Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | José J. Ceron | -- | 3456 | 2023-06-30 20:17:46 | | | |
| 2 | Camila Xu | Meta information modification | 3456 | 2023-07-03 03:30:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cerón, J.J.; Ortín-Bustillo, A.; López-Martínez, M.J.; Martínez-Subiela, S.; Eckersall, P.D.; Tecles, F.; Tvarijonaviciute, A.; Muñoz-Prieto, A. S-100 Proteins. Encyclopedia. Available online: https://encyclopedia.pub/entry/46287 (accessed on 07 February 2026).
Cerón JJ, Ortín-Bustillo A, López-Martínez MJ, Martínez-Subiela S, Eckersall PD, Tecles F, et al. S-100 Proteins. Encyclopedia. Available at: https://encyclopedia.pub/entry/46287. Accessed February 07, 2026.
Cerón, José Joaquín, Alba Ortín-Bustillo, María José López-Martínez, Silvia Martínez-Subiela, Peter David Eckersall, Fernando Tecles, Asta Tvarijonaviciute, Alberto Muñoz-Prieto. "S-100 Proteins" Encyclopedia, https://encyclopedia.pub/entry/46287 (accessed February 07, 2026).
Cerón, J.J., Ortín-Bustillo, A., López-Martínez, M.J., Martínez-Subiela, S., Eckersall, P.D., Tecles, F., Tvarijonaviciute, A., & Muñoz-Prieto, A. (2023, June 30). S-100 Proteins. In Encyclopedia. https://encyclopedia.pub/entry/46287
Cerón, José Joaquín, et al. "S-100 Proteins." Encyclopedia. Web. 30 June, 2023.
Copy Citation
S100s are a group of calcium-binding proteins which received this name because of their solubility in a 100% saturated solution of ammonium sulphate at neutral pH. All members of the S100 protein family have a similar molecular mass of 10–12 KDa, and they each share 25–65% similarity in their amino acid sequence.
calgranulins
S100
calprotectin
biomarkers
inflammation
1. General Concepts and Functions of S100 Proteins
1.1. Definition
S100s are a group of calcium-binding proteins which received this name because of their solubility in a 100% saturated solution of ammonium sulphate at neutral pH [1]. All members of the S100 protein family have a similar molecular mass of 10–12 KDa, and they each share 25–65% similarity in their amino acid sequence [2]. They belong to the superfamily of the calcium-binding EF-hand proteins, the EF-hand being a helix-loop-helix structural domain or motif [3]. These proteins have charged amino acid residues, resulting in their affinity for divalent ions such as Ca2+, Zn2+, and Cu2+ [4]. Ca2+ binds to the EF-hand sites, while Zn2+ and Cu2+ bind to separate sites. They have the ability to form homodimers, heterodimers, and oligomeric assemblies which can have different physiological activities [5][6].
Although initially they were thought to be of neural origin since they were purified from the bovine brain, they are expressed in many tissues, and to date 25 different types of S100 proteins have been identified [2], and crystal structure forms of some of them have been described [7]. The main characteristics of each of the different S100s are summarized in Table 1.
Table 1. S100 proteins, names, human diseases in which they have been studied, general reviews published, and veterinary studies (when available). There are 23 proteins in the table. S100A17 and S100A18 are also described, giving a total of 25 proteins in the group of S100 [5], but they are not included in this table since, to the authors’ knowledge, their functions and roles in diseases are still not well understood.
| S100 Protein (Alternative Name) |
Examples of Diseases in Which It Is Involved in Humans | General Reviews, Examples of Veterinary Studies |
|---|---|---|
| S100A1 (S100 alpha) |
Neuroinflammation, Alzheimer’s disease [8] Vascular function and hypertension [9] Cardiotoxicity [10] |
Structure, function, and therapeutic potential [11] Role in cardiovascular health and disease [12] |
| S100A2 (S100L, CAN19) |
Endometrial carcinoma [13] Pancreatic cancer [14] Lung cancer [15] Keratinocyte damage in response to any inflammatory or toxic condition [16] |
Influence in human diseases [17] |
| S100A3 (S100E) |
Colorectal cancer [18] Pulmonary fibrosis [19] |
|
| S100A4 (Metastasin1, Calvasculin) |
Prostatic cancer [20] Rheumatoid arthritis [21] |
Cancer progression and metastasis [22] Pathophysiology processes such as fibrosis, inflammation, immune response, neuroprotection, angiogenesis [23] Role in health and disease [24] Veterinary studies: Canine mammary carcinomas [25] Expressed in canine melanoma [26] |
| S100A5 (S100D) |
Bladder cancer [27] Meningioma [28] |
|
| S100A6 [Calcyclin (CACY)] |
Osteosarcoma [29] Pancreatic cancer [30] Alzheimer’s disease [31] |
Potential therapeutic target for acute myocardial infarction [32] Neurodegenerative diseases [33] Veterinary studies: Porcine reproductive and respiratory syndrome virus [34] Atopic dogs [35] Toxoplasma gondii infection [36] |
| S100A7 (Psoriasin1) |
Psoriasis and inflammatory skin diseases [37] Squamous cell carcinomas and large cell lung carcinomas [38] Alzheimer disease [39] Wound healing process [40] |
Role in breast cancer [41] Veterinary studies: Mastitis in goat [42] |
| S100A8 (Calgranulin A) S100A9 (Calgraunlin B) S100A8/A9 (Calprotectin) |
Gastrointestinal disease, inflammation, autoimmune and septic conditions, among others. See details for specific diseases in humans and veterinary in the text. |
Use of fecal calprotectin [43] Biological function and use as biomarker [44] Diagnosis of sepsis [45] |
| S100A10 (Calpactin-1 Plasminogen receptor S100A10) |
Breast cancer [46] | Role in disease [47] Depression and antidepressant actions [48] Oncogenesis [49] |
| S100A11 (Calgizzarin S100C) |
Reumatoid arthritis [50] Muscle damage and autoimmune disease [51] Ovarian cancer [52] |
Role in disease [53] |
| S100A12 (Calgranulin C) |
Inflammation, autoimmune and septic conditions. See details for specific diseases in humans and veterinary in the text | Digestive diseases [54] Atherosclerosis [55] Inflammation [56] |
| S100A13 | Lung cancer [57] Melanoma [58] Thyroid cancer [59] |
|
| S100A14 | Breast cancer [60] Gastric cancer [61] Hepatocellular carcinoma [62] |
Human cancer [63] |
| S100A15 (Koebnerisin) |
Psoriasis [64] Hidradenitis suppurativa [65] Lung adenocarcinoma [66] |
|
| S100A16 | Breast Cancer [60] Lung adenocarcinoma [67] Gastric cancer [68] Insulin sensitivity [69] |
Digestive system [70] |
| S100B | General diseases [71] Neurological disorders [72] Traumatic brain injuries [73] Sport-related concussion [74] Veterinary studies: Presence in milk of various species [75] Neurological diseases in calves [76] |
|
| S100G (Calbindin-DK9) |
Pancreatitis [77] | |
| S100P (S100E) |
Melanoma [78] Prostatic cancer [79] |
Cancer [80] |
| S100Z | Liver cancer (with S100G) [81] | |
| Others Repetin Filaggrin Trichohyalin |
Atopic dermatitis [82] Atopic dermatitis [83] Dermatological disorders [84] |
1.2. Functions
S100 proteins have important roles and functions in live organisms at both intracellular and extracellular levels.
1.2.1. Intracellular
S100 proteins have many intracellular roles through their interaction with several effector proteins within cells and their binding with Ca2+, Zn2+, and Cu2+. Therefore, they are involved in the regulation of multiple cellular processes such as contraction, motility, cell growth, division and differentiation, transcription, enzyme activation, and also in the structure of membranes, and dynamics of cytoskeleton constituents. In addition, they have a role in protection from oxidative cell damage and protein phosphorylation and secretion [85]. In many cases, S100 proteins function as Ca2+ sensor proteins, which on binding Ca2+, change their structure, and in this state they can interact with various target proteins changing their activity. The roles of Zn2+ and Cu2+ are not yet clear [86].
1.2.2. Extracellular
In situations related to cell damage and/or activation, S100 proteins can be released into the extracellular space and promote various paracrine and autocrine functions such as inflammation, autoimmunity, and also cell proliferation and survival (including neuronal survival and extension) [85]. These roles would be related to the elevated S100 protein concentrations that are found in inflammatory processes, autoimmune diseases, neurodegeneration, and neoplasms.
2. Mechanism of Action
S100s and especially calgranulins mediate, among other processes, the inflammatory response, being considered important proinflammatory factors of innate immunity, which has a key role in host defense and in initiating inflammation. For a better understanding, two main phases can be differentiated in the mechanism of action of these proteins (Figure 1).
