Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Manop Suphantharika and Version 2 by Camila Xu.
Proso millet starch (PMS) as an unconventional and underutilized millet starch is becoming increasingly popular worldwide due to its health-promoting properties. PMS can be isolated from proso millet grains by acidic, alkaline, or enzymatic extraction. PMS exhibits typical A-type polymorphic diffraction patterns and shows polygonal and spherical granular structures with a granule size of 0.3–17 µm. PMS is modified by chemical, physical, and biological methods. The native and modified PMS are analyzed for swelling power, solubility, pasting properties, thermal properties, retrogradation, freeze–thaw stability, and in vitro digestibility.
- proso millet starch
- extraction
- characterization
- modification
- in vitro digestibility
- application
1. Introduction
Global production of cereal grains has reached record levels. Cereal grains play an important role in the human diet as a primary source of energy. The Food and Agriculture Organization (FAO) [1] reported in 2020 that the global production of cereal grains in 2019 reached a record high of 2715 million tons. At the same time, the global community is facing climatic changes, pollution, water scarcity, rising food costs, population growth, and other socioeconomic issues. These negative aspects can affect regional agricultural progress and limit grain production, leading to high food prices and serious food security concerns worldwide [2]. Moreover, smallholder farmers facing these conditions become economically vulnerable due to their limited resources and have difficulty maintaining their yields and profitability [3]. As a consequence of unfavorable global phenomena and their adverse impacts that constrain agricultural production, there is an urgent need among experts in nutrition and technology to identify a suitable cereal crop that could serve as a viable food source to address these challenges [4]. Under these circumstances, millets may be a nutritious option to supplement the nutritional needs of a growing world population in an uncertain global environment [5].
Millet belongs to the Poaceae family and is cultivated in subtropical and tropical regions of marginal drylands. Over 10,000 years ago, prior to the widespread consumption of wheat, maize, barley, and rice, this food item served as a staple for the people of that era. Currently, the most commonly cultivated species include proso millet (Panicum miliaceum), pearl millet (Pennisetum glaucum), and finger millet (Setaria italica) [6]. Millet is abundant in proteins, fats, carbohydrates, fiber, minerals, vitamins, and phenolic compounds [7]. Nutritionally, millet contains proteins (6–19%), carbohydrates (60–70%), fats (1.5–5%), minerals (2–4%), dietary fiber (12–20%), and various phytochemicals [8]. In addition, millet is gluten-free. This is desirable for people with celiac disease, and because of millet’s blood sugar-lowering properties, it is also effective in treating type II diabetes [8].
Proso millet (Panicum miliaceum L.) is also known as common millet, hog millet, Russian millet, and broomcorn millet in certain areas [9]. Proso millet is characterized by its adaptability to unfavorable environmental conditions (such as salt, drought, temperature, and pH). It also has a short lifecycle (about 12 weeks) and is grown in slightly acidic, saline, sandy, and low-fertility soils with limited nitrogen and carbon dioxide [10][11][12][10,11,12]. Proso millet contains carbohydrates (70–74%), proteins (9.4–9.9%), ash (1.2–3.8%), and fats (1.2–3.8%), along with a variety of phytochemicals and vital minerals [13].
Starch is a major constituent of millet and is divided into two types, namely, amylose and amylopectin. Based on the amylose content, millets are classified into nonwaxy (high amylose content) and waxy (low amylose content) [14]. Yang et al. [15] measured the range of starch content in nonwaxy (high amylose content) proso millet as 59–77% and for waxy millet as 55–69%. Starch serves as a crucial energy source for humans and is extensively utilized in the food and food-related industries. It is a renewable, biodegradable, economical, and natural material used to modify the textural properties of various foods. It can be modified into thickeners, stabilizers, and sweeteners, and can also serve as a water-retention agent [9].
A number of researchers conducted an analysis comparing various types of millet starches, but, unfortunately, they did not provide a thorough study of proso millet starch (PMS) [6][16][17][6,16,17]. According to Banger et al. [18], a comprehensive account of PMS, including its physiochemical and functional properties, modification, and applications, was presented. However, it was noted that more detailed information on isolation, digestibility, and recent advances in its applications is lacking.
