of millet starches. Cereal Chem. 2014, 91, 286–292.
- Wen, Y.; Liu, J.; Meng, X.; Zhang, D.; Zhao, G. Characterization of proso millet starches from different geographical origins of China. Food Sci. Biotechnol. 2014, 23, 1371–1377.
- Yañez, G.A.; Walker, C.E.; Nelson, L.A. Some chemical and physical properties of proso millet (Panicum milliaceum) starch. J. Cereal Sci. 1991, 13, 299–305.
- Singh, M.; Adedeji, A.A. Characterization of hydrothermal and acid modified proso millet starch. LWT-Food Sci. Technol. 2017, 79, 21–26.
- Wu, Y.; Lin, Q.; Cui, T.; Xiao, H. Structural and physical properties of starches isolated from six varieties of millet grown in China. Int. J. Food Prop. 2014, 17, 2344–2360.
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starke 2014, 66, 41–57.
- Zarnkow, M.; Mauch, A.; Back, W.; Arendt, E.K.; Kreisz, S. Proso millet (Panicum miliaceum L.): An evaluation of the microstructural changes in the endosperm during the malting process by using scanning-electron and confocal laser microscopy. J. Inst. Brew. 2007, 113, 355–364.
- Nor Nadiha, M.Z.; Fazilah, A.; Bhat, R.; Karim, A.A. Comparative susceptibilities of sago, potato and corn starches to alkali treatment. Food Chem. 2010, 121, 1053–1059.
- Li, W.; Gao, J.; Saleh, A.S.M.; Tian, X.; Wang, P.; Jiang, H.; Zhang, G. The modifications in physicochemical and functional properties of proso millet starch after ultra-high pressure (UHP) process. Starke 2018, 70, 1700235.
- Kim, S.K.; Choi, H.J.; Kang, D.K.; Kim, H.Y. Starch properties of native proso millet (Panicum miliaceum L.). Agron. Res. 2012, 10, 311–318.
- Kumari, S.K.; Thayumanavan, B. Characterization of starches of proso, foxtail, barnyard, kodo, and little millets. Plant Foods Hum. Nutr. 1998, 53, 47–56.
- Li, Y.; Hu, A.; Wang, X.; Zheng, J. Physicochemical and in vitro digestion of millet starch: Effect of moisture content in microwave. Int. J. Biol. Macromol. 2019, 134, 308–315.
- Chao, G.; Gao, J.; Liu, R.; Wang, L.; Li, C.; Wang, Y.; Qu, Y.; Feng, B. Starch physicochemical properties of waxy proso millet (Panicum Miliaceum L.). Starke 2014, 66, 1005–1012.
- Mir, S.A.; Bosco, S.J.D. Cultivar difference in physicochemical properties of starches and flours from temperate rice of Indian Himalayas. Food Chem. 2014, 157, 448–456.
- Yoo, S.H.; Jane, J.L. Structural and physical characteristics of waxy and other wheat starches. Carbohydr. Polym. 2002, 49, 297–305.
- Hoover, R. Composition, molecular structure, and physicochemical properties of tuber and root starches: A review. Carbohydr. Polym. 2001, 45, 253–267.
- Xiao, Y.; Zheng, M.; Yang, S.; Li, Z.; Liu, M.; Yang, X.; Lin, N.; Liu, J. Physicochemical properties and in vitro digestibility of proso millet starch after addition of Proanthocyanidins. Int. J. Biol. Macromol. 2021, 168, 784–791.
- Wang, Y.; Chen, L.; Yang, T.; Ma, Y.; McClements, D.J.; Ren, F.; Tian, Y.; Jin, Z. A review of structural transformations and properties changes in starch during thermal processing of foods. Food Hydrocoll. 2021, 113, 106543.
- Brites, C.M.; Santos, C.A.L.D.; Bagulho, A.S.; Beirão-Da-Costa, M.L. Effect of wheat puroindoline alleles on functional properties of starch. Eur. Food Res. Technol. 2008, 226, 1205–1212.
- Li, K.; Zhang, T.; Narayanamoorthy, S.; Jin, C.; Sui, Z.; Li, Z.; Li, S.; Wu, K.; Liu, G.; Corke, H. Diversity analysis of starch physicochemical properties in 95 proso millet (Panicum miliaceum L.) accessions. Food Chem. 2020, 324, 126863.
- Koch, K.; Jane, J. Morphological changes of granules of different starches by surface gelatinization with calcium chloride. Cereal Chem. 2000, 77, 115–120.
- Gao, H.; Cai, J.; Han, W.; Huai, H.; Chen, Y.; Wei, C. Comparison of starches isolated from three different Trapa species. Food Hydrocoll. 2014, 37, 174–181.
- Fujita, S.; Fujiyama, G. The study of melting temperature and enthalpy of starch from rice, barley, wheat, foxtail-and proso-millets. Starke 1993, 45, 436–441.