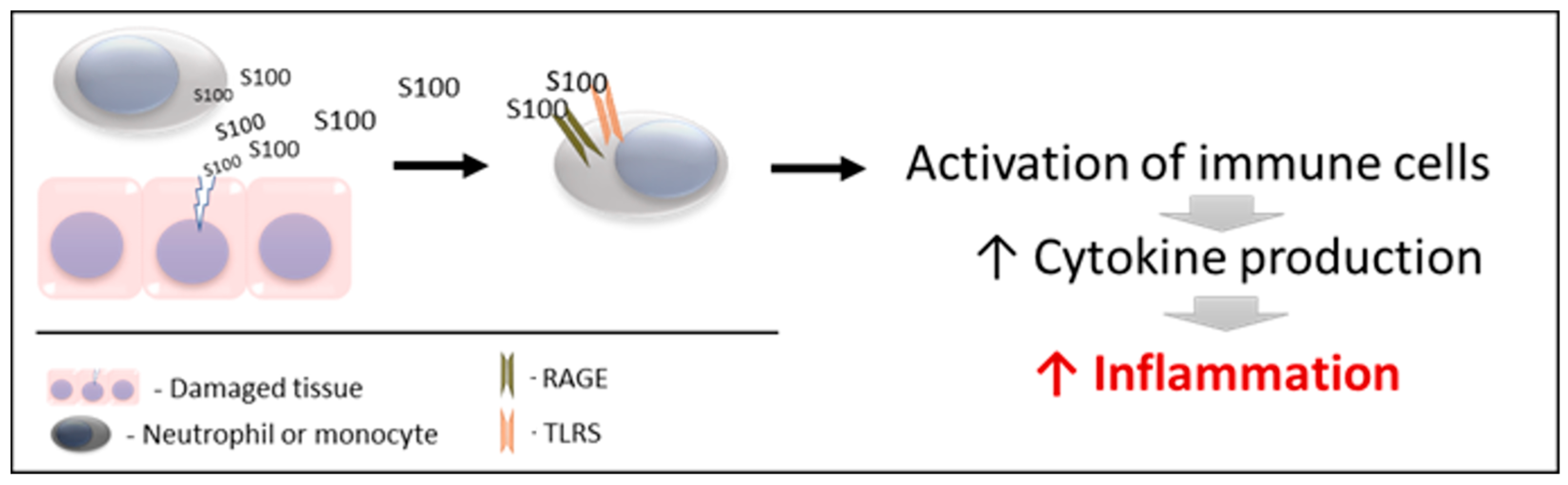
Figure 1. Schematic mechanism of action of S100 proteins. RAGE: receptors for advanced glycation end products. TLRs: toll-like receptors.
2.1. Action 1
S100 proteins are released by damaged and/or activated cells, under conditions of cell stress. Due to this mechanism of liberation, S100 proteins are considered to be “damage-associated molecular pattern proteins (DAMPs)”, “endokines”, or “alarmins” [87]; referring to compounds that, after any cell damage/stress and/or activation of immune cells (such as neutrophils and macrophages) are released to the extracellular space, where they play a key role in the regulation of several immune and inflammatory processes [88].
Interestingly, the three calgranulins do not have the structural elements required for secretion via the classical endoplasmic reticulum and Golgi-dependent secretory pathway. Therefore, they are released by cells by necrosis or cell death and also utilize an active cytoskeleton-dependent non-classical secretion that is used by some cytokines after cell activation [89].
It is interesting to point out that this mechanism is different from other mediators of innate immunity that are also used as biomarkers of inflammation and innate response such as the acute phase proteins, which are produced mainly by the liver mediated by interleukins, after an inflammatory stimulus [90]. This could explain why in some cases DAMPs can provide information on cell lesions that do not correlate with other inflammatory markers such as interleukins or acute phase proteins [91][92]. Therefore, acute phase proteins and calgranulins could be considered biomarkers that could provide complementary information about inflammation and innate immunity.
2.2. Action 2
The released S100 molecules of Action 1 are able to activate immune cells, promoting cytokine production and the inflammatory response. This is achieved by binding and interacting with the PRRS which are present in these cells (such as TLRs and RAGE). Therefore, they can interact and activate receptors similar to those activated by pathogen-associated molecular patterns (PAMPs) [93][94][95]. Recently, it has been demonstrated that S100 proteins can interact directly with cytokines, and such interactions can affect the functioning of these cytokines [96].
Although there is not any data about the half-life for all S100 proteins, the biological half-life of S100B is approximately 30 min, being eliminated mainly by the kidneys [85]. If this finding is similar to other S100 proteins, it can be stated that any persistent elevation of S100 serum levels would indicate their continuous release from affected tissues or activated cells. In addition, potential alterations in renal function could reduce their excretion and produce increases in these proteins.
3. Measurement
Usually, S100 proteins in humans are measured with immunological assays using specific antibodies and ELISA formats. These assays can be commercially available [97][98] or developed in-house [99]. In the case of calprotectin, in addition to ELISAs, it can be measured with automated turbidimetric immunoassays, obtainable from various suppliers and run on clinical biochemistry analyzers [100][101], with the advantages that the automation can have in clinical routine settings, such as higher precision and sample throughput.
These proteins can be measured in different sample types such as serum [102], feces [103], saliva [98][104], urine [105], or bronchoalveolar lavage fluid (BALF) [106]. As an example, in a report that studied serum, saliva, and urine in systemic lupus erythematosus in humans, the measurement of S100A8 showed good diagnostic ability in all sample types [97].
High-performance liquid chromatography coupled with electrospray ionization mass spectrometry has been used to simultaneously analyze several S100 proteins and their posttranslational modifications, complexes, and isoforms. This technique was able to identify in saliva different isoforms of S100 proteins, such as four isoforms of S100A8/S100A9 and two isoforms of S100A12 [107][108]. In addition, top-down and bottom-up proteomics detected S100A8 and A9 in different circulating complexes and proteoforms, which discriminated between survivors and non-survivors in septic shock patients [109].
In animals, species-specific assays have been used in different species such as dogs for the measurement of S100A8/A9 [92] and S100A12 [110] and cats for S100A12 [111]. In addition, a human turbidimetric immunoassay has been validated for the measurement of calprotectin in the saliva of pigs [112] and horses [113], and also in feces of dogs and cats [114] and pigs [115]. Therefore, there is the possibility of using heterologous immunoassays for the measurement of calprotectin, as used for other analytes such as acute phase proteins [116], providing the necessary analytical validation has been undertaken.
4. General Applications in Humans
In humans, calgranulins have been widely studied, and they have been described as biomarkers of different diseases and conditions. Various reviews can be found in the literature about these proteins and their clinical applications [45][85][117][118][119]. Overall, calgranulins have been evaluated in a variety of human diseases.
4.1. Gastrointestinal Disease
The extravasated neutrophils, which can be found in abundant number in the gastrointestinal system of patients with active inflammatory bowel disease (IBD), are a rich source of released S100A8/A9 which is detectable in feces. Therefore, calprotectin in feces has been used as a biomarker in these patients, being a disease activity indicator [120][121]. Measurement of S100A8/S100A9 in feces is considered a reliable method to distinguish IBD patients from those without chronic intestinal inflammation and it is, together with serum levels of S100A8/S100A9, useful in monitoring inflammation in patients with Crohn’s disease or ulcerative colitis [122]. In addition, S100A12 has been found to be massively expressed in inflamed tissue from patients with active IBD, and its serum concentrations correlate well with disease activity in individual patients [123].
4.2. Inflammation and Sepsis
After bacterial infection, neutrophils, macrophages, and monocytes intensely express and secrete calgranulins, with the role of modulating the inflammatory response, leading to the induction of inflammatory cytokines and also to the release of reactive oxygen species. Although still not totally clarified, S100A8 and S100A9 can have antibacterial potential due to their ability to bind Zn [4]. Of relevance is that deficiency of calprotectin has been related to the progress of pneumonia in Staphylococcus aureus infection in mice [124]. In addition, high concentrations of S100A9 have been involved in phagocyte hyperresponsiveness in sepsis and inflammatory conditions that can result in enhanced survival from septic shock [125]. However, there is some controversy to this, since other reports indicated that calgranulin can facilitate bacterial growth [126].
In sepsis, values of calprotectin were reported to be higher than in non-septic inflammation [127] and also correlate with the severity of the disease [128]. In addition, high plasma values of S100A8/A9 and S100A12 at admission can indicate a higher risk of death in septic shock patients [129]. In general, it is considered that calprotectin measurement allows early diagnosis of sepsis on admission of patients to intensive care units (ICU), being a tool that can aid timely sepsis management, reducing mortality rates and avoiding unnecessary antibiotic treatment, thus improving antibiotic stewardship [45].
4.3. Immunomediated Diseases
In systemic lupus erythematosus (SLE), there are increases in calprotectin that correlated with the SLE disease activity index [130]. Similarly, in rheumatoid arthritis (RA) and psoriatic arthritis serum concentrations of S100A8/S100A9 are related to the inflammatory activity of arthritis, being superior to other biomarkers such as C-reactive protein and erythrocyte sedimentation rate [131][132]. Additionally, S100A12 serum concentrations correlate with disease activity in RA [133], and calgranulins are also at high concentrations in synovial fluid in patients with this disease [87].
In addition, in dermatomyositis, polymyositis, and inclusion body myositis there is an association between S100A8 and S100A9 expression (possibly produced by the infiltration of macrophages) and degeneration of myofibers [134].
4.4. Obesity and Endocrine Disorders
The S100A8/A9 complex is implicated in the pathophysiology of obesity-promoting macrophage-based inflammation. In addition, serum levels of S100A8/A9 and S100A12 correlate with insulin resistance/type 2 diabetes, metabolic risk score, and fat cell size [135]. Additionally, calprotectin in type 2 diabetes is related to the degree of microvascular alteration at the glomerular and retinal bed, being a potential biomarker for microcirculatory defects associated with this disease [136].
4.5. Other Diseases
Other diseases in which calgranulins are increased are cardiovascular, neoplastic, skin, and neurological disorders including traumatic brain injuries and chronic neurodegenerative disorders such as Alzheimer’s disease [85][117][137]. Of further interest, S100A9 has been shown to be involved in CD 36 signaling in platelets, which is reported to be a key signal in arterial thrombosis, but not for physiologic hemostasis. Therefore, immunization of mice with a S100A9 vaccine resulted in long-term inhibition of thrombus formation through inhibition of increased S100A9/CD36 signaling, without risk of bleeding or adverse autoimmune responses [138].