2. Isolation, Yield, and Composition of Proso Millet Starch
The starch granules within proso millet grains exhibit strong binding affinity to the surrounding protein matrix. Various methods and chemical reagents are used to extract starch and solubilize the proteins in the grain [19]. Generally, starch extraction methods consist of three phases, i.e., fragmentation, cell disruption, and purification or separation [20]. Millet starch is usually isolated by the wet milling method. The grain or flour is soaked in an aqueous solution (water, alkali, or acid) for a certain time, depending on its chemical properties and composition [16]. The particular method of starch extraction (e.g., acidic, alkaline, or enzymatic) has a significant effect on starch yield. Starch isolation methods vary widely and depend on the inherent starch content of the grain and the initial soaking conditions (neutral, alkaline, or acidic) [21]. The procedure for isolating proso millet starch (PMS) is depicted in Figure 1.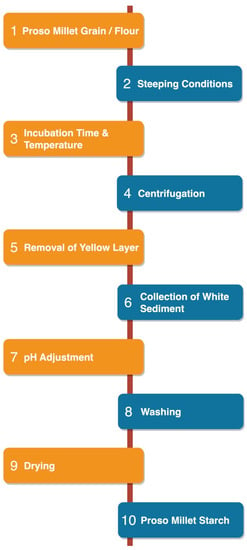
Figure 1.
Isolation of proso millet starch.
Table 1.
Starch yield and chemical composition of proso millet starch.
3. Morphology and Crystallinity of Proso Millet Starch
The size of starch granules in millet varies depending on the plant species. Despite being generally spherical and polygonal in shape (as indicated in Table 2), the dimensions of these granules range from 0.3–17 µm. The polygonal shapes are also larger and have more indentations than the spherical shapes [25], and the morphology of the starch is strongly influenced by its treatment and/or biomodification [30]. In addition, differences in particle size of PMS obtained from proso millet grown in different regions can be due to local environmental aspects. An increase in altitude and reduced mean temperature can lead to bigger granules [26]. Additionally, the morphology of starch is influenced by the arrangement of starch granules inside the endosperm of the grain [31]. Cavities are dispersed randomly throughout the entire outer layer of the starch granules due to surface pores and protein bodies. These pores are connected to the central cavity of the granules, enabling specific molecules from the external environment to penetrate the granules [16]. From a starch modification perspective, this phenomenon is helpful. These pores allow OH ions or water to enter the granules, destroying the amylose-containing amorphous region. Consequently, the restrictive qualities of amylose are reduced, leading to enhanced starch swelling and hydration properties [32].Table 2.
Proso millet starch’s native and modified morphological properties.
| Starch Source | Type | Size (µm) | Shape | Reference |
|---|---|---|---|---|
| Proso millet | NS | 3–10 | Oval, polygonal, irregular, and spherical | [ |
4.2. Pasting Properties
In the majority of cases, rheological evaluation of starch was carried out using both the Rapid Visco Analyzer (RVA) and the Brabender Visco-Amylograph (BVA), and the findings are presented in Table 3. This technique involves heating starch with a substantial quantity of water under continuous shear. The viscosity changes at a given temperature cycle are recorded. Pasting is affected by several parameters, including starch structure, water content, temperature program, and shear rate, which are closely monitored. The amount of starch used in the studies that was examined ranged from 6 to 10% [6]. Three sections can be identified in a typical pasting curve, each representing a specific phase of starch granule transformation during the pasting process [9]. The first phase involves the gradual absorption of water by the starch granules, causing them to expand; the second phase involves the leaching of the amylose component; and the final phase involves the loss of structural integrity of the expanded starch granules, causing them to disintegrate into fragments [42]. The pasting properties and attributes of starch paste are subject to the influence of several factors, including the concentration of starch, its composition in terms of amylose content and amylose-to-amylopectin ratio, and cooking and cooling temperatures, as well as the presence of solutes such as pH, lipids, and sugars. For instance, waxy starch has a greater tendency to absorb water and expand quickly, enabling it to attain its maximum pasting temperature in a shorter duration as compared to starches with a higher amylose content [43]. Yang et al. [14] reported that the peak viscosity (PV), trough viscosity (TV), and breakdown viscosity (BD) of waxy proso millet starch were greater, while the setback viscosity (SB) and pasting temperature (PT) were lower compared to nonwaxy millet starch. The study conducted on proso millet starch revealed that amylose content had a strong negative correlation with PV, TV, and BD, but a substantial positive correlation with SB and PT. A lower SB indicates better stability, and a lower BD indicates high shear resistance. Waxy proso millet starch demonstrates superior stability, making it a desirable choice for frozen food and thickening applications. On the other hand, nonwaxy proso millet starch exhibits higher temperature stability and improved shear resistance, indicating its potential suitability for medicinal resources [14].Table 3.