- Tomita, Y.; Sugimoto, Y.; Sakamoto, S.; Fuwa, H. Some properties of starches of grain amaranths and several millets. J. Nutr. Sci. Vitaminol. 1981, 27, 471–484.
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585.
- Srichuwong, S.; Isono, N.; Jiang, H.; Mishima, T.; Hisamatsu, M. Freeze–thaw stability of starches from different botanical sources: Correlation with structural features. Carbohydr. Polym. 2012, 87, 1275–1279.
- Magallanes-Cruz, P.A.; Flores-Silva, P.C.; Bello-Perez, L.A. Starch structure influences its digestibility: A review. J. Food Sci. 2017, 82, 2016–2023.
- Leong, S.Y.; Duque, S.M.; Muhammad Abduh, S.B.; Oey, I. Carbohydrates. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Barba, F.J., Saraiva, J.M.A., Cravotto, G., Lorenzo, J.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 171–206.
- Chai, Y.; Wang, M.; Zhang, G. Interaction between amylose and tea polyphenols modulates the postprandial glycemic response to high-amylose maize starch. J. Agric. Food Chem. 2013, 61, 8608–8615.
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch–A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17.
- Chang, L.; Zhao, N.; Jiang, F.; Ji, X.; Feng, B.; Liang, J.; Yu, X.; Du, S.-K. Structure, physicochemical, functional and in vitro digestibility properties of non-waxy and waxy proso millet starches. Int. J. Biol. Macromol. 2023, 224, 594–603.
- Jyothi, A.N.; Moorthy, S.N.; Rajasekharan, K.N. Effect of cross-linking with epichlorohydrin on the properties of cassava (Manihot esculenta Crantz) starch. Starke 2006, 58, 292–299.
- Hoover, R. Acid-treated starches. Food Rev. Int. 2000, 16, 369–392.
- Hoover, R. The impact of heat-moisture treatment on molecular structures and properties of starches isolated from different botanical sources. Crit. Rev. Food Sci. Nutr. 2010, 50, 835–847.
- Kumar, S.R.; Tangsrianugul, N.; Sriprablom, J.; Wongsagonsup, R.; Wansuksri, R.; Suphantharika, M. Effect of heat-moisture treatment on the physicochemical properties and digestibility of proso millet flour and starch. Carbohydr. Polym. 2023, 307, 120630.
- Zheng, M.-Z.; Xiao, Y.; Yang, S.; Liu, H.-M.; Liu, M.-H.; Yaqoob, S.; Xu, X.-Y.; Liu, J.-S. Effects of heat–moisture, autoclaving, and microwave treatments on physicochemical properties of proso millet starch. Food Sci. Nutr. 2020, 8, 735–743.
- Pratiwi, M.; Faridah, D.N.; Lioe, H.N. Structural changes to starch after acid hydrolysis, debranching, autoclaving-cooling cycles, and heat moisture treatment (HMT): A review. Starke 2018, 70, 1700028.
- Zeng, F.; Ma, F.; Kong, F.; Gao, Q.; Yu, S. Physicochemical properties and digestibility of hydrothermally treated waxy rice starch. Food Chem. 2015, 172, 92–98.
- Błaszczak, W.; Fornal, J.; Kiseleva, V.I.; Yuryev, V.P.; Sergeev, A.I.; Sadowska, J. Effect of high pressure on thermal, structural and osmotic properties of waxy maize and Hylon VII starch blends. Carbohydr. Polym. 2007, 68, 387–396.
- Li, W.; Bai, Y.; Mousaa, S.A.S.; Zhang, Q.; Shen, Q. Effect of high hydrostatic pressure on physicochemical and structural properties of rice starch. Food Bioprocess Technol. 2012, 5, 2233–2241.
- Bian, X.; Chen, J.-R.; Yang, Y.; Yu, D.-H.; Ma, Z.-Q.; Ren, L.-K.; Wu, N.; Chen, F.-L.; Liu, X.-F.; Wang, B.; et al. Effects of fermentation on the structure and physical properties of glutinous proso millet starch. Food Hydrocoll. 2022, 123, 107144.
- Sun, X.; Saleh, A.S.M.; Lu, Y.; Sun, Z.; Zhang, X.; Ge, X.; Shen, H.; Yu, X.; Li, W. Effects of ultra-high pressure combined with cold plasma on structural, physicochemical, and digestive properties of proso millet starch. Int. J. Biol. Macromol. 2022, 212, 146–154.
- Chaiwat, W.; Wongsagonsup, R.; Tangpanichyanon, N.; Jariyaporn, T.; Deeyai, P.; Suphantharika, M.; Fuongfuchat, A.; Nisoa, M.; Dangtip, S. Argon plasma treatment of tapioca starch using a semi-continuous downer reactor. Food Bioprocess Technol. 2016, 9, 1125–1134.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
990
Revisions:
2 times
(View History)
Update Date:
03 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