Overall, in humans, S100s are considered an important group of both molecular key players and biomarkers in the etiology, progression, manifestation, and therapy of many disorders in which they have been studied [6]. Therefore, it can be stated that their study and analysis can provide information about physiopathology and also their use as possible biomarkers of disease activity and treatment monitoring in a high number of clinical disorders.
5. Gastrointestinal Disease in Animals
The use of calprotectin for the evaluation of gastrointestinal disease is the best-known and most frequent application of S100 proteins in veterinary medicine, having been used in several different animal species.
5.1. Dogs
In dogs with idiopathic IBD, fecal calprotectin increases and then decreases after treatment, with a significant correlation of r = 0.60 between fecal calprotectin and canine inflammatory bowel disease activity index (CIBDAI) scores [139]. In addition, in another group of dogs with chronic enteropathy, fecal calprotectin was correlated (r = 0.27) with disease activity and showed a high correlation (r = 0.9) with fecal S100A12 [140].
5.2. Cats
In cats, the role of calgranulins in the pathogenesis of chronic inflammatory enteropathy and intestinal lymphoma has been suggested [141]. In addition, fecal S100A12 concentrations at the time of diagnosis were higher in cats with chronic inflammatory enteropathy (CIE) and alimentary lymphoma (LSA) than in healthy controls, but did not differ between cats with LSA and those with CIE [141].
5.3. Porcine
In pigs, calprotectin expression in jejunal mucosa was decreased after treatment and tended to reduce intestinal inflammation in weaned pigs challenged with enterotoxigenic Escherichia coli (E. coli) [142]. Furthermore, fecal calprotectin levels in pigs were increased following the development of colitis, but do not significantly change due to enteritis [115].
5.4. Equine
In horses, serum calprotectin concentration was increased in animals with systemic inflammation produced by large colon ischemia and reperfusion [143]. Calprotectin expression in the colon was also increased in a case of colon inflammation after black walnut extract administration, which is associated with a systemic inflammatory response [144]. In equine saliva, calprotectin had a higher concentration in horses with equine gastric ulcer syndrome (EGUS) compared with healthy horses, although the concentration did not allow differentiation of horses with EGUS from horses with similar clinical signs due to other gastrointestinal causes [113].
5.5. Ruminants
In bovines, increases in serum calprotectin concentrations have been detected in calves with diarrhea caused by coronavirus but not in diarrhea caused by Escherichia coli or healthy calves [145].
5.6. Avian
Calprotectin in the serum and feces of broiler chickens has been described with a potential for the detection of low-grade chronic intestinal inflammation, which has a negative impact on production by the decrease in nutrient absorption in this species [146].
6. Inflammation and Sepsis in Animals
The S100 proteins have been involved in the following inflammatory conditions in animals.
6.1. Glomerulonephritis
Serum concentrations of S100A8/A9 are increased in mice with glomerulonephritis, whereas mice deficient in this protein were protected from this disease. This protein can exert and amplify inflammation through its interaction with different renal cells, and its blockade could be a therapeutic target in glomerulonephritis [147].
6.2. Lung Inflammation
In a mouse model of tuberculosis, it was demonstrated that the exacerbated lung inflammation of this disease is dependent on S100A8/A9 protein; which induces the production of proinflammatory cytokines and neutrophilic accumulation. These authors indicate that targeting S100A8/A9 has the potential to decrease lung tissue damage in this pathology [148]. In addition, in calves with pneumonia, S100A9 was increased in serum due to the associated systemic inflammatory response and also in BALF in which was a useful biomarker of pulmonary inflammation and damage [149].
6.3. Liver Inflammation
Using a mouse model of malaria, it was demonstrated that S100A9 is one of the key molecules involved in liver inflammation that is associated with this disease [150].
6.4. Sepsis
In a pilot study, serum calprotectin and S100A12 were higher in dogs with sepsis and dogs with systemic inflammatory response syndrome (SIRS) compared to healthy controls. In this study, the values of these analytes at the time of hospital admission did not differentiate between dogs with sepsis and SIRS, although they showed a different evolution in both processes during the following 24–48 h [92]. In pigs, calprotectin in saliva has been recently found to be increased in experimentally induced sepsis by E. coli lipopolysaccharide (LPS) administration, with higher increases found than in the saliva of pigs observed to have a non-septic inflammation [112].
7. Immunomediated Diseases in Animals
Calgranulins are increased in various immunomediated diseases, both naturally occurring and experimentally induced.
7.1. Atopic Dermatitis
Dogs with this disease show significantly higher serum concentrations of S100A8 than healthy dogs [151]. Similar findings have been described in humans in whom S100A8 and S100A9 increase in expression in both injured skin and serum from patients with atopic dermatitis, with the expression correlating with disease severity. Interestingly, house dust mites triggered the expression of both proteins [152]. It is important to point out that many S100 proteins are expressed in the epidermis and keratinocytes in humans [137] and therefore they can have applications as biomarkers of skin disorders which may also be the case in animals.
7.2. Autoimmune Uveitis
Rats with experimentally induced autoimmune uveitis showed an increase in expression of S100A8 in the eye, and S100A8 blockade could be a therapeutic agent in this disease [153].
7.3. Arthritis Rheumatoid (RA)
In a mouse model in which osteoarthritis was induced, S100A8/A9 was elevated in the synovium [154]. Of relevance is that S100A89, released from the synovium upon inflammation in experimental synovitis in mice, is an important mediator of pain response in the knee during the acute phase of inflammation [155]. Furthermore, in mice with experimentally induced arthritis, serum levels of S100A8/A9 were significantly increased and correlated with macroscopic joint swelling and histological inflammation, while serum levels of pro-inflammatory cytokines did not correlate with joint swelling. In addition, early serum S100A8/A9 levels were prognostic for disease outcomes at a later stage [156].
8. Obesity and Endocrine Disorders in Animals
In a report on human and mice obesity, serum calprotectin correlated with the visceral and subcutaneous fat area and body mass index in men, and S100A8 and S100A9 were overexpressed in adipose tissue in mice. This indicates that possibly adipocytes can be involved in the production of these S100 proteins, which could be considered potential markers of subclinical inflammation associated with obesity [157].
In miniature Schnauzers with idiopathic hyperlipidemia, there was an increase in serum calprotectin and S100A12, with the increase in serum calprotectin being associated with hyperlipidemia [158]. In dogs with diabetes mellitus, S100A12 was one of the proteins that showed a higher expression in saliva compared to healthy dogs [159].
In addition, in some endocrinopathies such as diabetes mellitus, a rat model showed that an increase in calprotectin in serum can indicate complications such as ischemic colitis [160].
Overall, this is really an area in which additional studies are needed in order to elucidate the role of calgranulins in obesity, insulin resistance and endocrine diseases in animals, and their possible use as biomarkers.
References
- Moore, B.W. A Soluble Protein Characteristic of the Nervous System. Biochem. Biophys. Res. Commun. 1965, 19, 739–744.
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 Proteins in Health and Disease. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2020, 1867, 118677.
- Kazakov, A.S.; Deryusheva, E.I.; Permyakova, M.E.; Sokolov, A.S.; Rastrygina, V.A.; Uversky, V.N.; Permyakov, E.A.; Permyakov, S.E. Calcium-Bound S100P Protein Is a Promiscuous Binding Partner of the Four-Helical Cytokines. Int. J. Mol. Sci. 2022, 23, 12000.
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298.
- Donato, R. Functional Roles of S100 Proteins, Calcium-Binding Proteins of the EF-Hand Type. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 1999, 1450, 191–231.
- Pietzsch, J. S100 Proteins in Health and Disease. Amino Acids 2011, 41, 755–760.
- Heizmann, C.W. S100 Proteins Structure Functions and Pathology. Front. Biosci. 2002, 7, A846.
- Afanador, L.; Roltsch, E.A.; Holcomb, L.; Campbell, K.S.; Keeling, D.A.; Zhang, Y.; Zimmer, D.B. The Ca2+ Sensor S100A1 Modulates Neuroinflammation, Histopathology and Akt Activity in the PSAPP Alzheimer’s Disease Mouse Model. Cell Calcium 2014, 56, 68–80.
- Pleger, S.T.; Harris, D.M.; Shan, C.; Vinge, L.E.; Chuprun, J.K.; Berzins, B.; Pleger, W.; Druckman, C.; Völkers, M.; Heierhorst, J.; et al. Endothelial S100A1 Modulates Vascular Function via Nitric Oxide. Circ. Res. 2008, 102, 786–794.
- Eryilmaz, U.; Demirci, B.; Aksun, S.; Boyacioglu, M.; Akgullu, C.; Ilgenli, T.F.; Yalinkilinc, H.S.; Bilgen, M. S100A1 as a Potential Diagnostic Biomarker for Assessing Cardiotoxicity and Implications for the Chemotherapy of Certain Cancers. PLoS ONE 2015, 10, e0145418.
- Wright, N.T.; Cannon, B.R.; Zimmer, D.B.; Weber, D.J. S100A1: Structure, Function, and Therapeutic Potential. Curr. Chem. Biol. 2009, 3, 138–145.
- Kraus, C.; Rohde, D.; Weidenhammer, C.; Qiu, G.; Pleger, S.T.; Voelkers, M.; Boerries, M.; Remppis, A.; Katus, H.A.; Most, P. S100A1 in Cardiovascular Health and Disease: Closing the Gap between Basic Science and Clinical Therapy. J. Mol. Cell Cardiol 2009, 47, 445–455.