Pasting properties of proso millet starch.
| Starch (g/mL) |
Method | Unit | PV | BD | SB | PT (°C) | Reference |
|---|---|---|---|---|---|---|---|
| - | BVA | BU |
Table 4.
Thermal properties of proso millet starch determined by differential scanning calorimetry (DSC).
| Starch Water Ratio (w/w) | Heating Rate (°C/min) |
To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [ | 25 | ] | ||||||||||||||
| 33 | ] | 72.5–74.5 | [ | 27 | ] | 92.19–94.60 | 0.27–0.67 | 14.92–17.37 | ||||||||
| 1:3 | 5 | 68.4 | 72.2 | 13.1 | [25] | |||||||||||
| UHP | - | - | BVA | 0.13–0.25 | Structural disruption, gel-like structure formed | [ | BU | 26 | 520 | ] | ||||||
| [ | 33 | ] | 50 | 330 | 75.8 | - | 0.69–4.31 | |||||||||
| 1:3 | 10 | 72.7–73.6 | 75.8–77.6 | [ | 27.2–29.1 | 0.59–0.6 | [23] | |||||||||
| 35 | ] | |||||||||||||||
| 84.4–89.5 | 13.2–14.8 | [ | 23 | ] | NS | 2.5–17 | Few spherical and mostly polygonal | [25] | 3.5/25 | DHR | Pa.s | 4.60 | 2.60 | 1.69 | 79.23 | [28] |
| 1:2 | 5 | 65.8–80.2 | 6.4–11.4 | [47] | 61.8–68.2 | 1.1–2.1 | 29.2–32.6 | |||||||||
| NS | - | 0.3–12 | Few small spherical granules and mainly uniform large or small polygonal | [ | 3/25 | 26 | RVA | cP | ] | 2807 | ||||||
| 1:2.7 | 10 | 67.8–69.0 | 69–73.9 | [27] | ||||||||||||
| 1746 | 1634 | 57.40 | [ | 75.5–81.8 | 33 | ] | 87.27–94.60 | |||||||||
| 13.2–14.8 | [ | 27 | ] | 1.07–1.30 | 2.80–32.80 | 0.01 | [14] | |||||||||
| NS | 3–10 | 3/25 | RVAFew small spheres and large polygonal shape | cP | [29] | 2372 | 1792 | 582 | - | [29] | - | 0.48 | 1.61 | 0.01 | ||
| 1:2 | 5 | 62–69 | 67–74 | 77–78 | 9.6–12.6 | [22] | ||||||||||
| [ | 48 | ] | NS | 3/25 | 4.3–8.9 | Mostly polygonal with some elliptical granules having rounded edges and surface pores | [34] | RVA | cP | 2822 | 1854 | 501 | 76 | [22] | 54.1 | 1.21 |
| 2.5/25 | ||||||||||||||||
| 1:2 | 10 | 68.65 | 71.37 | 80.04 | 15.03 | [22] | 28.51 | 0.27 | ||||||||
| NS | 5–12 | [ | Round and smooth | [22] | ||||||||||||
| RVA | cP | 2284.5 | 913 | |||||||||||||
| 1:2 | 372.5 | 10 | 71.95 | 77.36 | 87.42 | 79.18 | 14.98[41] | |||||||||
| [ | 41 | ] | DHT | 2/25 | - | Smooth and plump surface with large lumps | [22] | |||||||||
| RVA | cP | 2134–3515 | 488–967 | 197–1102 | 63.60–63.80 | [ | 37] | |||||||||
| 3/25 | RVA | cP | ||||||||||||||
| 1:2 | 10 | 68.56 | 74.53 | 82.43 | 5.16 | [29] | NS | 1.8–13.5 | Bimodal distribution, small spherical and large polygonal | [27] | ||||||
| NS | 1.3–8 | Bimodal distribution, large polygonal, small and large spherical | [35] | |||||||||||||
| NS | 4.49–4.70 | Regular, polygonal, and round shape, along with the characteristic Maltese cross structure | [14] | |||||||||||||
| NS | 1.54–11.7 | Mainly polygonal and round shape, larger and smaller granules make honey-comb structure | [9] |
Key: NS, native starch; DHT, dry-heat-treated; UHP, ultra-high-pressure-treated.