- Zhang, Q.; Xia, T.; Qi, C.; Du, J.; Ye, C. High Expression of S100A2 Predicts Poor Prognosis in Patients with Endometrial Carcinoma. BMC Cancer 2022, 22, 77.
- Chen, Y.; Wang, C.; Song, J.; Xu, R.; Ruze, R.; Zhao, Y. S100A2 Is a Prognostic Biomarker Involved in Immune Infiltration and Predict Immunotherapy Response in Pancreatic Cancer. Front. Immunol. 2021, 12, 758004.
- Wang, H.; Zhang, Z.; Li, R.; Ang, K.K.; Zhang, H.; Caraway, N.P.; Katz, R.L.; Jiang, F. Overexpression of S100A2 Protein as a Prognostic Marker for Patients with Stage I Non Small Cell Lung Cancer. Int. J. Cancer 2005, 116, 285–290.
- Yoshioka, M.; Sawada, Y.; Saito-Sasaki, N.; Yoshioka, H.; Hama, K.; Omoto, D.; Ohmori, S.; Okada, E.; Nakamura, M. High S100A2 Expression in Keratinocytes in Patients with Drug Eruption. Sci. Rep. 2021, 11, 5493.
- Sugino, H.; Sawada, Y. Influence of S100A2 in Human Diseases. Diagnostics 2022, 12, 1756.
- Liu, B.; Sun, W.-Y.; Zhi, C.-Y.; Lu, T.-C.; Gao, H.-M.; Zhou, J.-H.; Yan, W.-Q.; Gao, H.-C. Role of S100A3 in Human Colorectal Cancer and the Anticancer Effect of Cantharidinate. Exp. Ther. Med. 2013, 6, 1499–1503.
- Al-Mutairy, E.A.; Imtiaz, F.A.; Khalid, M.; Al Qattan, S.; Saleh, S.; Mahmoud, L.M.; Al-Saif, M.M.; Al-Haj, L.; Al-Enazi, A.; AlJebreen, A.M.; et al. An Atypical Pulmonary Fibrosis Is Associated with Co-Inheritance of Mutations in the Calcium Binding Protein Genes S100A3 and S100A13. Eur. Respir. J. 2019, 54, 1802041.
- Ganaie, A.A.; Mansini, A.P.; Hussain, T.; Rao, A.; Siddique, H.R.; Shabaneh, A.; Ferrari, M.G.; Murugan, P.; Klingelhöfer, J.; Wang, J.; et al. Anti-S100A4 Antibody Therapy Is Efficient in Treating Aggressive Prostate Cancer and Reversing Immunosuppression: Serum and Biopsy S100A4 as a Clinical Predictor. Mol. Cancer Ther. 2020, 19, 2598–2611.
- Oslejskova, L.; Grigorian, M.; Hulejova, H.; Vencovsky, J.; Pavelka, K.; Klingelhofer, J.; Gay, S.; Neidhart, M.; Brabcova, H.; Suchy, D.; et al. Metastasis-Inducing S100A4 Protein Is Associated with the Disease Activity of Rheumatoid Arthritis. Rheumatology 2009, 48, 1590–1594.
- Fei, F.; Qu, J.; Zhang, M.; Li, Y.; Zhang, S. S100A4 in Cancer Progression and Metastasis: A Systematic Review. Oncotarget 2017, 8, 73219–73239.
- Fei, F.; Qu, J.; Li, C.; Wang, X.; Li, Y.; Zhang, S. Role of Metastasis-Induced Protein S100A4 in Human Non-Tumor Pathophysiologies. Cell BioSci. 2017, 7, 64.
- Barraclough, R. Calcium-Binding Protein S100A4 in Health and Disease. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 1998, 1448, 190–199.
- Yoshimura, H.; Otsuka, A.; Michishita, M.; Yamamoto, M.; Ashizawa, M.; Zushi, M.; Moriya, M.; Azakami, D.; Ochiai, K.; Matsuda, Y.; et al. Expression and Roles of S100A4 in Anaplastic Cells of Canine Mammary Carcinomas. Vet. Pathol. 2019, 56, 389–398.
- Grandi, F.; Rocha, R.M.; Miot, H.A.; Cogliati, B.; Rocha, N.S. Immunoexpression of S100A4 in Canine Skin Melanomas and Correlation with Histopathological Parameters. Vet. Q. 2014, 34, 98–104.
- Yao, R.; Lopez-Beltran, A.; Maclennan, G.T.; Montironi, R.; Eble, J.N.; Cheng, L. Expression of S100 Protein Family Members in the Pathogenesis of Bladder Tumors. Anticancer Res. 2007, 27, 3051–3058.
- Hancq, S.; Salmon, I.; Brotchi, J.; De Witte, O.; Gabius, H.-J.; Heizmann, C.W.; Kiss, R.; Decaestecker, C. S100A5: A Marker of Recurrence in WHO Grade I Meningiomas. Neuropathol. Appl. Neurobiol. 2004, 30, 178–187.
- Li, Y.; Wagner, E.R.; Yan, Z.; Wang, Z.; Luther, G.; Jiang, W.; Ye, J.; Wei, Q.; Wang, J.; Zhao, L.; et al. The Calcium-Binding Protein S100A6 Accelerates Human Osteosarcoma Growth by Promoting Cell Proliferation and Inhibiting Osteogenic Differentiation. Cell. Physiol. Biochem. 2015, 37, 2375–2392.
- Nedjadi, T.; Kitteringham, N.; Campbell, F.; Jenkins, R.E.; Park, B.K.; Navarro, P.; Ashcroft, F.; Tepikin, A.; Neoptolemos, J.P.; Costello, E. S100A6 Binds to Annexin 2 in Pancreatic Cancer Cells and Promotes Pancreatic Cancer Cell Motility. Br. J. Cancer 2009, 101, 1145–1154.
- Boom, A.; Pochet, R.; Authelet, M.; Pradier, L.; Borghgraef, P.; Van Leuven, F.; Heizmann, C.W.; Brion, J.-P. Astrocytic Calcium/Zinc Binding Protein S100A6 over Expression in Alzheimer’s Disease and in PS1/APP Transgenic Mice Models. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2004, 1742, 161–168.
- Mofid, A.; Newman, N.S.; Lee, P.J.H.; Abbasi, C.; Matkar, P.N.; Rudenko, D.; Kuliszewski, M.A.; Chen, H.H.; Afrasiabi, K.; Tsoporis, J.N.; et al. Cardiac Overexpression of S100A6 Attenuates Cardiomyocyte Apoptosis and Reduces Infarct Size After Myocardial Ischemia-Reperfusion. J. Am. Heart Assoc. 2017, 6, e004738.
- Filipek, A.; Leśniak, W. S100A6 and Its Brain Ligands in Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 3979.
- Zhou, X.; Wang, P.; Michal, J.J.; Wang, Y.; Zhao, J.; Jiang, Z.; Liu, B. Molecular Characterization of the Porcine S100A6 Gene and Analysis of Its Expression in Pigs Infected with Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (HP-PRRSV). J. Appl. Genet. 2015, 56, 355–363.
- Anturaniemi, J.; Zaldívar-López, S.; Savelkoul, H.F.J.; Elo, K.; Hielm-Björkman, A. The Effect of Atopic Dermatitis and Diet on the Skin Transcriptome in Staffordshire Bull Terriers. Front. Vet. Sci. 2020, 7, 552251.
- Zhou, L.-J.; Peng, J.; Chen, M.; Yao, L.-J.; Zou, W.-H.; He, C.Y.; Peng, H.-J. Toxoplasma Gondii SAG1 Targeting Host Cell S100A6 for Parasite Invasion and Host Immunity. iScience 2021, 24, 103514.
- Ekman, A.; Vegfors, J.; Eding, C.; Enerbäck, C. Overexpression of Psoriasin (S100A7) Contributes to Dysregulated Differentiation in Psoriasis. Acta Derm. Venereol. 2017, 97, 441–448.
- Zhang, H.; Zhao, Q.; Chen, Y.; Wang, Y.; Gao, S.; Mao, Y.; Li, M.; Peng, A.; He, D.; Xiao, X. Selective Expression of S100A7 in Lung Squamous Cell Carcinomas and Large Cell Carcinomas but Not in Adenocarcinomas and Small Cell Carcinomas. Thorax 2008, 63, 352–359.
- Qin, W.; Ho, L.; Wang, J.; Peskind, E.; Pasinetti, G.M. S100A7, a Novel Alzheimer’s Disease Biomarker with Non-Amyloidogenic α-Secretase Activity Acts via Selective Promotion of ADAM-10. PLoS ONE 2009, 4, e4183.
- Rangaraj, A.; Ye, L.; Sanders, A.J.; Price, P.E.; Harding, K.G.; Jiang, W.G. Molecular and Cellular Impact of Psoriasin (S100A7) on the Healing of Human Wounds. Exp. Ther. Med. 2017, 13, 2151–2160.
- Lyu, X.-J.; Li, H.-Z.; Ma, X.; Li, X.-T.; Gao, Y.; Ni, D.; Shen, D.-L.; Gu, L.-Y.; Wang, B.-J.; Zhang, Y.; et al. Elevated S100A6 (Calcyclin) Enhances Tumorigenesis and Suppresses CXCL14-Induced Apoptosis in Clear Cell Renal Cell Carcinoma. Oncotarget 2015, 6, 6656–6669.
- Fan, M.; Miao, Y.; Yan, Y.; Zhu, K.; Zhao, X.; Pan, M.; Ma, B.; Wei, Q. C-Type Natriuretic Peptide Regulates the Expression and Secretion of Antibacterial Peptide S100A7 in Goat Mammary Gland Through PKG/JNK/c-Jun Signaling Pathway. Front. Vet. Sci. 2022, 2, 822165.
- Brookes, M.J.; Whitehead, S.; Gaya, D.R.; Hawthorne, A.B. Practical Guidance on the Use of Faecal Calprotectin. Frontline Gastroenterol. 2018, 9, 87–91.
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From Biomarker to Biological Function. Gut 2021, 70, 1978–1988.
- Gao, R.-Y.; Jia, H.-M.; Han, Y.-Z.; Qian, B.-S.; You, P.; Zhang, X.-K.; Li, W.-X.; Huang, L.-F. Calprotectin as a Diagnostic Marker for Sepsis: A Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 1045636.
- Yanagi, H.; Watanabe, T.; Nishimura, T.; Hayashi, T.; Kono, S.; Tsuchida, H.; Hirata, M.; Kijima, Y.; Takao, S.; Okada, S.; et al. Upregulation of S100A10 in Metastasized Breast Cancer Stem Cells. Cancer Sci. 2020, 111, 4359–4370.
- Hedhli, N.; Falcone, D.J.; Huang, B.; Cesarman-Maus, G.; Kraemer, R.; Zhai, H.; Tsirka, S.E.; Santambrogio, L.; Hajjar, K.A. The Annexin A2/S100A10 System in Health and Disease: Emerging Paradigms. J. Biomed. Biotechnol. 2012, 2012, 406273.
- Chen, M.X.; Oh, Y.-S.; Kim, Y. S100A10 and Its Binding Partners in Depression and Antidepressant Actions. Front. Mol. NeuroSci. 2022, 15, 953066.
- Madureira, P.A.; O’Connell, P.A.; Surette, A.P.; Miller, V.A.; Waisman, D.M. The Biochemistry and Regulation of S100A10: A Multifunctional Plasminogen Receptor Involved in Oncogenesis. J. Biomed. Biotechnol. 2012, 2012, 353687.
- Andrés Cerezo, L.; Šumová, B.; Prajzlerová, K.; Veigl, D.; Damgaard, D.; Nielsen, C.H.; Pavelka, K.; Vencovský, J.; Šenolt, L. Calgizzarin (S100A11): A Novel Inflammatory Mediator Associated with Disease Activity of Rheumatoid Arthritis. Arthritis Res. Ther. 2017, 19, 79.
- Cerezo, L.A.; Hulejová, H.; Šumová, B.; Kropáčková, T.; Kryštůfková, O.; Klein, M.; Mann, H.F.; Zámečník, J.; Pecha, O.; Pavelka, K.; et al. Pro-Inflammatory S100A11 Is Elevated in Inflammatory Myopathies and Reflects Disease Activity and Extramuscular Manifestations in Myositis. Cytokine 2019, 116, 13–20.
- Li, Y.; Zhang, J. Expression of S100A11 Is a Prognostic Factor for Disease-Free Survival and Overall Survival in Patients with High-Grade Serous Ovarian Cancer. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 110–116.
- Zhang, L.; Zhu, T.; Miao, H.; Liang, B. The Calcium Binding Protein S100A11 and Its Roles in Diseases. Front. Cell Dev. Biol. 2021, 9, 693262.
- Carvalho, A.; Lu, J.; Francis, J.D.; Moore, R.E.; Haley, K.P.; Doster, R.S.; Townsend, S.D.; Johnson, J.G.; Damo, S.M.; Gaddy, J.A. S100A12 in Digestive Diseases and Health: A Scoping Review. Gastroenterol. Res. Pract. 2020, 2020, 2868373.
- Oesterle, A.; Bowman, M.A.H. S100A12 and the S100/Calgranulins. Arter. Thromb. Vasc. Biol. 2015, 35, 2496–2507.
- Meijer, B.; Gearry, R.B.; Day, A.S. The Role of S100A12 as a Systemic Marker of Inflammation. Int. J. Inflamm. 2012, 2012, 907078.
- Miao, S.; Qiu, T.; Zhao, Y.; Wang, H.; Sun, X.; Wang, Y.; Xuan, Y.; Qin, Y.; Jiao, W. Overexpression of S100A13 Protein Is Associated with Tumor Angiogenesis and Poor Survival in Patients with Early-Stage Non-Small Cell Lung Cancer. Thorac. Cancer 2018, 9, 1136–1144.
- Massi, D.; Landriscina, M.; Piscazzi, A.; Cosci, E.; Kirov, A.; Paglierani, M.; Di Serio, C.; Mourmouras, V.; Fumagalli, S.; Biagioli, M.; et al. S100A13 Is a New Angiogenic Marker in Human Melanoma. Mod. Pathol. 2010, 23, 804–813.
- Zhong, J.; Liu, C.; Chen, Y.; Zhang, Q.; Yang, J.; Kang, X.; Chen, S.-R.; Wen, G.; Zu, X.; Cao, R. The Association between S100A13 and HMGA1 in the Modulation of Thyroid Cancer Proliferation and Invasion. J. Transl. Med. 2016, 14, 80.
- Tanaka, M.; Ichikawa-Tomikawa, N.; Shishito, N.; Nishiura, K.; Miura, T.; Hozumi, A.; Chiba, H.; Yoshida, S.; Ohtake, T.; Sugino, T. Co-Expression of S100A14 and S100A16 Correlates with a Poor Prognosis in Human Breast Cancer and Promotes Cancer Cell Invasion. BMC Cancer 2015, 15, 53.
- Zhu, M.; Wang, H.; Cui, J.; Li, W.; An, G.; Pan, Y.; Zhang, Q.; Xing, R.; Lu, Y. Calcium-Binding Protein S100A14 Induces Differentiation and Suppresses Metastasis in Gastric Cancer. Cell Death Dis. 2017, 8, e2938.
- Mohamed, B.F.; Serag, W.M.; Abdelal, R.M.; Elsergany, H.F. S100A14 Protein as Diagnostic and Prognostic Marker in Hepatocellular Carcinoma. Egypt. Liver J. 2019, 9, 9.
- Basnet, S.; Sharma, S.; Costea, D.E.; Sapkota, D. Expression Profile and Functional Role of S100A14 in Human Cancer. Oncotarget 2019, 10, 2996–3012.
- Batycka-Baran, A.; Hattinger, E.; Zwicker, S.; Summer, B.; Howard, O.M.Z.; Thomas, P.; Szepietowski, J.C.; Ruzicka, T.; Prinz, J.C.; Wolf, R. Leukocyte-Derived Koebnerisin (S100A15) and Psoriasin (S100A7) Are Systemic Mediators of Inflammation in Psoriasis. J. Dermatol. Sci. 2015, 79, 214–221.
- Batycka-Baran, A.; Matusiak, Ł.; Nowicka-Suszko, D.; Szepietowski, J.C.; Baran, W. Increased Serum Levels of S100A4 and S100A15 in Individuals Suffering from Hidradenitis Suppurativa. J. Clin. Med. 2021, 10, 5320.
- Chen, Y.-C.; Lin, M.-C.; Hsiao, C.-C.; Zheng, Y.-X.; Chen, K.-D.; Sung, M.-T.; Chen, C.-J.; Wang, T.-Y.; Lin, Y.-Y.; Chang, H.-C.; et al. Increased S100A15 Expression and Decreased DNA Methylation of Its Gene Promoter Are Involved in High Metastasis Potential and Poor Outcome of Lung Adenocarcinoma. Oncotarget 2017, 8, 45710–45724.
- Katono, K.; Sato, Y.; Kobayashi, M.; Nagashio, R.; Ryuge, S.; Igawa, S.; Ichinoe, M.; Murakumo, Y.; Saegusa, M.; Masuda, N. S100A16, a Promising Candidate as a Prognostic Marker for Platinum-Based Adjuvant Chemotherapy in Resected Lung Adenocarcinoma. Onco Targets Ther. 2017, 10, 5273–5279.
- You, X.; Li, M.; Cai, H.; Zhang, W.; Hong, Y.; Gao, W.; Liu, Y.; Liang, X.; Wu, T.; Chen, F.; et al. Calcium Binding Protein S100A16 Expedites Proliferation, Invasion and Epithelial-Mesenchymal Transition Process in Gastric Cancer. Front. Cell Dev. Biol. 2021, 9, 736929.
- Liu, Y.; Zhang, R.; Xin, J.; Sun, Y.; Li, J.; Wei, D.; Zhao, A.Z. Identification of S100A16 as a Novel Adipogenesis Promoting Factor in 3T3-L1 Cells. Endocrinology 2011, 152, 903–911.
- Li, B.; Zhu, W.; Shi, D.; Che, H.; Lyu, Q.; Jiang, B. New Progress with Calcium-Binding Protein S100A16 in Digestive System Disease. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 263–272.
- Michetti, F.; D’Ambrosi, N.; Toesca, A.; Puglisi, M.A.; Serrano, A.; Marchese, E.; Corvino, V.; Geloso, M.C. The S100B Story: From Biomarker to Active Factor in Neural Injury. J. Neurochem. 2019, 148, 168–187.
- Yardan, T.; Erenler, A.K.; Baydin, A.; Aydin, K.; Cokluk, C. Usefulness of S100B Protein in Neurological Disorders. J. Pak. Med. Assoc. 2011, 61, 276–281.
- Thelin, E.P.; Nelson, D.W.; Bellander, B.-M. A Review of the Clinical Utility of Serum S100B Protein Levels in the Assessment of Traumatic Brain Injury. Acta Neurochir. 2017, 159, 209–225.