4. Physiological and Functional Properties
4.1. Swelling Power and Solubility
With an appropriate quantity of water, the starch is subjected to heating, causing the granules to absorb moisture and undergo swelling. In this process, the components of the starch granule are leached out and largely dissolved in the form of amylose. Eventually, the swollen starch granules break down and disintegrate when they continue to be exposed to high temperatures. This activity is influenced by several factors, including the physical associations of the chemical components in the granules, the molecular structure of amylose and amylopectin, the intrinsic phosphorus groups, and the restricting entanglement of the lipid–amylose complex [40]. Starch granules undergo swelling when exposed to temperatures between 50–90 °C in the presence of water. Studies have shown that the swelling power (SP) of millet is lower compared to that of rye, potato, and wheat. This indicates that millet starch has greater resistance towards swelling due to its relatively strong binding force between the granules [16]. Much research has been conducted on SP of PMS, and some representative results are presented below. Singh and Adedeji [28] studied the SP of PMS at different temperatures (70–90 °C) and recorded the percentage range of their size changes, i.e., native starch (4.69–24.99%), acid-modified starch (4.94–21.26%), and hydrothermally modified starch (5.29–10.37%). At 95 °C, Xiao et al. [41] studied the SP of native PMS (13.77 g/g) and PMS with proanthocyanidins (14.15–19.83 g/g). Wu et al. [29] reported that the SP of PMS in their research was greater than other millet varieties, such as foxtail, barnyard, and finger millet, as well as a hybrid of barnyard and pearl millet. Li et al. [33] studied the SP of PMS (2–35%) at 50–90 °C and found that after ultra-high pressure, the treated starch showed lower SP than native starch. The following solubility of PMS at different temperatures (70–90 °C) was observed for native starch (2.62–34.88%), acid-modified starch (18.97–86.17%), and hydrothermally modified starch (1.71–12.45%) [28]. The solubility of acid-modified starch was higher than that of native starch, which is due to the fact that increasing temperature causes structural weakening and depolarization of starch granules in the former [28]. Li et al. [33] observed the solubility of PMS in a temperature range of 50–90 °C and found that the ultra-high-pressure-treated starch exhibited lower solubility than the native starch at a higher temperature. At 95 °C, Xiao et al. [41] investigated the solubility of native PMS (5.32%) and found a higher solubility of PMS with proanthocyanidins (8.64–16.35%). Wu et al. [| 2110–3286 | ||||||||||||||
| 1114–2189 | ||||||||||||||
| 279–1478 | ||||||||||||||
| 77.8–80.9 | ||||||||||||||
| [ | 14 | ] | ||||||||||||
| 1:3 | 10 | 73.1–76.4 | 78.0–81.5 | 79.3–86.0 | 0.81–4.48 | [34] | 2/26 | RVA | cP | 2215–3585 | 511–1437 | 752–1435 | 78.8–82.8 | [44] |
| - | BVA | BU | 219–457 | 79.5–240 | 115.05–201.5 | - | [26] |
Key: RAV = Rapid Visco-Analyzer, DHR = Discovery Hybrid Rheometer, BVA = Brabender Visco-Amylograph. The viscosity units for RVA, BVA, and DHR are cP, BU, and Pa.s, respectively; PV = peak viscosity; BD = breakdown viscosity; SB = setback viscosity; PT = pasting temperature (°C).