- Schulte, S.; Podlog, L.W.; Hamson-Utley, J.J.; Strathmann, F.G.; Strüder, H.K. A Systematic Review of the Biomarker S100B: Implications for Sport-Related Concussion Management. J. Athl. Train. 2014, 49, 830–850.
- Gazzolo, D.; Galvano, F.; Frigiola, A.; Gagliardi, L.; Ciotti, S.; Bognanno, M.; Iacopino, A.M.; Nigro, F.; Tina, G.L.; Cavallaro, D.; et al. S100B Milk Concentration in Mammalian Species. Front. Biosci. 2009, E1, e51.
- Kojima, Y.; Chiba, S.; Horiuchi, N.; Kobayashi, Y.; Inokuma, H. Evaluation of S100B in Cerebrospinal Fluid as a Potential Biomarker for Neurological Diseases in Calves. J. Vet. Med. Sci. 2015, 77, 605–607.
- Mashima, H.; Takahashi, K.; Sekine, M.; Matsumoto, S.; Asano, T.; Uehara, T.; Fujiwara, J.; Otake, H.; Ishii, T.; Yoshikawa, S.; et al. The Role of Calcium-Binding Protein S100g (CalbindinD-9K) and Annexin A10 in Acute Pancreatitis. Biochem. Biophys. Res. Commun. 2020, 526, 692–698.
- Zhu, L.; Ito, T.; Nakahara, T.; Nagae, K.; Fuyuno, Y.; Nakao, M.; Akahoshi, M.; Nakagawa, R.; Tu, Y.; Uchi, H.; et al. Upregulation of S100P, Receptor for Advanced Glycation End Products and Ezrin in Malignant Melanoma. J. Dermatol. 2013, 40, 973–979.
- Basu, G.D.; Azorsa, D.O.; Kiefer, J.A.; Rojas, A.M.; Tuzmen, S.; Barrett, M.T.; Trent, J.M.; Kallioniemi, O.; Mousses, S. Functional Evidence Implicating S100P in Prostate Cancer Progression. Int. J. Cancer 2008, 123, 330–339.
- Tóthová, V.; Gibadulinová, A. S100P, a Peculiar Member of S100 Family of Calcium-Binding Proteins Implicated in Cancer. Acta Virol. 2013, 57, 238–246.
- Zhang, C.; Yao, R.; Chen, J.; Zou, Q.; Zeng, L. S100 Family Members: Potential Therapeutic Target in Patients with Hepatocellular Carcinoma. Medicine 2021, 100, e24135.
- Trzeciak, M.; Sakowicz-Burkiewicz, M.; Wesserling, M.; Gleń, J.; Dobaczewska, D.; Bandurski, T.; Nowicki, R.; Pawelczyk, T. Altered Expression of Genes Encoding Cornulin and Repetin in Atopic Dermatitis. Int. Arch. Allergy Immunol. 2017, 172, 11–19.
- Drislane, C.; Irvine, A.D. The Role of Filaggrin in Atopic Dermatitis and Allergic Disease. Ann. Allergy Asthma Immunol. 2020, 124, 36–43.
- Lee, J.-B.L.S.-C. Expression of Trichohyalin in Dermatological Disorders: A Comparative Study with Involucrin and Filaggrin by Immunohistochemical Staining. Acta Derm.-Venereol. 1999, 79, 122–126.
- Sedaghat, F.; Notopoulos, A. S100 Protein Family and Its Application in Clinical Practice. Hippokratia 2008, 12, 198–204.
- Zimmer, D.B.; Eubanks, J.O.; Ramakrishnan, D.; Criscitiello, M.F. Evolution of the S100 Family of Calcium Sensor Proteins. Cell Calcium 2013, 53, 170–179.
- Foell, D.; Wittkowski, H.; Vogl, T.; Roth, J. S100 Proteins Expressed in Phagocytes: A Novel Group of Damage-Associated Molecular Pattern Molecules. J. Leukoc. Biol. 2007, 81, 28–37.
- Xia, C.; Braunstein, Z.; Toomey, A.C.; Zhong, J.; Rao, X. S100 Proteins As an Important Regulator of Macrophage Inflammation. Front. Immunol. 2018, 8, 1908.
- Holzinger, D.; Foell, D.; Kessel, C. The Role of S100 Proteins in the Pathogenesis and Monitoring of Autoinflammatory Diseases. Mol. Cell. Pediatr. 2018, 5, 7.
- Cerón, J.J. Acute Phase Proteins, Saliva and Education in Laboratory Science: An Update and Some Reflections. BMC Vet. Res. 2019, 15, 197.
- Chen, G.Y.; Nuñez, G. Sterile Inflammation: Sensing and Reacting to Damage. Nat. Rev. Immunol. 2010, 10, 826–837.
- Thames, B.E.; Barr, J.W.; Suchodolski, J.S.; Steiner, J.M.; Heilmann, R.M. Prospective Evaluation of S100A12 and S100A8/A9 (Calprotectin) in Dogs with Sepsis or the Systemic Inflammatory Response Syndrome. J. Vet. Diagn. Investig. 2019, 31, 645–651.
- Stern, D.; Yan, S.D.; Yan, S.F.; Schmidt, A.M. Receptor for Advanced Glycation Endproducts: A Multiligand Receptor Magnifying Cell Stress in Diverse Pathologic Settings. Adv. Drug Deliv. Rev. 2002, 54, 1615–1625.
- Lotze, M.T.; Tracey, K.J. High-Mobility Group Box 1 Protein (HMGB1): Nuclear Weapon in the Immune Arsenal. Nat. Rev. Immunol. 2005, 5, 331–342.
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE Mediates a Novel Proinflammatory Axis. Cell 1999, 97, 889–901.
- Kazakov, A.S.; Sokolov, A.S.; Permyakova, M.E.; Litus, E.A.; Uversky, V.N.; Permyakov, E.A.; Permyakov, S.E. Specific Cytokines of Interleukin-6 Family Interact with S100 Proteins. Cell Calcium 2022, 101, 102520.
- Kim, J.-W.; Jung, J.-Y.; Lee, S.-W.; Baek, W.-Y.; Kim, H.-A.; Suh, C.-H. S100A8 in Serum, Urine, and Saliva as a Potential Biomarker for Systemic Lupus Erythematosus. Front. Immunol. 2022, 13, 886209.
- Majster, M.; Almer, S.; Boström, E.A. Salivary Calprotectin Is Elevated in Patients with Active Inflammatory Bowel Disease. Arch. Oral Biol. 2019, 107, 104528.
- Leach, S.T.; Yang, Z.; Messina, I.; Song, C.; Geczy, C.L.; Cunningham, A.M.; Day, A.S. Serum and Mucosal S100 Proteins, Calprotectin (S100A8/S100A9) and S100A12, Are Elevated at Diagnosis in Children with Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2007, 42, 1321–1331.
- Åsberg, A.; Løfblad, L.; Felic, A.; Hov, G.G. Measuring Calprotectin in Plasma and Blood with a Fully Automated Turbidimetric Assay. Scand. J. Clin. Lab. Investig. 2019, 79, 50–57.
- Bourgonje, A.R.; van den Berg, E.H.; Kieneker, L.M.; Nilsen, T.; Hidden, C.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F.; van Goor, H.; Abdulle, A.E. Plasma Calprotectin Levels Associate with Suspected Metabolic-Associated Fatty Liver Disease and All-Cause Mortality in the General Population. Int. J. Mol. Sci. 2022, 23, 15708.
- Bartáková, E.; Štefan, M.; Stráníková, A.; Pospíšilová, L.; Arientová, S.; Beran, O.; Blahutová, M.; Máca, J.; Holub, M. Calprotectin and Calgranulin C Serum Levels in Bacterial Sepsis. Diagn. Microbiol. Infect. Dis. 2019, 93, 219–226.
- Pathirana, W.G.W.; Chubb, S.P.; Gillett, M.J.; Vasikaran, S.D. Faecal Calprotectin. Clin. Biochem. Rev. 2018, 39, 77–90.
- Holmström, S.B.; Lira-Junior, R.; Zwicker, S.; Majster, M.; Gustafsson, A.; Åkerman, S.; Klinge, B.; Svensson, M.; Boström, E.A. MMP-12 and S100s in Saliva Reflect Different Aspects of Periodontal Inflammation. Cytokine 2019, 113, 155–161.
- Florio, P.; Marinoni, E.; Di Iorio, R.; Bashir, M.; Ciotti, S.; Sacchi, R.; Bruschettini, M.; Lituania, M.; Serra, G.; Michetti, F.; et al. Urinary S100B Protein Concentrations Are Increased in Intrauterine Growth-Retarded Newborns. Pediatrics 2006, 118, e747–e754.
- Hesselstrand, R.; Wildt, M.; Bozovic, G.; Andersson-Sjöland, A.; Andréasson, K.; Scheja, A.; Westergren-Thorsson, G.; Bjermer, L.; Wuttge, D.M. Biomarkers from Bronchoalveolar Lavage Fluid in Systemic Sclerosis Patients with Interstitial Lung Disease Relate to Severity of Lung Fibrosis. Respir. Med. 2013, 107, 1079–1086.
- Castagnola, M.; Cabras, T.; Iavarone, F.; Fanali, C.; Messana, I. Detection of Ca2+-Binding S100 Proteins in Human Saliva by HPLC-ESI-MS. In Calcium-Binding Proteins and RAGE; Humana Press: Totowa, NJ, USA, 2013; pp. 357–371.
- Castagnola, M.; Inzitari, R.; Fanali, C.; Iavarone, F.; Vitali, A.; Desiderio, C.; Vento, G.; Tirone, C.; Romagnoli, C.; Cabras, T.; et al. The Surprising Composition of the Salivary Proteome of Preterm Human Newborn. Mol. Cell. Proteom. 2011, 10, M110.003467.
- Dubois, C.; Payen, D.; Simon, S.; Junot, C.; Fenaille, F.; Morel, N.; Becher, F. Top-Down and Bottom-Up Proteomics of Circulating S100A8/S100A9 in Plasma of Septic Shock Patients. J. Proteome Res. 2020, 19, 914–925.
- Heilmann, R.M.; Cranford, S.M.; Ambrus, A.; Grützner, N.; Schellenberg, S.; Ruaux, C.G.; Suchodolski, J.S.; Steiner, J.M. Validation of an Enzyme-Linked Immunosorbent Assay (ELISA) for the Measurement of Canine S100A12. Vet. Clin. Pathol. 2016, 45, 135–147.
- Bridges, C.S.; Grützner, N.; Suchodolski, J.S.; Steiner, J.M.; Heilmann, R.M. Analytical Validation of an Enzyme-linked Immunosorbent Assay for the Quantification of S100A12 in the Serum and Feces of Cats. Vet. Clin. Pathol. 2019, 48, 754–761.
- López-Martínez, M.J.; Martínez-Subiela, S.; Cerón, J.J.; Ortín-Bustillo, A.; Ramis, G.; López-Arjona, M.; Martínez-Miró, S.; Manzanilla, E.G.; Eckersall, P.D.; Tecles, F.; et al. Measurement of Calprotectin (S100A8/A9) in the Saliva of Pigs: Validation Data of A Commercially Available Automated Assay and Changes in Sepsis, Inflammation, and Stress. Animals 2023, 13, 1190.
- Muñoz-Prieto, A.; Contreras-Aguilar, M.D.; Cerón, J.J.; de la Peña, I.A.; Martín-Cuervo, M.; Eckersall, P.D.; Henriksen, I.-M.H.; Tecles, F.; Hansen, S. Changes in Calprotectin (S100A8-A9) and Aldolase in the Saliva of Horses with Equine Gastric Ulcer Syndrome. Animals 2023, 13, 1367.
- Enderle, L.L.; Köller, G.; Heilmann, R.M. Verification of the FCAL Turbo Immunoturbidimetric Assay for Measurement of the Fecal Calprotectin Concentration in Dogs and Cats. J. Vet. Diagn. Investig. 2022, 34, 813–824.
- Barbosa, J.A.; Rodrigues, L.A.; Columbus, D.A.; Aguirre, J.C.P.; Harding, J.C.S.; Cantarelli, V.S.; Costa, M.d.O. Experimental Infectious Challenge in Pigs Leads to Elevated Fecal Calprotectin Levels Following Colitis, but Not Enteritis. Proc. Health Manag. 2021, 7, 48.
- Muñoz-Prieto, A.; Tvarijonaviciute, A.; Escribano, D.; Martínez-Subiela, S.; Cerón, J.J. Use of Heterologous Immunoassays for Quantification of Serum Proteins: The Case of Canine C-Reactive Protein. PLoS ONE 2017, 12, e0172188.
- Heizmann, C.W. S100 Proteins: Diagnostic and Prognostic Biomarkers in Laboratory Medicine. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2019, 1866, 1197–1206.
- Manolakis, A.C.; Kapsoritakis, A.N.; Tiaka, E.K.; Potamianos, S.P. Calprotectin, Calgranulin C, and Other Members of the S100 Protein Family in Inflammatory Bowel Disease. Dig. Dis Sci. 2011, 56, 1601–1611.
- Manfredi, M.; Van Hoovels, L.; Benucci, M.; De Luca, R.; Coccia, C.; Bernardini, P.; Russo, E.; Amedei, A.; Guiducci, S.; Grossi, V.; et al. Circulating Calprotectin (CCLP) in Autoimmune Diseases. Autoimmun. Rev. 2023, 22, 103295.
- Pruenster, M.; Vogl, T.; Roth, J.; Sperandio, M. S100A8/A9: From Basic Science to Clinical Application. Pharmacol. Ther. 2016, 167, 120–131.
- Ikhtaire, S.; Shajib, M.S.; Reinisch, W.; Khan, W.I. Fecal Calprotectin: Its Scope and Utility in the Management of Inflammatory Bowel Disease. J. Gastroenterol. 2016, 51, 434–446.
- Boehm, D.; Krzystek-Korpacka, M.; Neubauer, K.; Matusiewicz, M.; Berdowska, I.; Zielinski, B.; Paradowski, L.; Gamian, A. Paraoxonase-1 Status in Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel. Dis. 2009, 15, 93–99.
- Foell, D.; Kucharzik, T.; Kraft, M.; Vogl, T.; Sorg, C.; Domschke, W.; Roth, J. Neutrophil Derived Human S100A12 (EN-RAGE) Is Strongly Expressed during Chronic Active Inflammatory Bowel Disease. Gut 2003, 52, 847–853.
- Achouiti, A.; Vogl, T.; Van der Meer, A.J.; Stroo, I.; Florquin, S.; de Boer, O.J.; Roth, J.; Zeerleder, S.; van’t Veer, C.; de Vos, A.F.; et al. Myeloid-Related Protein-14 Deficiency Promotes Inflammation in Staphylococcal Pneumonia. Eur. Respir. J. 2015, 46, 464–473.
- Austermann, J.; Friesenhagen, J.; Fassl, S.K.; Ortkras, T.; Burgmann, J.; Barczyk-Kahlert, K.; Faist, E.; Zedler, S.; Pirr, S.; Rohde, C.; et al. Alarmins MRP8 and MRP14 Induce Stress Tolerance in Phagocytes under Sterile Inflammatory Conditions. Cell Rep. 2014, 9, 2112–2123.
- Achouiti, A.; Vogl, T.; Endeman, H.; Mortensen, B.L.; Laterre, P.-F.; Wittebole, X.; van Zoelen, M.A.D.; Zhang, Y.; Hoogerwerf, J.J.; Florquin, S.; et al. Myeloid-Related Protein-8/14 Facilitates Bacterial Growth during Pneumococcal Pneumonia. Thorax 2014, 69, 1034–1042.
- Simm, M.; Söderberg, E.; Larsson, A.; Castegren, M.; Nilsen, T.; Eriksson, M.; Lipcsey, M. Performance of Plasma Calprotectin as a Biomarker of Early Sepsis: A Pilot Study. Biomark Med. 2016, 10, 811–818.
- van Zoelen, M.A.D.; Vogl, T.; Foell, D.; Van Veen, S.Q.; van Till, J.W.O.; Florquin, S.; Tanck, M.W.; Wittebole, X.; Laterre, P.-F.; Boermeester, M.A.; et al. Expression and Role of Myeloid-Related Protein-14 in Clinical and Experimental Sepsis. Am. J. Respir. Crit. Care Med. 2009, 180, 1098–1106.
- Dubois, C.; Marcé, D.; Faivre, V.; Lukaszewicz, A.-C.; Junot, C.; Fenaille, F.; Simon, S.; Becher, F.; Morel, N.; Payen, D. High Plasma Level of S100A8/S100A9 and S100A12 at Admission Indicates a Higher Risk of Death in Septic Shock Patients. Sci. Rep. 2019, 9, 15660.
- Haga, H.-J.; Brun, J.G.; Berntzen, H.B.; Cervera, R.; Khamashta, M.; Hughes, G.R.V. Calprotectin in Patients with Systemic Lupus Erythematosus: Relation to Clinical and Laboratory Parameters of Disease Activity. Lupus 1993, 2, 47–50.
- Brun, J.G.; Jonsson, R.; Haga, H.J. Measurement of Plasma Calprotectin as an Indicator of Arthritis and Disease Activity in Patients with Inflammatory Rheumatic Diseases. J. Rheumatol. 1994, 21, 733–738.
- Kane, D.; Roth, J.; Frosch, M.; Vogl, T.; Bresnihan, B.; FitzGerald, O. Increased Perivascular Synovial Membrane Expression of Myeloid-Related Proteins in Psoriatic Arthritis. Arthritis Rheum. 2003, 48, 1676–1685.
- Foell, D.; Kane, D.; Bresnihan, B.; Vogl, T.; Nacken, W.; Sorg, C.; FitzGerald, O.; Roth, J. Expression of the Pro-Inflammatory Protein S100A12 (EN-RAGE) in Rheumatoid and Psoriatic Arthritis. Rheumatology 2003, 42, 1383–1389.
- Seeliger, S.; Vogl, T.; Engels, I.H.; Schröder, J.M.; Sorg, C.; Sunderkötter, C.; Roth, J. Expression of Calcium-Binding Proteins MRP8 and MRP14 in Inflammatory Muscle Diseases. Am. J. Pathol. 2003, 163, 947–956.
- Riuzzi, F.; Chiappalupi, S.; Arcuri, C.; Giambanco, I.; Sorci, G.; Donato, R. S100 Proteins in Obesity: Liaisons Dangereuses. Cell. Mol. Life Sci. 2020, 77, 129–147.
- Burkhardt, K.; Schwarz, S.; Pan, C.; Stelter, F.; Kotliar, K.; Von Eynatten, M.; Sollinger, D.; Lanzl, I.; Heemann, U.; Baumann, M. Myeloid-Related Protein 8/14 Complex Describes Microcirculatory Alterations in Patients with Type 2 Diabetes and Nephropathy. Cardiovasc. Diabetol. 2009, 8, 10.
- Büchau, A.S.; Hassan, M.; Kukova, G.; Lewerenz, V.; Kellermann, S.; Würthner, J.U.; Wolf, R.; Walz, M.; Gallo, R.L.; Ruzicka, T. S100A15, an Antimicrobial Protein of the Skin: Regulation by E. coli through Toll-Like Receptor 4. J. Investig. Dermatol. 2007, 127, 2596–2604.
- Kawano, T.; Shimamura, M.; Nakagami, H.; Iso, T.; Koriyama, H.; Takeda, S.; Baba, K.; Sasaki, T.; Sakaguchi, M.; Morishita, R.; et al. Therapeutic Vaccine Against S100A9 (S100 Calcium-Binding Protein A9) Inhibits Thrombosis Without Increasing the Risk of Bleeding in Ischemic Stroke in Mice. Hypertension 2018, 72, 1355–1364.
- Otoni, C.C.; Heilmann, R.M.; García-Sancho, M.; Sainz, A.; Ackermann, M.R.; Suchodolski, J.S.; Steiner, J.M.; Jergens, A.E. Serologic and Fecal Markers to Predict Response to Induction Therapy in Dogs with Idiopathic Inflammatory Bowel Disease. J. Vet. Intern. Med. 2018, 32, 999–1008.
- Heilmann, R.M.; Berghoff, N.; Mansell, J.; Grützner, N.; Parnell, N.K.; Gurtner, C.; Suchodolski, J.S.; Steiner, J.M. Association of Fecal Calprotectin Concentrations with Disease Severity, Response to Treatment, and Other Biomarkers in Dogs with Chronic Inflammatory Enteropathies. J. Vet. Intern. Med. 2018, 32, 679–692.
- Riggers, D.S.; Gurtner, C.; Protschka, M.; Böttcher, D.; von Bomhard, W.; Alber, G.; Winter, K.; Steiner, J.M.; Heilmann, R.M. Intestinal S100/Calgranulin Expression in Cats with Chronic Inflammatory Enteropathy and Intestinal Lymphoma. Animals 2022, 12, 2044.
- Xiao, D.; Wang, Y.; Liu, G.; He, J.; Qiu, W.; Hu, X.; Feng, Z.; Ran, M.; Nyachoti, C.M.; Kim, S.W.; et al. Effects of Chitosan on Intestinal Inflammation in Weaned Pigs Challenged by Enterotoxigenic Escherichia coli. PLoS ONE 2014, 9, e104192.
- Grosche, A.; Morton, A.J.; Graham, A.S.; Polyak, M.M.R.; Freeman, D.E. Effect of Large Colon Ischemia and Reperfusion on Concentrations of Calprotectin and Other Clinicopathologic Variables in Jugular and Colonic Venous Blood in Horses. Am. J. Vet. Res. 2013, 74, 1281–1290.
- Chiavaccini, L.; Hassel, D.M.; Shoemaker, M.L.; Charles, J.B.; Belknap, J.K.; Ehrhart, E.J. Detection of Calprotectin and Apoptotic Activity within the Equine Colon from Horses with Black Walnut Extract-Induced Laminitis. Vet. Immunol. Immunopathol. 2011, 144, 366–373.
- Aydin, O.; Ulas, N.; Genc, A.; Baysal, S.; Kandemir, O.; Aktas, M.S. Investigation of Hemogram, Oxidative Stress, and Some Inflammatory Marker Levels in Neonatal Calves with Escherichia Coli and Coronavirus Diarrhea. Microb. Pathog. 2022, 173, 105802.
- Dal Pont, G.C.; Belote, B.L.; Lee, A.; Bortoluzzi, C.; Eyng, C.; Sevastiyanova, M.; Khadem, A.; Santin, E.; Farnell, Y.Z.; Gougoulias, C.; et al. Novel Models for Chronic Intestinal Inflammation in Chickens: Intestinal Inflammation Pattern and Biomarkers. Front. Immunol. 2021, 12, 676628.
- Pepper, R.J.; Wang, H.-H.; Rajakaruna, G.K.; Papakrivopoulou, E.; Vogl, T.; Pusey, C.D.; Cook, H.T.; Salama, A.D. S100A8/A9 (Calprotectin) Is Critical for Development of Glomerulonephritis and Promotes Inflammatory Leukocyte–Renal Cell Interactions. Am. J. Pathol. 2015, 185, 1264–1274.
- Gopal, R.; Monin, L.; Torres, D.; Slight, S.; Mehra, S.; McKenna, K.C.; Junecko, B.A.F.; Reinhart, T.A.; Kolls, J.; Báez-Saldaña, R.; et al. S100A8/A9 Proteins Mediate Neutrophilic Inflammation and Lung Pathology during Tuberculosis. Am. J. Respir. Crit. Care Med. 2013, 188, 1137–1146.
- Ider, M.; Maden, M. Biomarkers of Infectious Pneumonia in Naturally Infected Calves. Am. J. Vet. Res. 2022, 83, 1–8.
- Mizobuchi, H.; Fujii, W.; Isokawa, S.; Ishizuka, K.; Wang, Y.; Watanabe, S.; Sanjoba, C.; Matsumoto, Y.; Goto, Y. Exacerbation of Hepatic Injury during Rodent Malaria by Myeloid-Related Protein 14. PLoS ONE 2018, 13, e0199111.
- Chung, T.-H.; Oh, J.-S.; Lee, Y.-S.; Kang, K.-S.; Jung, J.-W.; Youn, H.-Y.; Hwang, C.-Y. Elevated Serum Levels of S100 Calcium Binding Protein A8 (S100A8) Reflect Disease Severity in Canine Atopic Dermatitis. J. Vet. Med. Sci. 2010, 72, 693–700.
- Jin, S.; Park, C.O.; Shin, J.U.; Noh, J.Y.; Lee, Y.S.; Lee, N.R.; Kim, H.R.; Noh, S.; Lee, Y.; Lee, J.-H.; et al. DAMP Molecules S100A9 and S100A8 Activated by IL-17A and House-Dust Mites Are Increased in Atopic Dermatitis. Exp. Dermatol. 2014, 23, 938–941.
- Yun, J.; Xiao, T.; Zhou, L.; Beuerman, R.W.; Li, J.; Zhao, Y.; Hadayer, A.; Zhang, X.; Sun, D.; Kaplan, H.J.; et al. Local S100A8 Levels Correlate with Recurrence of Experimental Autoimmune Uveitis and Promote Pathogenic T Cell Activity. Investig. Opthalmol. Vis. Sci. 2018, 59, 1332.
- Cremers, N.A.J.; van den Bosch, M.H.J.; van Dalen, S.; Di Ceglie, I.; Ascone, G.; van de Loo, F.; Koenders, M.; van der Kraan, P.; Sloetjes, A.; Vogl, T.; et al. S100A8/A9 Increases the Mobilization of pro-Inflammatory Ly6Chigh Monocytes to the Synovium during Experimental Osteoarthritis. Arthritis Res. Ther. 2017, 19, 217.
- Blom, A.B.; van den Bosch, M.H.; Blaney Davidson, E.N.; Roth, J.; Vogl, T.; van de Loo, F.A.; Koenders, M.; van der Kraan, P.M.; Geven, E.J.; van Lent, P.L. The Alarmins S100A8 and S100A9 Mediate Acute Pain in Experimental Synovitis. Arthritis Res. Ther. 2020, 22, 199.
- Geven, E.J.W.; van den Bosch, M.H.J.; Di Ceglie, I.; Ascone, G.; Abdollahi-Roodsaz, S.; Sloetjes, A.W.; Hermann, S.; Schäfers, M.; van de Loo, F.A.J.; van der Kraan, P.M.; et al. S100A8/A9, a Potent Serum and Molecular Imaging Biomarker for Synovial Inflammation and Joint Destruction in Seronegative Experimental Arthritis. Arthritis Res. Ther. 2016, 18, 247.
- Sekimoto, R.; Kishida, K.; Nakatsuji, H.; Nakagawa, T.; Funahashi, T.; Shimomura, I. High Circulating Levels of S100A8/A9 Complex (Calprotectin) in Male Japanese with Abdominal Adiposity and Dysregulated Expression of S100A8 and S100A9 in Adipose Tissues of Obese Mice. Biochem. Biophys. Res. Commun. 2012, 419, 782–789.
- Heilmann, R.M.; Xenoulis, P.G.; Müller, K.; Stavroulaki, E.M.; Suchodolski, J.S.; Steiner, J.M. Association of Serum Calprotectin (S100A8/A9) Concentrations and Idiopathic Hyperlipidemia in Miniature Schnauzers. J. Vet. Intern. Med. 2019, 33, 578–587.
- Franco-Martínez, L.; Gelemanović, A.; Horvatić, A.; Contreras-Aguilar, M.D.; Mrljak, V.; Cerón, J.J.; Martínez-Subiela, S.; Tvarijonaviciute, A. The Serum and Saliva Proteome of Dogs with Diabetes Mellitus. Animals 2020, 10, 2261.
- Ozel, Y.; Elcioglu, H.K.; Cevikelli, Z.A.; Kudas, I.; Ahmad, S.; Uzun, H.; Topal, C.; Aktas, S.; Kabasakal, L. Ischemic Colitis of the Colon in Streptozotocin-Induced Diabetic Rats. Mol. Cell. Biochem. 2018, 439, 87–93.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
769
Revisions:
2 times
(View History)
Update Date:
03 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




